Abstract
Background
Transfusion of packed red blood cells (RBCs) produces a myriad of immunologic derangements, from suppressive to stimulatory. Proliferation of human T cells is suppressed in vitro after exposure to processed red blood cells (PRBCs). We hypothesized that this effect would be mitigated by using fresh RBCs. We also hypothesized that this suppressive effect was a generalized effect on lymphocyte proliferation and would be observed in both CD4+ and CD8+ T-cell subpopulations as well as B cells.
Materials and methods
We isolated human T cells from donor peripheral blood mononuclear cells and exposed them to either blood bank PRBCs or fresh RBCs from volunteer donors and stimulated them with anti-CD3/anti-CD28. Human B cells were stimulated with lipopolysaccharide and exposed to PRBCs or fresh RBCs. We measured proliferation of B cells by thymidine incorporation assays. We also treated RBCs with citrate-phosphate-dextrose (CPD) at different time points before culture them with stimulated T cells to determine the role of this common RBC storage solution in lymphocyte proliferation.
Results
In vitro proliferation of CD4+ and CD8+ T cells was suppressed by blood bank RBCs. This suppression is eliminated when fresh RBCs were used. The B cells showed inhibition of proliferation when exposed to similar conditions, which appeared to be consistent over serial dilutions. Fresh RBCs exposed to CPD did not appear suppressive in the first 6 h after exposure.
Conclusions
T-cell and B-cell proliferation inhibition by blood banked RBCs suggests a generalized effect of RBCs on cellular proliferation. The lack of suppression by fresh RBCs further suggests that something involved in blood banking alters RBC properties such that they attain a suppressive phenotype. One such blood banking component, CPD, does not appear to affect this suppressive phenotype within the first 6 h.
Keywords: Red blood cell, Transfusion, Immunomodulation, Critical care, Transfusion-related immunomodulation
Introduction
Although it is often necessary and life-saving in clinical practice, transfusion of packed red blood cells (PRBCs) alters the immune system, producing both proinflammatory and anti-inflammatory responses. Clinically, PRBC transfusion increases the risk of infection, length of stay, mechanical ventilation, and mortality [1]. One possible mechanism for these effects is that PRBC exposure suppresses T-cell proliferation, as was reported to occur in vitro in a dose-dependent manner without apoptosis or necrosis, in prior work from this laboratory [2]. Age of stored red blood cells (RBCs) has been shown to enhance both immunologic derangements and harmful postoperative outcomes in patients [3–5]. Studies have compared the effects of storage in RBCs <7 d old with RBCs stored for 7–30 d, but these studies used RBCs processed with conventional blood bank protocols [6–8]. Military use of warm fresh whole blood for transfusion has been associated with increased efficacy and decreased RBC storage lesions [9]. Murine studies indicate that fresh blood may abrogate some of the negative immune effects seen with stored RBCs, but fresh blood as a model has not been applied to human cells [6,10]. No studies of storage duration have yet isolated specific components and steps of preparation and storage, or measured their effects on immune parameters. Characterizing the changes induced in banked human RBCs, evaluating storage processes, and isolating components of the RBC storage process used in traditional banking may help identify a culprit component of suppressed T-cell proliferation and/or a chronologic point at which the “RBC storage lesion” of suppressed T-cell proliferation is triggered [6,11,12]. We previously showed that banked human RBCs suppress human T cells in a cell contact–dependent manner. However, human RBCs can suppress allogeneic or syngeneic human T cells (manuscript in preparation) as well as mouse T cells [2], which indicates a lack of specificity. The purposes of our study were to determine whether current blood bank processing contributed to the suppressive properties seen in stored RBCs, and to determine whether fresh RBCs could eliminate this effect. In the present study, we found that blood banked RBC could suppress the proliferation of both CD4+ and CD8+ T cells as well as B cells. In addition, we found that fresh RBCs lacked this suppressive property and that citrate-phosphate-dextrose (CPD) exposure of fresh RBCs had no effect within the first 6 h.
Materials and methods
This study was approved by the Institutional Review Board of the University of Kentucky Office of Research Integrity (Protocol 12-0966-P6 H).
T-cell isolation and culture
We performed T-cell isolation as previously described [2]. Human peripheral blood mononuclear cells (PBMCs) obtained from volunteer blood donors (Kentucky Blood Center, Lexington, KY) using an institutional review board (IRB)–exempt protocol were separated using Ficoll-Hypaque gradient. We isolated T cells from PBMCs by depleting all non–T cells using a magnetic bead Pan T cell kit (Miltenyi Biotec, Auburn, CA) and an autoMACS separator, and plated them at 1.0 x 106 cells/mL in Roswell Park Memorial Institute 1640 culture media (Life Technologies, Carlsbad, CA) supplemented with penicillin-streptomycin, 10% heat-inactivated fetal bovine serum (FBS), and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, henceforth termed CRPMI (complete RPMI). Then we stimulated the T cells with plate-bound anti-CD3 (1 μg/mL, OKT-3; Ortho Pharmaceutical, Raritan, NJ) and anti-CD28 (100 ng/mL, Becton Dickinson, San Jose, CA). These studies require longer continuous incubation periods; thus, we further supplemented the media with minimal essential media nonessential amino acid solution, sodium pyruvate, and glutamine (CRPMI). We analyzed random samples of T cells by flow cytometry with CD4+ and CD8+ antibody stains to ensure the purity of the separated populations. All cultures were maintained at 37°C and 5% CO2.
B-cell stimulation and culture
We cultured human PBMCs obtained from volunteer donors (Kentucky Blood Center) using IRB–exempt protocols at a concentration of 1.0 x 106 cells/mL in CRPMI media and stimulated B cells to proliferate by exposure to lipopolysaccharide (LPS) (Sigma, St. Louis, MO) at an initial concentration of 100 μg/mL. Lipopolysaccharide stimulation results in isolated proliferation of human B cells, although the cell cultures themselves contained all components of human PBMCs. Cells were cultured in 96-well plates for 72 h at 37°C and 5% CO2. We added 3H-thymidine for the final 18–20 h of incubation at 1 μCi/well, harvested cells on Perkin Elmer unifilter plates, and determined cell proliferation by scintillation counting.
Red blood cell cultures
To simulate transfusion, we obtained allogeneic human PRBCs (O−) from the University of Kentucky Hospital Blood Bank under an IRB-exempted protocol and added them to T-cell cultures. Following blood bank protocols, the PRBCs were leukocyte-reduced by filtration based on size and charge using a standard leukocyte-reduction filter that removes 99.9% of leukocytes. The PRBC units were not routinely irradiated. We added PRBCs (2- to 3-wk storage duration unless specified otherwise) to cultures. Initial hematocrit was 60%–80% in blood bank PRBC specimens used for experiments and was diluted in media to 2% by volume. The number of PRBC per well was standardized such that 2% hematocrit equated to 193 ± 17 PRBCs:1 T cell, and followed by serial dilutions. For carboxyfluorescein diacetate succinimidyl ester (CFSE) staining and culture, we counted RBCs by light microscopy using a hemocytometer and diluted them to an initial concentration of 2 × 108/mL, followed by serial dilutions.
We obtained human T cells by following the protocol for T-cell isolation and culture, counted them, and suspended them in phosphate-buffered saline/0.1% fetal bovine serum at a concentration of 5.0 × 106 cells/mL. We prepared 5 mmol/L CFSE by dissolving one vial of stock CFSE solution (CellTrace, Grand Island, NY) with 18 μL dimethyl sulfoxide. We then added CFSE at a volume of 0.25 μL/ mL T cells, rapidly inverted them, and stained them for 10 min at room temperature in the dark. Staining was quenched by addition of excess CRPMI. Cells were centrifuged at 300 g for 10 min at 18° C, supernatant was removed, and cells were recounted before suspension at a final concentration of 2.0 × 106 cells/mL. We stored CFSE-stained T cells in the dark at room temperature until they were ready for plating. To determine proliferation by CFSE dye dilution, harvested cells were stained with anti-CD4 Per CP and anti-CD8 PE (eBioscience, San Diego, CA) for 30 min at room temperature in the dark. Cells were then washed with and resuspended in phosphate-buffered saline/0.1% fetal bovine serum. Cells were placed on ice for immediate flow cytometry analysis.
Data analysis
Data collected after flow cytometric analysis included the percentage of total cells and percentage of proliferating cells. We did not compare numerical results across experiments, given the necessary use of different donor granulocyte units for each day. Each experiment contained unstimulated T-cell control conditions that allowed us to examine individual results essentially as a within-subject design. Results represent trends seen across multiple experiments. B-cell experiments rely on scintillation counts and are presented as the mean value of each condition, with standard deviations and standard error calculations performed in Microsoft Excel (Redmond, WA) databases.
Results
CD4 and CD8 T cells continue to proliferate after exposure to fresh RBCs
In thymidine incorporation assays measuring a variety of dilutions of PRBCs, human T cells stimulated with anti CD3/ CD28 did not proliferate after exposure to stored RBCs. Red blood cell concentrations of 100:1 (RBC:T cells), 50:1, 25:1, and 12.5:1, all showed near-complete suppression of T-cell proliferation [2]. In the present study, we used CFSE dye dilution as a direct measure of T-cell division in vitro. Purified T cells were CFSE labeled and stimulated with anti-CD3/CD28 in the presence of 100:1 blood banked or fresh RBCs. After 3 d, cells were harvested and stained for both CD4 and CD8, which allowed us to analyze both cell populations. In all conditions tested, PRBCs suppressed both CD4 and CD8 cell proliferation. When T cells were exposed to similar conditions using fresh RBCs that were leuko-reduced but unprocessed by blood bank standard protocols, CD4+ and CD8+ human T-cell proliferation continued uninhibited, similar to levels of positive control cells in various experiments (Fig. 1). Percentages of proliferating cells showed slight variation between experiments, but in multiple experiments using fresh RBCs, T cells showed proliferation similar to positive control conditions (T cells stimulated with CD3/CD28 and no RBC exposure). However, PRBCs suppressed T cells to proliferate at approximately one third of the positive control levels.
Fig. 1.
Packed red blood cells suppress proliferation of both CD4 and CD8 human T cells. Fresh RBCs restore proliferation of both CD4 and CD8 T cells. T cells were purified from human PBMCs, stained with CFSE, exposed to anti CD3/CD28, and then cultured with no RBCs, PRBCs (100:1), or fresh RBCs (100:1) for 4 d. Cells were then harvested, stained with anti-CD4 or anti-CD8, and analyzed by flow cytometry. Proliferation is assessed by the dilution of CFSE intensity.
Blood bank storage processes
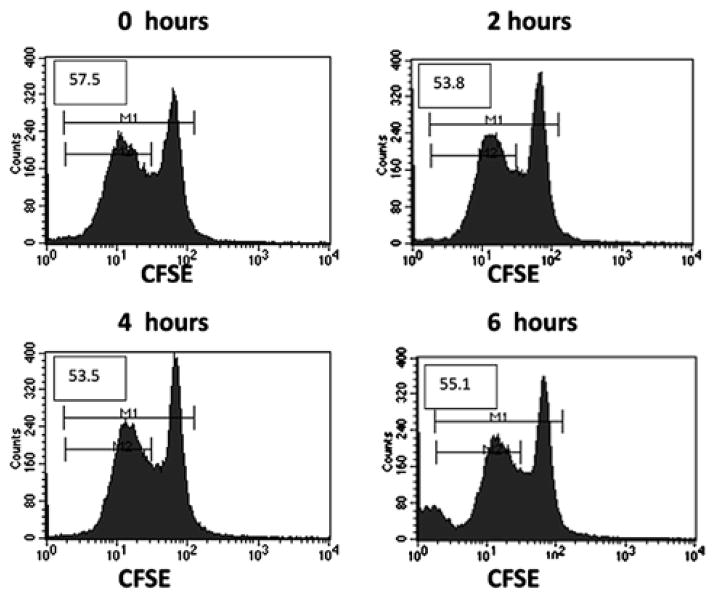
Published experiments implicate the blood bank storage process in the development of the RBC storage lesion; testing individual additives at varying time points will potentially elucidate the time at which RBCs become suppressive for T cells. To begin to assess the effect of additives, we exposed human T cells to fresh RBCs that had been treated with CPD, an anti-coagulant solution added to donor RBCs during early processing by blood banks. Citrate-phosphate-dextrose is the traditional anticoagulant solution used to store donated blood in the blood bank. Red blood cells stored in additive solutions are known to have altered aging processes [13,14]. At time points ranging from 0 h (exposure to CPD and then immediate culturing) to 6 h after CPD exposure, RBCs were treated and then placed in culture with T cells. We saw no inhibition of proliferation after exposure to CPD-treated RBCs. This indicates that the change in the RBCs may happen later in the processing and/or that storage conditions other than the presence of CPD are responsible (Fig. 2).
Fig. 2.
Addition of CPD to RBCs does not suppress T-cell proliferation within the first 6 h after exposure. We added fresh RBCs to anti-CD3/CD28 stimulation cultures of CFSE-labeled T cells after the indicated times of incubation with CPD. T cells were harvested after 3 d and proliferation was measured by CFSE dye dilution using flow cytometry. Initial CFSE-stained T-cell populations are demonstrated by the large peaks at a fluorescence intensity of 102; the leftward diminution of fluorescence intensity demonstrates continued proliferation. Numbers in each histogram represent the percentage of T-cell division (M2/M1 x 100).
Fresh RBCs alter B-cell proliferation
Because PRBCs inhibits CD4 and CD8 T cells, both syngeneic and allogeneic, as well as mouse T cells, we reasoned that proliferation inhibition might be a generalized effect on lymphocytes by PRBC. Thus, we examined human B-cell proliferation after exposure to both PRBCs and fresh RBCs. Using 3H-thymidine incorporation to measure proliferation and LPS to stimulate proliferation of B cells from human PBMCs, we demonstrated suppression of proliferation after exposure to both PRBCs and fresh RBCs. We observed suppression of B-cell proliferation by ≥50% at RBC/PBMC ratios of 100:1, 50:1, 25:1, and 12:1 (Fig. 3). The overall magnitude of proliferation was low because of the weak stimulation of human B cells by LPS. These experiments are also limited because we did not use a pure human B-cell population. Thus, although these experiments suggest an inhibitory effect of PRBC on human B-cell proliferation, further studies better characterizing the effect of RBCs, both fresh and stored, on pure B-cell populations will be necessary.
Fig. 3.
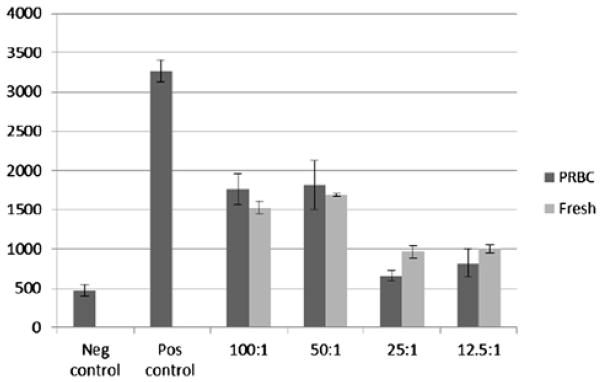
Red blood cells have a suppressive effect on B-cell proliferation. B cells within human PBMCs were stimulated to proliferate by the addition of LPS at 100 μg/mL. Cells were then exposed to blood bank PRBCs and fresh donor RBCs at an initial concentration of 100 RBCs to one PBMC, followed by serial dilutions. Cells were cultured in 96-well plates with CRPMI media, pulsed with tritiated thymidine at 48 h, harvested, and read by scintillation counter at 72 h. Overall, cells exposed to RBCs showed significant inhibition of proliferation. No difference was appreciated between exposure to PRBCs versus fresh RBCs.
Discussion
Transfusion-related immunomodulation persists despite leuko-reduction, which implicates the RBC itself as a major causative agent. Transfusion-related immunomodulation consists of both immunostimulatory and immunosuppressive phenomena. Proinflammatory responses (i.e., systemic inflammatory responses and multiple organ failure) are often seen after massive transfusions, whereas the immunosuppressive effects are seen in lower-volume transfusion of as small as a single unit of PRBCs. Likewise, the two-hit model of post-transfusion immune stimulation has been proposed, including an underlying inflammatory state that primes the recipient immune system with subsequent PRBC transfusion leading to marked proinflammatory responses [5,15]. This study clarifies known suppressive effects of PRBCs on human T cells, both CD4+ and CD8+, and demonstrates an ability to eliminate this phenomenon by using fresh RBCs not exposed to traditional blood banking methods. In examining the blood banking protocols, we were not able to induce a suppressive profile in RBCs after 6 h of exposure to CPD, which suggests that a CPD effect, if it occurs, does not develop immediately upon processing [16,17]. To further understand the suppressive effects of PRBC on different lymphocyte subsets, we have begun examining the effects of RBCs on human B cells. Early data indicate that both stored and fresh RBCs potentially suppress B-cell proliferation. Although these concepts require more detailed analysis, they offer some insight into the breadth of immunosuppressive mechanisms associated with transfusion-related immunomodulation. A previous study established that the immunosuppressive effects from transfusing a single unit of RBCs results in increases in surgical site infections, length of stay, days of mechanical ventilation, and overall mortality [1]. Identifying the potential culprit in blood banking protocols offers great potential for modification of current practices in transfusion medicine. Further characterization of each component used in the process is under way.
Acknowledgments
This project was supported by the National Center for Advancing Translational Sciences, grant UL1TR000117; and the Dean of the College of Medicine, University of Kentucky.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of Kentucky.
References
- 1.Bernard AC, Davenport DL, Chang PK, Vaughn TB, Zwischenberger JB. Intraoperative transfusion of 1U to 2U packed red blood cells is associated with increased 30-day mortality, surgical site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Bernard A, Meier C, Ward M, et al. Packed red blood cells suppress T-cell proliferation through a process involving cell-cell contact. Trauma. 2010;69:320. doi: 10.1097/TA.0b013e3181e401f0. [DOI] [PubMed] [Google Scholar]
- 3.Middleburg RA, van de Watering LM, Briet E, van der Bom JG, et al. Storage time of red blood cells and mortality of transfusion recipients. Transfus Med Rev. 2013;27:36. doi: 10.1016/j.tmrv.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Koch CG, Li L, Blackstone EH, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 5.Sparrow RL. Red blood cell storage and transfusion-related immunomodulation: a second hit in an inflammatory cascade? Vox Sang. 2010;95:261. doi: 10.1111/j.1423-0410.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 6.Hendrickson JE, Eldad AH, Zimring JC, et al. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion. 2011;51:695. doi: 10.1111/j.1537-2995.2011.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparrow R. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10:s7. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajashekharaiah V, Koshy AA, Koushik AK, et al. The efficacy of erythrocytes isolated from blood stored under blood bank conditions. Transfus Apher Sci. 2012;47:359. doi: 10.1016/j.transci.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Spinella PC, Strandenes G, Hervig T, et al. Symposium on fresh whole blood for severe hemorrhagic shock: from in-hospital to far forward resuscitations. Transfus Apher Sci. 2012;46:113. doi: 10.1016/j.transci.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Aubron C, Syres G, Cooper DJ, et al. A pilot feasibility trial of allocation of freshest available red blood cells versus standard care in critically ill patients. Transfusion. 2012;52:196. doi: 10.1111/j.1537-2995.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- 11.Hendrickson JE, Chadwick TE, Zimring JC, et al. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 12.Clark DA, Gorczynski RM, Blajchman MA. Transfusion-related immunomodulation due to peripheral blood dendritic cells expressing the CD200 tolerance signaling molecule and alloantigen. Transfusion. 2008;48:814. doi: 10.1111/j.1537-2995.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- 13.Antonelou MH, Kriebardis AG, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50:376. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 14.Moroff G, Dende D. Characterization of biochemical changes occurring during storage of red cells: comparative studies with CPD and CPDA-1 anticoagulant-preservative solutions. Transfusion. 1983;23:484. doi: 10.1046/j.1537-2995.1983.23684074268.x. [DOI] [PubMed] [Google Scholar]
- 15.Lannan KL, Sahler J, Spinelli SL, Phipps RP, Blumberg N. Transfusion immunomodulation—the case for leuko reduced and (perhaps) washed transfusions. Blood Cells Mol Dis. 2013;50:61. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamel N, Goubran F, Ramsis N, Ahmed AS. Effects of storage time and leukocyte burden of packed and buffy-coat depleted red blood cell units on red cell storage lesion. Blood Transfus. 2010;8:260. doi: 10.2450/2009.0131-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess JR. Red cell storage. J Proteomics. 2010;73:368. doi: 10.1016/j.jprot.2009.11.005. [DOI] [PubMed] [Google Scholar]