Abstract
Recent outbreaks of influenza A highlight the importance of rapid and sufficient supply for pandemic and inter-pandemic vaccines. Classical manufacturing methods for influenza vaccines fail to satisfy this demand. Alternatively, cell culture-based production systems and virus-like particle (VLP)-based technologies have been established. We developed swine-origin pandemic H1N1 influenza VLPs consisting of hemagglutinin (A/California/04/2009) and matrix protein. Hemagglutinin and matrix protein were co-expressed in insect cells by the baculovirus expression system. VLPs were harvested from infection supernatants, purified and used for intraperitoneal immunization of BALB/c mice. Immunization induced high serum antibody titers against A/California/04/2009 as well as hemagglutination inhibiting antibodies. Additionally, we compared VLP production in two different insect cell lines, Sf9 and BTI-TN5B1-4 (High Five™). Taken together VLPs represent a potential strategy for the fight against new pandemic influenza viruses.
Keywords: H1N1, Influenza vaccine, Insect cells, Pandemic influenza, Virus-like particle
1 Introduction
In June 2009, the World Health Organization raised the pandemic level for swine-origin H1N1 influenza A virus to phase 6. As demonstrated by this latest outbreak, influenza A viruses represent a continuous pandemic threat [1-3]. Vaccination has been the most effective way to prevent the disease resulting from influenza virus infections. Classical egg-derived influenza vaccines can be produced in quantities that are needed in an inter-pandemic seasonal influenza situation but, due to limited egg supply, may be insufficient in case of a pandemic. Alternatively, cell culture-based methods have been established for faster and higher yield production. However, biosafety issues and difficulties with growth and yield of certain isolates may cause constraints during production. Besides whole virus vaccines, subunit vaccines comprising hemagglutinin (HA) and virus-like particles (VLPs) are considered as alternative strategies and have been shown to successfully induce neutralizing immune response [4-13].
Influenza A virus is a negative-sense single-stranded RNA virus belonging to the Orthomyxoviridae family. The virion consists of a host cell-derived envelope, which is lined on the inner side by the matrix protein (M1). On the surface there are multiple copies of HA, which is responsible for attachment to the host cell surface receptors, and neuraminidase (NA), which causes the release of new virus particles from the host cell [14]. HA and NA are both glycoproteins and are the major antigens of influenza A viruses. Successful prophylactic influenza vaccines have to induce neutralizing antibodies that prevent HA from binding to cellular receptors.
Insect cell-derived VLPs have been produced previously and shown to be promising candidates for vaccination [15].VLPs are able to induce B cell-mediated immune responses as well as cytotoxic T cell responses and CD4+ proliferation [15]. The market entry of GlaxoSmithKline’s human papilloma virus vaccine (Cervarix™) finally demonstrated the potency of insect cell-derived VLP vaccines.
Influenza VLPs have been expressed in various cell systems including mammalian cells, plants and insect cells [4-13, 16]. Insect cell-derived influenza A VLPs have been produced and studied by various investigators. Co-expression of HA, NA and M1 or HA and M1, respectively, in insect cells by the baculoviral expression system has been shown to lead to the formation of VLPs [7-13, 16, 17].VLPs of HA subtypes H1, H3, H5, H7 and H9 have been shown to elicit protective immune responses against influenza A virus when administered intranasally, intraperitoneally or intramuscularly [4, 6-12, 16].
In this study we generated VLPs as an alternative vaccine candidate for swine-origin H1N1 pandemic influenza A virus.VLP production in two insect cell lines, Sf9 and BTI-TN5B1-4 (High Five™), was compared in terms of yield and purity. Furthermore, mice were immunized with BTI-TN5B1-4-derived particles. Induction of neutralizing antibodies and IgG levels were analyzed.
2 Materials and methods
2.1 Cells
Spodoptera frugiperda Sf9 cells (ATCC CRL-1711) were maintained as adherent cultures in Roux flasks in modified insect cell medium IPL-41 [supplemented with lipid mixture (Sigma, St. Louis, USA) and yeast extract (Sigma)] and 3% fetal calf serum (FCS) at 27°C [18]. Sf9 cells dedicated to VLP production were cultivated in the same media in suspension in 500-mL shaker flasks at 100 rpm. Trichoplusia ni BTI-TN5B1-4 cells (ATCC CRL-10859) were maintained in shaker flasks in serum-free modified IPL-41 media at 27°C shaking at 100 rpm [18].
2.2 Cloning and recombinant baculovirus generation
The A/California/04/2009 HA gene (GenBank: GQ117044.1) was synthesized by Geneart (Regensburg, Germany). The synthesis vector pGA was digested by the restriction endonucleases EcoRV and NotI. The hemagglutinin fragment was ligated into a StuI and NotI-digested pBacPAK8 (Clontech, CA, USA), resulting in pBacPAK8-SF.
The M1 gene from strain A/Udorn/307/1972 (H3N2) (GenBank: DQ508932.1) was synthesized by Sloning (Puchheim, Germany). The synthesis vector pPCR-M1 was digested by the restriction endonucleases XhoI and NotI. The M1 fragment was ligated into an XhoI and NotI-digested pBac-PAK8, resulting in pBacPAK8-UM. The green fluorescent protein (GFP) gene was ligated into an HindIII and XhoI-digested pBac-5 (Merck, Darmstadt, Germany), resulting in pBac-5-GFP. Recombinant baculoviruses (rBVs) were generated by cotransfection of pBacPAK8-SF, pBacPAK8-UM, or pBac-5-GFP together with Baculogold Autographa californica nuclear polyhedrosis virus (AcNPV) linearized DNA (Pharmingen, San Diego, USA) into Sf9 cells by Cellfectin (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions.
2.3 Production, purification and characterization of VLPs
BTI-TN5B1-4 or Sf9 cells were co-infected with rBV expressing HA and M1 at a multiplicity of infection of 10 and a cell density of 1 × 106 cells/mL in 500-mL shaker flasks. Cells were harvested 72 h post infection and separated from supernatant by low-speed centrifugation for 10 min at 2000 × g at room temperature. The supernatant was tested for VLP content by hemagglutination assay and for baculovirus titer by plaque assay on Sf9 cells [19]. VLPs were pelleted by ultracentrifugation at 136 000 × g at 20°C for 90 min. Pellets were resuspended in PBS for 1 h at room temperature. VLPs were further purified by discontinuous sucrose density gradient centrifugation (20%, 30%, 40%, 50%, 60%) at 190 000 × g at 4°C for 16 h. Gradients were split into fractions, which were analyzed by SDS-PAGE, Western blot and protein quantification with Bradford reagent (Bio-Rad, Hercules, USA).The two fractions containing most of the VLP material were diluted with PBS and were pelleted by ultracentrifugation at 4°C at 136 000 × g for 90 min. The pellets were resuspended in SPGN buffer (6% sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM l-glutamic acid, 75 mM NaCl, pH 7.5) and tested for VLP content by hemagglutination assay and for baculovirus titer by plaque assay on Sf9 cells. An rBV expressing GFP as negative control for animal testing was produced by three-step ultracentrifugation from similar gradient fractions as for VLPs.
2.4 SDS-PAGE and blots
Sucrose gradient fractions, infection supernatants and vaccination preparation were examined by SDS-PAGE and Coomassie staining [20]. Western Blot analysis was performed using anti-M1 (Institute of Virology, Bratislava) and anti-H1 antibodies (Institute of Virology, Bratislava) and a secondary anti-mouse IgG alkaline phosphatase-labeled goat antibody (Sigma).
2.5 Transmission electron microscopy
Sucrose gradient fractions were used for electron microscopy.VLPs were adsorbed for 1 min on copper grids directly from sucrose gradient fraction 7 and washed with PBS containing 1% BSA. Negative staining was performed using uranyl acetate (pH 4.5) [21]. Samples were examined on a transmission electron microscope at various magnifications.
2.6 Hemagglutination assay
VLPs were serially twofold diluted in PBS and incubated at room temperature for 2 h with 50 μL 1.25% human red blood cells (RBC, blood group 0). The hemagglutination extent was estimated visually. The hemagglutination titer designated as hemagglutination units (HAU) was determined by the reciprocal of the highest dilution without complete sedimentation of RBC.
2.7 Animals and vaccination
BTI-TN5B1-4-derived influenza VLPs were pooled from sucrose gradient fractions, diluted in PBS and pelleted by ultracentrifugation at 136 000 × g at 4°C. Pellets were resuspended in SPGN buffer (6% sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM l-glutamic acid, 75 mM NaCl, pH 7.5).
For immunization, 6-week-old female BALB/c mice were used. Mice (three to four mice per group) were vaccinated with purified VLPs (0.25 μg HA), with GFP-expressing rBV (~100 ng, the same amount as the baculovirus background found in the VLP preparation) and with SPGN, respectively, via intraperitoneal injection at week 0. Mice were boosted on day 25 with VLPs carrying total 3 μg HA, whereas the negative control animals received baculovirus preparation or SPGN. On days 0, 25 and 47, blood was collected from anesthetized mice via the orbit and transferred to a microfuge tube. Tubes were centrifuged and sera was removed and frozen at −20°C. Immunological tests in mice were approved by the Austrian Federal Ministry of Science and Research according to Austrian law (ZI. 200910116).
2.8 Evaluation of humoral immune response
A/California/04/2009-specific serum antibodies were determined by ELISA. The 96-well microtiter plates (Nunc-Immuno Plate Maxisorp) were coated with 2 μg/mL A/California/07/2009 standard (NIBSC, London, UK) in coating buffer for 2 h at room temperature. Plates were blocked with PBS containing 3% BSA and 0.005% Tween 20 (room temperature, 2 h) and incubated with serial twofold dilutions of each 1:3200 pre-diluted serum samples (room temperature, 2 h). Afterwards, plates were washed with PBS containing 0.005% Tween 20 and incubated with peroxidase-conjugated anti-mouse IgG antibody (Sigma, A3673) in PBS containing 1% BSA and 0.005% Tween 20 (room temperature, 1 h). Unbound antibody was removed and plates were washed. Samples were incubated with substrate (o-phenylenediamine and H2O2), reaction was stopped using H2SO4 and the colorimetric change was measured at 492 nm.
2.9 Hemagglutination inhibition assay
Sera of immunized mice were treated with receptor-destroying enzyme (Szabo Scandic, Vienna, Austria) overnight at 37°C and heat-inactivated at 56°C for 30 min [22]. Serial twofold dilutions were prepared in a 96-well microtiter plate and 25 μL containing 4 HAU antigen A/Texas/5/2009 re-as-sortant (IDCDC-RG15 CDC) – or A/California/04/2009 – presenting VLPs, respectively, was added. After 30 min of incubation 50 μL 0.5% chicken erythrocytes solution (derived from adult chicken blood) was added. The assay was incubated for 2 h at +4°C and hemagglutination extent was estimated visually. The hemagglutination inhibition (HAI) titration end point was determined by the highest dilution of antiserum that still inhibits complete hemagglutination of chicken erythrocytes.The presented value is the reciprocal of the last serum dilution inhibiting virus hemagglutination. For a better comparison of the two antigens (re-assortant virus and VLPs) geometric mean titers (GMT) of all analyzed groups were calculated.
3 Results
3.1 Production and characterization of influenza A/California/04/2009 VLPs in two different insect cell lines
VLPs were generated by co-infection of Sf9 or BTI-TN5B1-4 cells with HA(A/California/04/2009) and M1(A/Udorn/307/1972) expressing baculoviruses. VLPs were detected and quantified in the supernatant by hemagglutination assay, while the concentration of baculoviruses was measured by plaque assay. Baculovirus titers were ~1 × 108 PFU/mL in Sf9 supernatants and ~1 × 106 PFU/mL in BTI-TN5B1-4 supernatants (Table 1). Sf9 and BTI-TN5B1-4 supernatants showed a biological activity of 16 HAU/50 μL, although BTI-TN5B1-4 supernatants contained a sevenfold higher HA concentration when analyzed by Western blotting (Table 1).Thus, a total higher content of HA protein was detected in BTI-TN5B1-4-derived VLPs, in spite of the same hemagglutination titer as for Sf9-derived VLPs.
Table 1.
Comparison between Sf9 and BTI-TN5B1-4 cells as production platforms for influenza A VLPs
| Sf9 | BTI-TN5B1-4 | |
|---|---|---|
| HAU/mL | 320 | 320 |
| HA protein yield in μg/mL | 0.4 | 3 |
| Baculovirus titer PFU/mL | ~108 | ~106 |
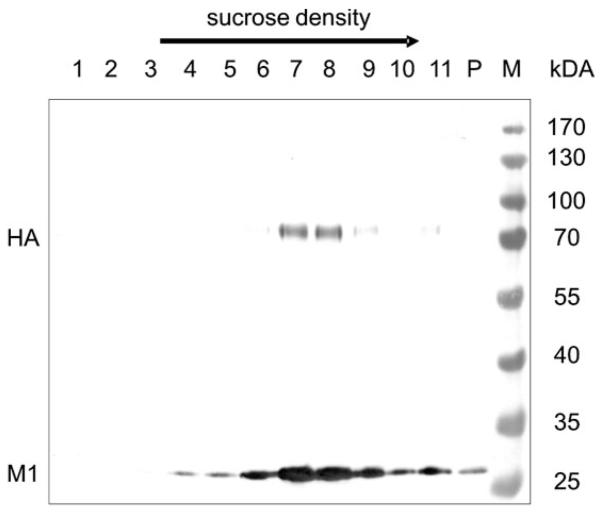
For investigating the size and shape of the virions, VLPs produced in both cell systems were purified and compared with respect to their density in a sucrose gradient.VLPs were harvested from supernatants by ultracentrifugation, resuspended pellets were further purified by sucrose density gradient centrifugation and different fractions were analyzed by Western blot analysis. As shown in Fig. 1, the HA protein was detected as an approximately 70-kDa protein and the M1 protein was detected as a 29-kDa protein. In the VLP preparation derived from the BTI-TN5B1-4 cell culture supernatant (Fig. 1), M1 was found in fractions 4-11 and in the pellet.The highest concentrations of HA and therefore of VLPs were found in fraction 7 and 8 (~40–45% sucrose) in the BTI-TN5B1-4 preparation and in fraction 6 and 7 (~35–40% sucrose) in the Sf9 preparation (data not shown). To confirm the structure of insect cell-derived VLPs, electron microscopy was performed. Particles from fraction 7 of both purifications were stained with uranyl acetate (pH 4.5) as described and used for transmission electron microscopy (Fig. 2) [21]. Both cell lines produced spheric and partially polymorphic VLPs of 80–120 nm diameter, which is consistent with the morphology of wildtype H1 influenza virions. VLPs containing HAs from various strains were produced in Sf9 cells, and all fractions of the sucrose gradient showed a similar picture (80-120 nm in diameter) when analyzed by electron microscopy (data not shown).
Figure 1.
Western blot of BTI-TN5B1-4 cell culture-derived A/California/04/2009 VLPs in a discontinuous sucrose density gradient (20–60% sucrose). The blot was developed using anti-M1 and anti-H1 mAbs and an alkaline phosphatase-conjugated anti-mouse IgG mAb, respectively. Lane 1 is the top fractions with the lowest sucrose concentration, lane 11 harbors the highest sucrose concentration, P indicates the pellet. Positions of HA and M1 are indicated.
Figure 2.
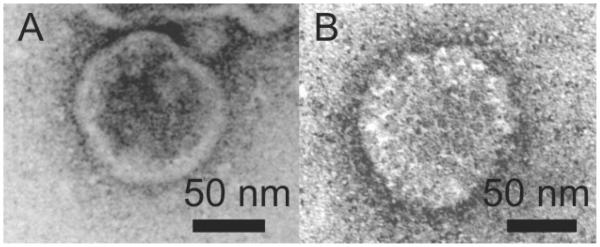
Transmission electron micrographs of negatively stained A/California/04/2009 VLPs derived from BTI-TN5B1-4 cells (A) and Sf9 cells (B).
3.2 Analysis of immune response
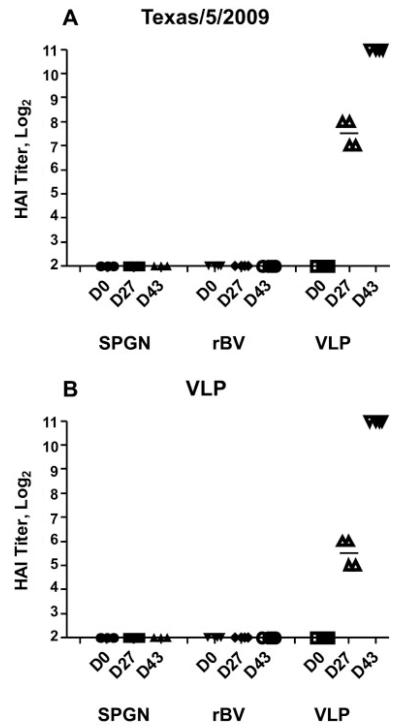
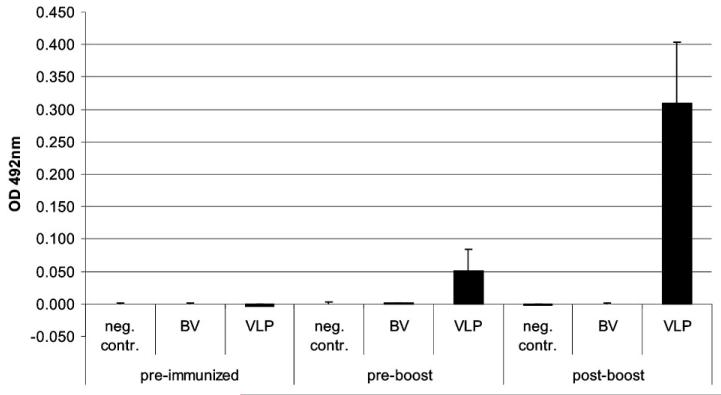
Immunogenicity of VLPs was examined in BALB/c mice (6 weeks) by intraperitoneal injection, at days 0 and 25, of purified antigen (VLPs carrying total 0.25 and 3 μg HA, four animals), of purified GFP-expressing rBV (~100 ng, four animals) and of SPGN (sucrose-phosphate-glutamine-NaCl buffer; three animals). Pre-immune sera and post-vaccination antisera 25 days after the first vaccination and 22 days after the boost were evaluated for the induction of specific immune response by ELISA and by HAI assay. Serum antibodies with the ability to prevent virus-induced agglutination of chicken RBCs are a main indicator for vaccine-induced immune response and consequently for the efficiency of vaccines. Four out of four mice sero-converted after the first immunization and responses significantly increased after boosting. As can be seen in Fig. 3, sera from pre-immunized mice and from the negative controls showed no immune response, whereas sera from mice single immunized with VLPs (0.25 μg HA) showed a GMT of 181 (titers between 128 and 256 HAI units) with A/Texas/5/2009 re-assortant as antigen, and a GMT of 45 (titers between 32 and 64 HAI units) with A/California/04/2009 VLPs used as antigen (Fig. 3). After the second immunization with VLPs carrying a total of 3 μg HA, the GMT titers with both antigens increased to 2048 HAI units (Fig. 3). IgG levels against A/California/04/2009 were tested by IgG ELISA. ELISA titers of 1:3200 pre-diluted sera (absorbance at 492 nm) ranged from 0.027 to 0.098 in immunized mice, whereas sera from animals from both negative controls showed only background reactivity (−0.004 to 0.001).After boosting, a significant rise of titers from immunized mice could be observed. Titers reached values between 0.215 and 0.439; both negative controls showed again only background reactivity (−0.004 to 0.002) (Fig. 4).
Figure 3.
HAI titers. Day 0, 25 (post-immunized) and 47 (22 days post-boost) serum HAI antibody responses were assessed against an H1N1 A/Texas/5/2009 re-assortant (A) and H1N1 A/California/04/2009-presenting VLPs (B). Bars indicate GMT. Negative controls (neg. contr.) and baculovirus controls (BV) showed only background levels at all time points, whereas A/California/04/2009 VLPs (VLP) raised serum antibodies able to prevent virus-induced agglutination of chicken RBCs. Antibody levels increased to 2048 HAI units after boosting.
Figure 4.
Influenza HA-specific total serum IgG responses. Day 0, 25 (post-immunized) and 47 (22 days post-boost) serum IgG antibody responses were assessed by ELISA against H1N1 A/California/07/2009 standard antigen (NIBSC). Sera were 1:3200 pre-diluted. Negative controls (neg. contr.) and baculovirus controls (BV) showed only background levels of specific anti-HA IgG at all time points, whereas A/California/04/2009 VLPs (VLP) raised high levels of specific anti-HA antibodies. Absorbances were read at 492 nm and results are expressed as arithmetic mean titer.
4 Discussion
This report describes the production and the immunogenicity of swine-origin pandemic H1N1 A/California/04/2009 HA-containing influenza A VLPs. VLPs produced in the two cell lines Sf9 and in BTI-TN5B1-4 (High Five™) were compared. Supernatants from both cell lines showed a similar and constant biological activity of 16 HAU/50 μL, but BTI-TN5B1-4 supernatants contained a seven-fold higher HA concentration when analyzed by Western blot analysis (Table 1).Additional analysis by electron microscopy and by gradient centrifugation showed that VLPs from both cell lines had a similar size and shape; however, the main portion of BTI-TN5B1-4-derived VLPs accumulated at a higher density during centrifugation. We assume that two preparations of similar VLPs accumulating at different densities have different numbers of HA molecules integrated in their membranes.This fact could explain the higher amount of HA protein found in the BTI-TN5B1-4 cell culture supernatant.
Another main difference between both production platforms was the concentration of baculoviruses in the production supernatants. Sf9 supernatants contained approximately ~1 × 108 PFU baculovirus/mL, whereas BTI-TN5B1-4 supernatants contained ~1 × 106 PFU/mL. The presence of baculoviruses and hence DNA contamination is considered a major problem for insect cell-derived influenza VLP vaccine production [11]. Baculovirus has been shown to transduce a variety of mammalian cells very efficiently and in vivo provokes expression of various kinds of cytokines, which may lead to an un-intended adjuvant effect as well as to unwanted inflammatory reactions [23, 24]. Additionally, a theoretical risk of integration of baculovirus DNA into the cellular genome of the vaccinee and consequential DNA damage cannot be ruled out [23]. So far, generation of insect cell-derived influenza VLP vaccines have usually been carried out in Sf9 cells. In terms of downstream processing, it has to be noted that Sf9 cells were cultivated in the presence of FCS, while BTI-TN5B1-4 cells are easily cultivated in serum-free media. We consider the establishment of BTI-TN5B1-4 cells an advantageous production system, as it decreases the protein background (FCS) as well as the baculovirus background and, hence, DNA contamination. Additionally, Hi-5 Rix4446 cells, which are derived from a BTI-TN5B1-4 cell subclone, are used as production cell line for the production of a human papilloma virus vaccine (Cervarix™). Cervarix™ was approved by the European Medicines Agency (EMEA). Consequently, our further immunological studies were performed with BTI-TN5B1-4 cell-derived VLPs.
Fractions of homogeneous consistency were achieved using purification of the influenza VLPs by sucrose gradient centrifugation (Fig. 1). The calculated mass of A/California/04/2009 HA was 63 kDa. Nevertheless, the HA band in the blot appears slightly above 70 kDa, which leads to the conclusion that the HA is glycosylated. Insect cells perform complex glycosylation but in a slightly different manner than mammalian cells. Insect cells are not able to add sialic acid or galactose to their terminal N-glycan structures; generally they show a paucimannose type of glycosylation [25]. In the case of BTI-TN5B1-4 cells some galactosylation activity has been shown previously [26]. Nevertheless, a negative effect of the different glycosylation pattern found in insect cells, as compared to mammalian cells, on the immunogenicity of the recombinant HA in the in vivo assay has not yet been reported.
Neutralizing antibodies against the influenza HA are the main indicator for protective immunity against infection. We demonstrated that vaccination with A/California/04/2009 VLPs lead to seroconversion of four out of four tested mice. HAI assays showed high titers of neutralizing antibodies in all four vaccinated mice. Both negative control groups showed no or just background antibody activity. Hemagglutination inhibition assay was carried out with two different antigens, an A/Texas/5/2009 H1N1 re-assortant and A/California/04/2009 HA presenting VLPs. At lower titers the hemagglutination inhibition assay seems to have a higher sensitivity with the A/Texas/5/2009 re-assortant as antigen. On the other hand the use of VLPs as antigen in the hemagglutination assay has advantages from the safety point of view. In general, ELISA data on anti-HA IgG reflects the results from HAI assay (Fig. 4). Influenza A VLPs have been produced in insect cells (Sf9) displaying various subtypes of HA before [7, 9, 11, 27]. Although, different experimental settings were applied, in all cases IgG levels in mice increased after boosting. The HAI titers shown in our study compare to reports by others. For example, Pushko et al. [11] showed HAI titers in the range 320–640 after a second boosting, while Ross et al. [9] were able to induce HAI titers up to 2048. Virus neutralization assay and challenge studies will give additional and definite information about protective immunity induced by A/California/04/2009 VLPs in mice; however, for the scope of this first study we considered fast production and proof of principle as the main goals. As a first promising step towards an alternative pandemic influenza vaccine strategy, we demonstrated that A/California/04/2009 VLPs were able to induce high levels of hemagglutination inhibiting antibodies and a high anti-HA serum IgG level in absence of adjuvants.
Not only efficacy, but also time is a very critical factor in vaccine production, especially in a pandemic situation. It took us 51 days from beginning of the cloning work to the production of the first VLP batch for mouse tests.Taken into account that gene synthesis of a 1700-bp gene will take approximately 2 weeks, the vaccine production for a new influenza subtype could be started within less than 10 weeks after first isolation of the RNA sequence of a new strain.VLP vaccines circumvent problems like slow growth of isolates and therefore un-predictable yields, mutation of HA through host adaption and the need for biosafety level (BSL)-3 facilities, which are obligatory in cell culture-based production of inactivated pandemic influenza vaccines. VLP vaccines could be produced in any standard-equipped good manufacturing practice BSL-1 laboratory with an additional ultracentrifuge, a laminar flow hood and a shaker or a wave bag device. Insect cell-derived influenza VLPs not only represent a very promising candidate for inter-pandemic annual influenza vaccine, they are also a very fast, safe and effective alternative vaccine approach especially for newly emerging pandemic influenza strains like swine-origin H1N1 or avian H5N1.
Acknowledgments
We thank Ingo Klancnik for excellent assistance regarding ELISA experiment design. This work was supported by the Austrian Science Fund (FWF).
Abbreviations
- FCS
fetal calf serum
- GFP
green fluorescent protein
- GMT
geometric mean titer
- H1–H9
HA subtypes 1–9
- HA
hemagglutinin
- HAI
HA inhibition
- HAU
HA unit
- M1
matrix protein
- NA
neuraminidase
- RBC
red blood cells
- rBV
recombinant baculovirus
- VLP
virus-like particle
Footnotes
The authors have declared no conflict of interest.
References
- [1].Schnitzler S, Schnitzler P. An update on swine-origin influenza virus A/H1N1: A review. Virus Genes. 2009 doi: 10.1007/s11262-009-0404-8. in press. DOI: 10.1007/s11262-009-0404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Michaelis M, Doerr H, Cinatl JJ. Novel swine-origin influenza A virus in humans: Another pandemic knocking at the door. Med. Microbiol. Immunol. 2009;198:175–183. doi: 10.1007/s00430-009-0118-5. [DOI] [PubMed] [Google Scholar]
- [3].Peiris J, Tu W, Yen H. A novel H1N1 virus causes the first pandemic of the 21(st) century. Eur. J. Immunol. 2009;39:2946–2954. doi: 10.1002/eji.200939911. [DOI] [PubMed] [Google Scholar]
- [4].Cox M. Progress on baculovirus-derived influenza vaccines. Curr. Opin. Mol. Ther. 2008;10:56–61. [PubMed] [Google Scholar]
- [5].D’Aoust M, Lavoie P, Couture M, Trépanier S, et al. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 2008;6:930–940. doi: 10.1111/j.1467-7652.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- [6].Kang S, Song J, Quan F, Compans R. Influenza vaccines based on virus-like particles. Virus Res. 2009;143:140–146. doi: 10.1016/j.virusres.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bright R, Carter D, Daniluk S, Toapanta F, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- [8].Mahmood K, Bright R, Mytle N, Carter D, et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- [9].Ross T, Mahmood K, Crevar C, Schneider-Ohrum K, et al. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One. 2009;4:e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perrone L, Ahmad A, Veguilla V, Lu X, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J.Virol. 2009;83:5726–5734. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pushko P, Tumpey T, Bu F, Knell J, et al. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–5759. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- [12].Quan F, Huang C, Compans R, Kang S. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Galarza J, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:244–251. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- [14].Nayak D, Balogun R, Yamada H, Zhou Z, Barman S. Influenza virus morphogenesis and budding. Virus Res. 2009;143:147–161. doi: 10.1016/j.virusres.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roy P, Noad R. Virus-like particles as a vaccine delivery system: Myths and facts. Hum.Vaccin. 2008;4:5–12. doi: 10.4161/hv.4.1.5559. [DOI] [PubMed] [Google Scholar]
- [16].Szécsi J, Boson B, Johnsson P, Dupeyrot-Lacas P, et al. Induction of neutralising antibodies by virus-like particles harbouring surface proteins from highly pathogenic H5N1 and H7N1 influenza viruses. Virol. J. 2006;3:70. doi: 10.1186/1743-422X-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Latham T, Galarza J. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 2001;75:6154–6165. doi: 10.1128/JVI.75.13.6154-6165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wickham T, Davis T, Granados R, Shuler M, Wood H. Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotechnol. Prog. 8:391–396. doi: 10.1021/bp00017a003. [DOI] [PubMed] [Google Scholar]
- [19].King L, Hitchman R, Possee R. Recombinant baculovirus isolation. Methods Mol. Biol. 2007;388:77–94. doi: 10.1007/978-1-59745-457-5_4. [DOI] [PubMed] [Google Scholar]
- [20].Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [21].Sarkar N, Manthey W, Sheffield J. The morphology of murine oncornaviruses following different methods of preparation for electron microscopy. Cancer Res. 1975;35:740–749. [PubMed] [Google Scholar]
- [22].Romanova J, Krenn B, Wolschek M, Ferko B, et al. Preclinical evaluation of a replication-deficient intranasal DeltaNS1 H5N1 influenza vaccine. PLoS One. 2009;4:e5984. doi: 10.1371/journal.pone.0005984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hu Y. Baculoviral vectors for gene delivery: A review. Curr. Gene Ther. 2008;8:54–65. doi: 10.2174/156652308783688509. [DOI] [PubMed] [Google Scholar]
- [24].Kost T, Condreay J. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 2002;20:173–180. doi: 10.1016/s0167-7799(01)01911-4. [DOI] [PubMed] [Google Scholar]
- [25].Kost T, Condreay J, Jarvis D. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Altmann F, Staudacher E, Wilson I, März L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- [27].Haynes J, Dokken L, Wiley J, Cawthon A, et al. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2009;27:530–541. doi: 10.1016/j.vaccine.2008.11.011. [DOI] [PubMed] [Google Scholar]