Abstract
Extracellular matrix (ECM), a major component of the cellular microenvironment, plays critical roles in normal tissue morphogenesis and disease progression. Binding of ECM to membrane receptor proteins, such as integrin, discoidin domain receptors, and dystroglycan, elicits biochemical and biomechanical signals that control cellular architecture and gene expression. These ECM signals cooperate with growth factors and hormones to regulate cell migration, differentiation, and transformation. ECM signaling is tightly regulated during normal mammary gland development. Deposition and alignment of fibrillar collagens direct migration and invasion of mammary epithelial cells during branching morphogenesis. Basement membrane proteins are required for polarized acinar morphogenesis and milk protein expression. Deregulation of ECM proteins in the long run is sufficient to promote breast cancer development and progression. Recent studies demonstrate that the integrated biophysical and biochemical signals from ECM and soluble factors are crucial for normal mammary gland development as well as breast cancer progression.
Keywords: extracelluar matrix, mammary gland development, breast cancer progression, mechanotransduction
Introduction
Cells in vivo are surrounded by or adhere to the extracellular matrix (ECM). ECM is the non-cellular component present within all tissues and organs, and contains fibrous proteins and polysaccharides such as collagen, laminin, fibronectin and hyaluronan (Naba et al., 2012). These ECM molecules are classified into two subgroups: basement membrane (BM) and interstitial/stromal ECM (Guo & Giancotti, 2004). Basement membranes are thin layers of ECM which usually underlie epithelial or endothelial cells, while the interstitial ECM fills in the intercellular space. Cell-ECM adhesion is mediated by the ECM receptors, including integrins, discoidin domain receptors (DDR), dystoglycans, syndecans, CD44, and Rhamm (Xu, Boudreau, et al., 2009). Binding of ECM to the receptors induces a cascade of both biochemical and biomechanical signals which transmit from the cell membrane to the nucleus (Figure 1), necessary for cellular architecture and function (Xu, Boudreau, et al., 2009).
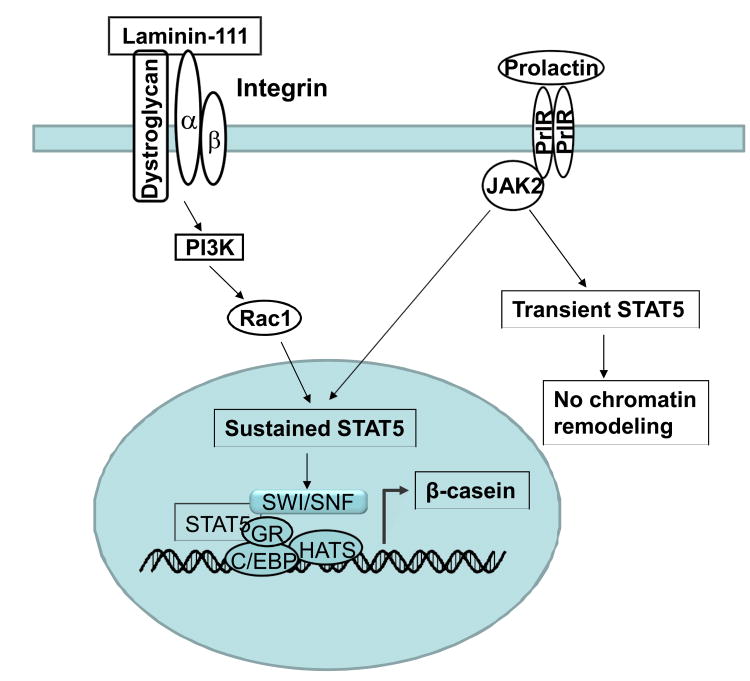
Figure 1.
Mammary gland epithelial cells form polarized acinar structures when cultured in 3D matrigel. Treatment with prolactin can activate JAK2-STAT5 pathway. Without laminin-111, STAT5 only shows a transient phosphorylation, which is not suffcient for chromatin remodeling and milk gene expression. After laminin-111 binds integrins and dystroglycan, PI3K re-localize to the basal surface. Rac1 is a downsteam target of PI3K and required for sustained activation of STAT5. Prolactin, together with laminin-111, induces histone acetylation, binding of the SWI/SNF and transcription factors to the promoter and initiates transcription of casein.
The majority of mammary gland development occurs postnatally, which provides a powerful model to investigate role of ECM proteins in normal tissue development. In mammary tissue, luminal and basal epithelial cells form bi-layer tubular or acinar structures where basal cells adhere to a BM. The BM is comprised largely of laminins, type IV collagen, entactin/nidogen, and proteoglycans (Prince et al., 2002, Aumailley et al., 2005, Xu & Mao, 2011). These proteins, especially laminin-111, are required for the milk protein expression and secretion. Outside of the BM, stromal cells, adipocytes, and immune cells can produce a variety of stromal ECM proteins and small molecules to affect epithelial behaviors. The stromal ECM proteins include a set of fiber forming collagens, such as type I, II, and III collagen, as well as fibronectin, vitronectin, and elastin (Akalu & Brooks, 2004). Fibrillar collagens have been detected mainly around large mammary ducts, and recently studies showed that orientation of collagen I directs epithelial branching (Ingman et al., 2006, Brownfield et al., 2013). Therefore, ECM not only provides mechanical cues to support mammary gland structure, but also serves as a communicating bridge between mammary epithelia and their local and global environment throughout this organ's development (Bissell et al., 1982).
As an important component of tumor microenvironment, ECM also plays critical roles in breast cancer development and progression. For instance, the BM acts as a mechanical barrier and prevent malignant cells from invasion during the breast cancer progression (Liotta et al., 1980), whereas fibril collagen I contributes greatly to the strength of tissues and promotes tumor growth, invasion, and metastasis (Provenzano et al., 2008, Conklin et al., 2011).
In this review, we discuss recent findings regarding the ECM in mammary gland biology. We focus on the roles of integrated ECM and other microenvironmental signals in regulating mammary-specific tissue function, mammary tissue morphogenesis, and breast cancer progression. And more specifically, we discuss how the biochemical and biomechanical cues from the ECM cooperate to dictate normal and malignant tissue architecture and function.
Roles of ECM in normal mammary gland development
During mammary gland branching, alveologenesis, lactation, and involution, the expression and/or activitation of collagens, laminin, and matrix metalloproteinases (MMPs) are tightly regulated both temporally and spatially (reviewed in (Xu, Boudreau, et al., 2009), Table 1). A variety of growth factors and hormones such as estrogen, progesterone, and prolactin also play important roles in mammary gland development by regulating cell proliferation and differentiation. However, the mammary epithelial cells have distinct responses to the growth factors and hormones when adhering to different ECM molecules, suggesting that ECM receptors also play central roles in regulating these processes. In fact, a number of studies have shown that the signals from ECM and soluble factors cooperate to regulate acinar morphogenesis and mammary specific gene expression (Streuli et al., 1995, Wang et al., 1998, Akhtar & Streuli, 2006, Guo et al., 2006, Xu, Nelson, et al., 2009), supporting the concept that tissue architecture and function are determined by integrated microenvironmental signals.
Table 1. Components of ECM in mammary gland development and breast cancer.
| Development | Tumor | |
|---|---|---|
| Collagens | ||
| Collagen I | Abundant around larger mammary ducts (Keely et al., 1995), direct branch orientation (Brownfield et al., 2013) | Promote tumor progress (Kauppila et al., 1998) |
| Collagen III | Promote tumor progress (Kauppila et al., 1998) | |
| Collagen IV | Regulate ERα expression and function (Novaro et al., 2003) | Promote tumor progress (Nakano et al., 1999) |
| Collagen V | Regulate expression of apoptotic and stress response genes (Luparello et al., 2003, Luparello & Sirchia, 2005) | |
| Collagen VI | Contribute to tumor growth at early stages (Iyengar et al., 2005) | |
| Collagen XV | Lost early in the development of invasive tumors (Amenta et al., 2003) | |
| Glycoproteins | ||
| DMBT1 | Suppress breast cancer (Mollenhauer et al., 2004) | |
| FN | Increased in puberty and sexual maturity, remaining high during pregnancy and lactation (Woodward et al., 2001) | Stimulate proliferation and promote EMT (Williams et al., 2008, Park & Schwarzbauer, 2013) |
| Laminin 111 | Expressed near growing end buds and alveoli (Keely et al., 1995), necessary for formation of acinar structure and β-casein expression (Xu, Nelson, et al., 2009) | |
| Laminin 332 | Induce adhesive contacts in epithelial cells (Ewald et al., 2008) | Associated with aggressive features (Carpenter et al., 2009) |
| Nidogen | Promote the ability of Laminin-111 inducing β-casein expression (Pujuguet et al., 2000) | |
| Periostin | Elevated serum level with bone metastases (Sasaki et al., 2003), allow cancer stem cell maintenance (Malanchi et al., 2012) | |
| SPARC | Highly expressed in breast cancer tissue (Watkins et al., 2005). SPARC expression inhibits cancer cell metastasis (Koblinski et al., 2005). | |
| Tenascin C | Promote the survival and growth of pulmonary metastases (Oskarsson et al., 2011) | |
| Vitronectin | IGF-I binds vitronectin enhance breast cell migration and survival (Kashyap et al., 2011) | |
Laminin cooperates with prolactin to regulate mammary gland function
Prolactin, a lactogenic hormone mainly produced in the pituitary gland, is required for the alveologenesis and milk production (Goffin et al., 2002). Binding of prolactin to its receptor induces STAT5 phosphorylation through JAK2 (Gouilleux et al., 1994, Gouilleux et al., 1995). Phosphorylated STAT5 dimerizes and translocates to the nucleus then induces the related milk gene expression. As a downstream transcription factor of prolactin receptor (PrlR), STAT5 is essential for maximal expression of milk protein genes. STAT5a is a principal obligate mediator of mammopoietic and lactogenic signaling. In STAT5a knockout mice, mammary lobuloalveolar outgrowth during pregnancy was curtailed, and females failed to lactate after parturition because of a failure of terminal differentiation (Liu et al., 1997). The phenotype of the PrlR knockout mouse closely resembles that of the STAT5a knockout mouse (Ormandy et al., 1997).
Interestingly, prolactin treatment only induces a transient STAT5 phosphorylation and nuclear translocation when the mammary epithelial cells are isolated and cultured in 2D or in suspension, and the transient STAT5 activation fails to differentiate and turn on milk protein expression. When cultured in 3D laminin-rich ECM gels, the cells form polarized acinar structures with a central lumen and functionally differentiate and express milk proteins, such as β- and γ-caseins with the addition of lactogenic hormones (Barcellos-Hoff et al., 1989). Laminin-111 is required for the mammary epithelial cells to form polarized acinar structures and milk protein expression (Alcaraz et al., 2008). In the presence of laminin-111, prolactin treatment induced sustained STAT5 activation in mammary epithelial cells cultured in suspension, which leads to transcription of β- and γ-casein genes (Xu, Nelson, et al., 2009). Dystroglycan and β1-integrin are involved in cell-laminin interaction. The extracellular domain of dystroglycan binds to prominent extracellular matrix proteins including laminins, perlecan and agrin. Knockout of dystroglycan expression in the mammary gland impedes epithelial outgrowth and leads a failure of lactation in vivo. Dystroglycan regulates STAT5 signaling in a manner that is dependent on laminin-111 binding (Leonoudakis et al., 2010). Knockout of β1-integrin also impairs function differentiation of mammary epithelial cells and inhibits STAT5 activation (Naylor et al., 2005). These results indicate that integrated laminin and lactogenic hormone signals are critical for mammary specific function.
PI3K is an important mediator of integrin signaling to regulate cellular architecture and proliferation (Liu et al., 2004). PI3K is basally localized in polarized mammary gland epithelial cells in 3D culture (Liu et al., 2004, Xu et al., 2010). Rac1 is a downsteam target of PI3K (Cantley, 2002, Kolsch et al., 2008). PI3K-Rac1 signaling axis is required for the activation of PrlR/STAT5 signaling cascade (Akhtar & Streuli, 2006, Xu et al., 2010). Laminin-111 treatment enhances the Rac1 activity and induces binding of Rac1 to STAT5. The inhibition of PI3K blocks laminin-dependent sustained STAT5 phosphorylation and mammary-specific gene expression (Xu et al., 2010). In addition, the PI3K pathway may induce secretion of autocrine prolactin and downstream activation of the PrlR-STAT5 pathway via Akt (Chen et al., 2012).
Transcription of mammary-specific genes requires not only activation of transcription factors, but also chromatin remodeling. Histone modification and ATP-dependent chromatin remodeling are two types of chromatin remodeling that contribute to transcriptional regulation of milk gene expression. Acetylated histones are associated with ‘open’ chromatin structure and promote gene transcription (Shahbazian & Grunstein, 2007). Laminin- and prolactin-dependent sustained STAT5 phosphorylation is necessary for histone acetylation in the promoters of casein genes, and also enhances binding of the SWI/SNF ATP-dependent chromatin remodeling complex to the promoters of β- and γ-casein (Xu et al., 2007). These findings reveal a pathway (Figure 1) in which integrated ECM and hormone signals regulate functional differentiation of mammary epithelial cells via modulating transcription factor activity and chromatin remodeling.
Roles of Collagen and MMPs in mammary gland branch morphogenesis
The mammary ducts remain quiescent until the beginning of puberty. During puberty, the mammary ductal epithelial cells proliferate and invade into stromal fat pad, forming extensive branches (Sternlicht et al., 2006), and cell-matrix interactions have a critical role throughout this process.
Fibrillar collagen is mainly produced by stromal cells in mouse mammary glands. Collagen I fibers in the mammary pad are axially oriented prior to branching morphogenesis (Ingman et al., 2006). This orientation of collagen fibers is crucial for the branching morphogenesis. Macrophage deficiency reduces the amount of collagen I organized into long fibers and shortens terminal end buds, indicating that macrophages contribute to collagen fibrillogenesis and organization of the structure of terminal end buds (Ingman et al., 2006). Using the prestretched malleable wells to direct orientation of collagen fibers, a recent study demonstrates that collagen fiber orientation is sufficient to control the branching direction of mammary epithelial cells (Brownfield et al., 2013). Rac1 is activated at the leading edge of nascent branches and required for branch extension (Zhu & Nelson, 2013). Expression of a constitutively-active form of Rac1 decreased branch orientation of mammary epithelial aggregates, indicating that Rac1 is a modulator of collagen I orientation during branching morphogenesis (Brownfield et al., 2013). Meanwhile, ROCK-mediated contractions contribute to generation collagen I fiber orientation (Brownfield et al., 2013). The Rho-ROCK pathway is a potential mediator of ECM signals in regulating mammary epithelial cell tubulogenesis. ROCK-mediated contractility diminished Rho activity in a floating 3D collagen gel, which in turn promotes mammary tubulogenesis. A decrease in focal adhesion formation is also observed in in vitro breast epithelial tubulogenesis (Wozniak et al., 2003).
Although it remains obscure how the orientation of collagen directs branching morphogenesis, accumulated evidence suggest that PI3K is involved in this process. There are two ubiquitously expressed PI3K isoforms: p110a and p110b (Engelman et al., 2006, Vanhaesebroeck et al., 2010). Homozygous ablation of p110a dramatically impaired mammary duct outgrowth and branching during puberty and significantly decreased post-partum lactation. In contrast to p110a, p110b is dispensable for the development of a functional mammary gland (Utermark et al., 2012). In vitro study shows mechanical stress leads to sustained phosphorylation of Akt at branch sites, and this activation is required for branch initiation (Zhu & Nelson, 2013). The levels of pAkt are controlled by PTEN, which in turn is regulated by mechanical signaling via SPRY2 (Zhu & Nelson, 2013). Through a PI3K phosphotyrosine-binding site, ErbB3 is able to recruit PI3K and initiates the PI3K/AKT signaling pathway (Soltoff et al., 1994). Mice with a mutant ErbB3 allele lacking the PI3K-binding sites exhibit an initial early growth defect and a dramatic impairment of mammary epithelial outgrowth (Lahlou et al., 2012). These results suggest that PI3K integrates collagen and growth factor signals to direct mammary branching morphogenesis.
Roles of ECM in breast cancer development and progression
Breast cancer development and progression requires extensive remodeling of the ECM microenvironment. As a major component of the tumor microenvironment, ECM regulates many pathways in cancer cells, including Wnt, PI3K/AKT, ERK, JNK, Src-FAK, and Rho-GTPases(Levental et al., 2009, Malanchi et al., 2012). In addition, increased deposition and crosslinking of collagens associated with tumor formation enhances the tissue stiffness (Provenzano et al., 2008, Levental et al., 2009). These ECM-dependent biochemical and biomechanical signals together compose the complex environmental cues to promote breast cancer development and progression (Cox & Erler, 2011).
ECM-dependent biomechanical cues in cancer progression
Increasing mammographic density is associated with breast cancer risk (McCormack & dos Santos Silva, 2006). Breast cancer tumors are more rigid compared to normal mammary tissue because they have a stiff stroma. It has been shown that enhanced collagen crosslinking and deposition correlates with dense mammography and rigidity in tumor tissue (Martin & Boyd, 2008, Levental et al., 2009). Lysyl oxidase (LOX) is a copper-dependent amine oxidase (Kagan & Li, 2003) that initiates the process of collagen crosslinking (Yamauchi & Shiiba, 2008). LOXs can be induced by hypoxia inducible factor and TGF (Postovit et al., 2008). Upregulation of LOXs promotes mammary tumor growth and metastasis by enhancing collagen crosslinking and stiffness (Levental et al., 2009, Pickup et al., 2013). The stiff ECM substrata elevate Rho-dependent cytoskeletal tension, disrupt tissue polarity, and enhance tumor growth (Paszek et al., 2005). Collagen prolyl hydroxylases, an enzyme necessary for collagen synthesis, is also highly expressed in breast cancer tissues and correlates with poor clinical outcomes. Silencing collagen prolyl hydroxylases reduced collagen deposition and alignment, resulting in decreased invasion and metastasis to lymph nodes and lungs (Gilkes, Bajpai, et al., 2013, Gilkes, Chaturvedi, et al., 2013, Xiong et al., 2014). Thus, increased ECM stiffness caused by collagen deposition and crosslinking may be considered a driving force of tumor progression.
Mechanotransduction from ECM to cytoskeleton enables cells to sense and adapt to external forces and physical constraints, which in turn modulate a variety of cellular functions (Vogel & Sheetz, 2006). It has been shown that stiff ECM induces integrin clustering and enhances growth factor-dependent ERK activation (Paszek et al., 2005). Activated ERK could facilitate malignant transformation by increasing focal adhesion assembly through Rho (Paszek et al., 2005). Expression of clustered integrin in mammary epithelial cells enhances EGF-stimulated Akt activity (Levental et al., 2009). Introducing auto-clustered integrin β1 (V373N) also promotes invasion of a Ha-ras mammary epithelium (Levental et al., 2009). Therefore, integrin clustering may be the key mediator of mechanotransduction to promote breast cancer progression.
A recent study demonstrates that matrix stiffness regulates a switch in prolactin signals from normal mammary function to protumorigenic. In a soft laminin-rich matrix, prolactin treatment stimulates milk protein expression via inducing STAT5 activation (Alcaraz et al., 2008). However, in stiff matrices, prolactin treatment increases SRC phosphorylated FAK, stimulates MMP-2 expression and activity (Barcus et al., 2013), and subsequently enhances cell invasion. Matrix stiffness also modulates activity of YAP and TAZ transcriptional regulators. This regulation requires Rho GTPase activity and tension of the actomyosin cytoskeleton, but is independent of Hippo/LATS cascade (Dupont et al., 2011). YAP regulates the expression of several cytoskeletal regulators, including ANLN, DIAPH3, MYL9, and MYH10 (Calvo et al., 2013). Together these downstream targets may generate a positive feedback loop to maintain cellular tension.
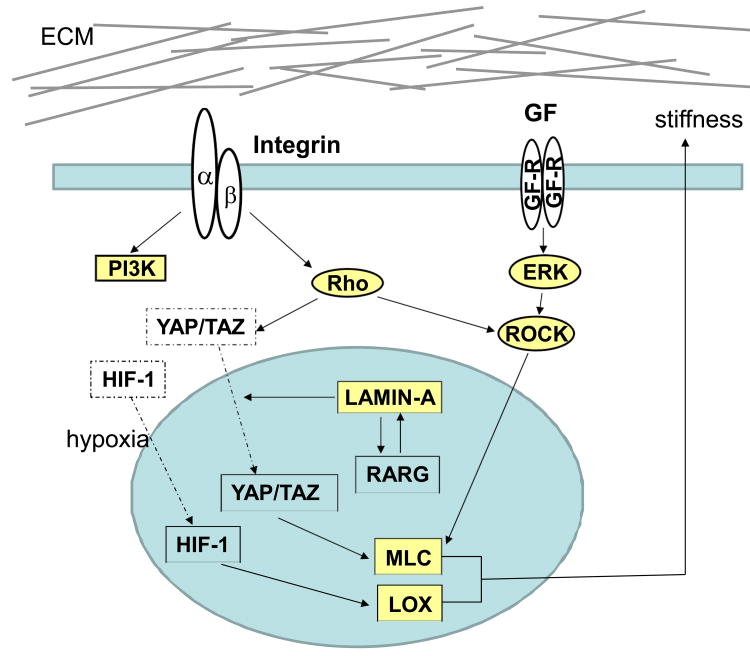
Altering cell tension has been show to regulate nuclear morphology and chromatin structure. Cells cultured in 3D matrigel or cells in suspension show reduced levels of both acetylated histones H3 and H4 when compared to cells cultured in the stiff microenvironment of 2D culture (Le Beyec et al., 2007). The results suggest low intracellular tension has profound effect on chromatin structure. Increased cell tension also reduces the turnover of lamin A in the nuclear lamina, which subsequently causes accumulation of YAP (Swift et al., 2013). An increase in lamin A also triggers the serum response factor (SRF) signaling pathway and drives translocation of the retinoic acid receptor into the nucleus to regulate gene expression and lineage differentiation (Swift et al., 2013). These findings reveal a novel link between ECM-controlled cell tension and nuclear structure. (Figure 2) However, how this link contributes to breast cancer development and progression still remains to be determined.
Figure 2.
Matrix stiffness induces integrin clustering and activation of PI3K and Rho in breast cancer cells. Integrin clustering enhances growth factor-dependent ERK activation and increases ROCK expression lever. Increased cell tension reduces turnover of lamin A. Accumulation of lamin A drives translocation of the retinoic acid receptor (RARG) into nucleus and RARG lead the transcription of Lamin-A. Rho and Lamin-A can translocate YAP/TAZ. YAP regulates the expression of several cytoskeletal regulators, including MLC.
Biochemical signals from the ECM niche in cancer progression and metastasis
A number of ECM proteins, such as periostin and tenascin C, are important components of the metastatic niche. Periostin is mainly produced by fibroblasts in the tumor stroma (Gillan et al., 2002, Contie et al., 2011). Deletion of periostin has little effect on normal tissue development and primary tumor growth (Saga et al., 1992, Malanchi et al., 2012); however, periostin promotes colonization of cancer stem cells in the distant organ by recruiting Wnt lignads and inducing Wnt signaling (Malanchi et al., 2012). Therefore, reducing its expression prevents metastasis (Malanchi et al., 2012). Tenascin C has been detected in both primary breast cancer and the invasive front of lung metastasis nodules (Oskarsson et al., 2011). Both cancer and stromal cells express a significant amount of tenascin C (Oskarsson et al., 2011). Tenascin C modulates cancer cell stem cell signaling by enhancing expression of musashi homolog 1 (MSI1) and leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5). These two proteins are key regulators of the Notch and Wnt pathways, respectively (Oskarsson et al., 2011). Cancer cell-derived tenascin C promotes the survival and outgrowth of breast cancer cells at distance organs, such as the lung (Oskarsson et al., 2011). These findings link ECM molecules to biochemical signaling that supports the survival and proliferation of tumor initiating cells at metastatic sites.
Increased expression and deposition of fibronectin and collagen have been detected in breast cancer tissue (Christensen, 1992, Provenzano et al., 2008). Fibronectin is a marker of epithelial-mesenchymal transition (EMT) and has been detected in the stem cell niche. Through Src kinase and the ERK/MAP kinase pathway, fibronectin induces cells to undergo EMT and enhances cancer metastasis (Saad et al., 2002, Park & Schwarzbauer, 2013). Binding of type I collagen to DDR enhances SNAIL stability by stimulating ERK2 activity. Activated ERK2 directly phosphorylates SNAIL1 leading to SNAIL1 nuclear accumulation, subsequently promotes breast cancer cell invasion and metastasis (Zhang et al., 2013). These studies indicate that ERK is critical a pathway downstream of ECM cues to promote breast cancer progression.
ECM proteins have a profound effect on stromal cells in tumor tissue. This has been well-demonstrated in the angiogenesis process. For instance, binding of fibroblast growth factors (FGFs) and vascular endothelial growth factors (VEGFs) to heparin– a component of ECM proteoglycans–mediates sequestration, stabilization and high affinity receptor binding and signaling of the factors (Vlodavsky et al., 1996). The initial burst of MMP production, especially of MMP-9, releases BM-bound VEGF and other factors that initiate tumor angiogenesis (Bergers et al., 2000). In addition, ECM is involved in angiogenesis signal transduction as precursor of biologically active signaling fragments. A large group of functional fragments, including endostatin, arrestin, vastatin, tumstatin and canstatin are derived from collagen XVIII, IV, and VIII and demonstrate anti-angiogenic effect (Colorado et al., 2000, Xu et al., 2001, Mott & Werb, 2004). Enrichment and differentiation of immune cells are also influenced by ECM microenvironment during cancer progression. Selective cleavage of collagen I by coordinated efforts of MMP-8, MMP-9 and prolyl endopeptidase produces tripeptide Pro-Gly-Pro (Gaggar et al., 2008). N-acetylated Pro-Gly-Pro shares sequence and structure homology with CXCL8 (Weathington et al., 2006), and causes chemotaxis and promotes neutrophil recruitment to the inflammation sites (Weathington et al., 2006). Therefore, cancer development and progression may require the coordinated action of ECM and stromal cells in the tumor microenvironment.
Conclusions
Microenvironmental signals generated from ECM, hormones, and growth factors are integrated at the extra- and intracellular level. This synergetic action of microenvironmental cues is crucial for both normal mammary gland development and for breast malignancy. ECM-dependent biochemical and biomechanical signals are transduced by cell surface receptors to modulate nuclear structure and gene expression. Further investigation how these signals are integrated to regulate mammary gland morphogenesis and breast cancer progression is crucial for the comprehensive understanding of ECM function.
Acknowledgments
This study was supported by grants from ACS (IRG 85-001-22 to R. Xu) and AHA (12SDG8600000 to R. Xu). This publication was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (Grant UL1TR000117). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Akalu A, Brooks PC. Matrix, extracellular and interstitial. In: Meyers RA, editor. Encyclopedia of molecular cell biology and molecular medicine. 2nd. Wiley-VCH Verlag; Weinheim: 2004. [Google Scholar]
- Akhtar N, Streuli CH. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. The Journal of cell biology. 2006;173:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C, Bissell MJ. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. The EMBO journal. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta PS, Hadad S, Lee MT, Barnard N, Li D, Myers JC. Loss of types xv and xix collagen precedes basement membrane invasion in ductal carcinoma of the female breast. The Journal of pathology. 2003;199:298–308. doi: 10.1002/path.1303. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix biology : journal of the International Society for Matrix Biology. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. The Journal of biological chemistry. 2013;288:12722–12732. doi: 10.1074/jbc.M112.447631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature cell biology. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? Journal of theoretical biology. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Brownfield DG, Venugopalan G, Lo A, Mori H, Tanner K, Fletcher DA, Bissell MJ. Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Current biology : CB. 2013;23:703–709. doi: 10.1016/j.cub.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and yap-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature cell biology. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carpenter PM, Dao AV, Arain ZS, Chang MK, Nguyen HP, Arain S, Wang-Rodriguez J, Kwon SY, Wilczynski SP. Motility induction in breast carcinoma by mammary epithelial laminin 332 (laminin 5) Molecular cancer research : MCR. 2009;7:462–475. doi: 10.1158/1541-7786.MCR-08-0148. [DOI] [PubMed] [Google Scholar]
- Chen CC, Stairs DB, Boxer RB, Belka GK, Horseman ND, Alvarez JV, Chodosh LA. Autocrine prolactin induced by the pten-akt pathway is required for lactation initiation and provides a direct link between the akt and stat5 pathways. Genes & development. 2012;26:2154–2168. doi: 10.1101/gad.197343.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L. The distribution of fibronectin, laminin and tetranectin in human breast cancer with special attention to the extracellular matrix. APMIS Supplementum. 1992;26:1–39. [PubMed] [Google Scholar]
- Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Anti-angiogenic cues from vascular basement membrane collagen. Cancer research. 2000;60:2520–2526. [PubMed] [Google Scholar]
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American journal of pathology. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contie S, Voorzanger-Rousselot N, Litvin J, Clezardin P, Garnero P. Increased expression and serum levels of the stromal cell-secreted protein periostin in breast cancer bone metastases. International journal of cancer. Journal international du cancer. 2011;128:352–360. doi: 10.1002/ijc.25591. [DOI] [PubMed] [Google Scholar]
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Disease models & mechanisms. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of yap/taz in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature reviews Genetics. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Developmental cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. Journal of immunology. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (hif-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing p4ha1, p4ha2, and plod2 expression in fibroblasts. The Journal of biological chemistry. 2013;288:10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, Hubbi ME, Wirtz D, Semenza GL. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer research. 2013;73:3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(v)beta(3) and alpha(v)beta(5) integrins and promotes cell motility. Cancer research. 2002;62:5358–5364. [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: The new biology of an old hormone. Annu Rev Physiol. 2002;64:47–67. doi: 10.1146/annurev.physiol.64.081501.131049. [DOI] [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of tyr694 of stat5 (mgf), a prerequisite for DNA binding and induction of transcription. The EMBO journal. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F, Pallard C, Dusanter-Fourt I, Wakao H, Haldosen LA, Norstedt G, Levy D, Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce mgf-stat5 DNA binding activity. The EMBO journal. 1995;14:2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nature reviews Molecular cell biology. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta 4 integrin amplifies erbb2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, Chopra N, Russell RG, Sasisekharan R, Trock BJ, Lippman M, Calvert VS, Petricoin EF, 3rd, Liotta L, Dadachova E, Pestell RG, Lisanti MP, Bonaldo P, Scherer PE. Adipocyte-derived collagen vi affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. The Journal of clinical investigation. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. Journal of cellular biochemistry. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kashyap AS, Hollier BG, Manton KJ, Satyamoorthy K, Leavesley DI, Upton Z. Insulin-like growth factor-i:Vitronectin complex-induced changes in gene expression effect breast cell survival and migration. Endocrinology. 2011;152:1388–1401. doi: 10.1210/en.2010-0897. [DOI] [PubMed] [Google Scholar]
- Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. Aberrant type i and type iii collagen gene expression in human breast cancer in vivo. The Journal of pathology. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen i, collagen iv, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation; research in biological diversity. 1995;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- Koblinski JE, Kaplan-Singer BR, VanOsdol SJ, Wu M, Engbring JA, Wang S, Goldsmith CM, Piper JT, Vostal JG, Harms JF, Welch DR, Kleinman HK. Endogenous osteonectin/sparc/bm-40 expression inhibits mda-mb-231 breast cancer cell metastasis. Cancer research. 2005;65:7370–7377. doi: 10.1158/0008-5472.CAN-05-0807. [DOI] [PubMed] [Google Scholar]
- Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. Journal of cell science. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Muller T, Sanguin-Gendreau V, Birchmeier C, Muller WJ. Uncoupling of pi3k from erbb3 impairs mammary gland development but does not impact on erbb2-induced mammary tumorigenesis. Cancer research. 2012;72:3080–3090. doi: 10.1158/0008-5472.CAN-11-3513. [DOI] [PubMed] [Google Scholar]
- Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Experimental cell research. 2007;313:3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Singh M, Mohajer R, Mohajer P, Fata JE, Campbell KP, Muschler JL. Dystroglycan controls signaling of multiple hormones through modulation of stat5 activity. Journal of cell science. 2010;123:3683–3692. doi: 10.1242/jcs.070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of pi3-kinase in breast epithelial tumor cells. The Journal of cell biology. 2004;164:603–612. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes & development. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Luparello C, Sirchia R. Type v collagen regulates the expression of apoptotic and stress response genes by breast cancer cells. Journal of cellular physiology. 2005;202:411–421. doi: 10.1002/jcp.20131. [DOI] [PubMed] [Google Scholar]
- Luparello C, David F, Campisi G, Sirchia R. T47-d cells and type v collagen: A model for the study of apoptotic gene expression by breast cancer cells. Biological chemistry. 2003;384:965–975. doi: 10.1515/BC.2003.109. [DOI] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast cancer research : BCR. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J, Helmke B, Medina D, Bergmann G, Gassler N, Muller H, Lyer S, Diedrichs L, Renner M, Wittig R, Blaich S, Hamann U, Madsen J, Holmskov U, Bikker F, Ligtenberg A, Carlen A, Olsson J, Otto HF, O'Malley B, Poustka A. Carcinogen inducibility in vivo and down-regulation of dmbt1 during breast carcinogenesis. Genes, chromosomes & cancer. 2004;39:185–194. doi: 10.1002/gcc.10309. [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Current opinion in cell biology. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Hoersch S, Hynes RO. Towards definition of an ecm parts list: An advance on go categories. Matrix biology : journal of the International Society for Matrix Biology. 2012;31:371–372. doi: 10.1016/j.matbio.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S, Iyama K, Ogawa M, Yoshioka H, Sado Y, Oohashi T, Ninomiya Y. Differential tissular expression and localization of type iv collagen alpha1(iv), alpha2(iv), alpha5(iv), and alpha6(iv) chains and their mrna in normal breast and in benign and malignant breast tumors. Laboratory investigation; a journal of technical methods and pathology. 1999;79:281–292. [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. The Journal of cell biology. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaro V, Roskelley CD, Bissell MJ. Collagen-iv and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. Journal of cell science. 2003;116:2975–2986. doi: 10.1242/jcs.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes & development. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin c as a metastatic niche component to colonize the lungs. Nature medicine. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene. 2013 doi: 10.1038/onc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pickup MW, Laklai H, Acerbi I, Owens P, Gorska AE, Chytil A, Aakre M, Weaver VM, Moses HL. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer research. 2013;73:5336–5346. doi: 10.1158/0008-5472.CAN-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ, Kirschmann DA. Hypoxia/reoxygenation: A dynamic regulator of lysyl oxidase-facilitated breast cancer migration. Journal of cellular biochemistry. 2008;103:1369–1378. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- Prince JM, Klinowska TC, Marshman E, Lowe ET, Mayer U, Miner J, Aberdam D, Vestweber D, Gusterson B, Streuli CH. Cell-matrix interactions during development and apoptosis of the mouse mammary gland in vivo. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;223:497–516. doi: 10.1002/dvdy.10070. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC medicine. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujuguet P, Simian M, Liaw J, Timpl R, Werb Z, Bissell MJ. Nidogen-1 regulates laminin-1-dependent mammary-specific gene expression. Journal of cell science. 2000;113(Pt 5):849–858. doi: 10.1242/jcs.113.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad S, Gottlieb DJ, Bradstock KF, Overall CM, Bendall LJ. Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer research. 2002;62:283–289. [PubMed] [Google Scholar]
- Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes & development. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Yu CY, Dai M, Tam C, Loda M, Auclair D, Chen LB, Elias A. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast cancer research and treatment. 2003;77:245–252. doi: 10.1023/a:1021899904332. [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annual review of biochemistry. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Soltoff SP, Carraway KL, 3rd, Prigent SA, Gullick WG, Cantley LC. Erbb3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Molecular and cellular biology. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation; research in biological diversity. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, Schindler C, Watson CJ. Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995;270:21639–21644. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-a scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, Iglehart JD, Roberts TM, Muller WJ, Zhao JJ. The p110alpha and p110beta isoforms of pi3k play divergent roles in mammary gland development and tumorigenesis. Genes & development. 2012;26:1573–1586. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific pi3k signalling. Nature reviews Molecular cell biology. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Miao HQ, Medalion B, Danagher P, Ron D. Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer metastasis reviews. 1996;15:177–186. doi: 10.1007/BF00437470. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature reviews Molecular cell biology. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins G, Douglas-Jones A, Bryce R, Mansel RE, Jiang WG. Increased levels of sparc (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins, leukotrienes, and essential fatty acids. 2005;72:267–272. doi: 10.1016/j.plefa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide cxcr ligand derived from extracellular matrix degradation during airway inflammation. Nature medicine. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer research. 2008;68:3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TL, Mienaltowski AS, Modi RR, Bennett JM, Haslam SZ. Fibronectin and the alpha(5)beta(1) integrin are under developmental and ovarian steroid regulation in the normal mouse mammary gland. Endocrinology. 2001;142:3214–3222. doi: 10.1210/endo.142.7.8273. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. Rock-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. The Journal of cell biology. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G, Deng L, Zhu J, Rychahou PG, Xu R. Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC cancer. 2014;14:1. doi: 10.1186/1471-2407-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Mao JH. Gene transcriptional networks integrate microenvironmental signals in human breast cancer. Integr Biol (Camb) 2011;3:368–374. doi: 10.1039/c0ib00087f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Spencer VA, Bissell MJ. Extracellular matrix-regulated gene expression requires cooperation of swi/snf and transcription factors. The Journal of biological chemistry. 2007;282:14992–14999. doi: 10.1074/jbc.M610316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Spencer VA, Groesser DL, Bissell MJ. Laminin regulates pi3k basal localization and activation to sustain stat5 activation. Cell cycle. 2010;9:4315–4322. doi: 10.4161/cc.9.21.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Yao ZY, Xin L, Zhang Q, Li TP, Gan RB. Nc1 domain of human type viii collagen (alpha 1) inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Biochemical and biophysical research communications. 2001;289:264–268. doi: 10.1006/bbrc.2001.5970. [DOI] [PubMed] [Google Scholar]
- Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of stat5 is essential for chromatin remodeling and maintenance of mammary-specific function. The Journal of cell biology. 2009;184:57–66. doi: 10.1083/jcb.200807021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Shiiba M. Lysine hydroxylation and cross-linking of collagen. Methods in molecular biology. 2008;446:95–108. doi: 10.1007/978-1-60327-084-7_7. [DOI] [PubMed] [Google Scholar]
- Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes snail1 to facilitate breast cancer metastasis. Nature cell biology. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Nelson CM. Pi3k regulates branch initiation and extension of cultured mammary epithelia via akt and rac1 respectively. Developmental biology. 2013;379:235–245. doi: 10.1016/j.ydbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]