Abstract
Cancers due to germline mutations in the BRCA1 gene tend to lack targets for approved chemoprevention agents. This study aimed at a targeted chemoprevention strategy for BRCA1-associated malignancies. Mutant BRCA1 limits the base-excision DNA repair activity that addresses oxidative DNA damage, the accumulation of which heightens one’s risk for cancer. Therefore, we conducted a high-throughput chemical screen to identify drug candidates that could attenuate the inhibitory effects of mutant BRCA1 on this repair activity, thereby describing a new class of DNA repair-activating chemopreventive agents. In the screen design, such drugs functioned by enhancing base-excision DNA repair of oxidative DNA damage in the presence of mutant BRCA1, with minimal cytotoxicity. We identified at least one new agent that decreased malignant properties associated with tumorigenesis, including anchorage-independent growth and tumor progression. This work offers a preclinical proof-of-concept for a wholly new approach to chemoprevention in carriers of BRCA1 mutations, as a strategy to reduce the prevalence of BRCA1-associated malignancy.
Keywords: Breast cancer, BRCA1, oxidative DNA damage, base-excision DNA repair, chemoprevention
Introduction
The Breast Cancer Susceptibility Gene 1 (BRCA1) encodes a tumor suppressor that acts to maintain genetic stability. Individuals that inherit a mutation in BRCA1 are predisposed to developing breast, ovarian, and other cancers. In fact, carriers of BRCA1 mutations have a 44–75% lifetime risk for developing breast cancer (1–2) and up to an approximate 45% risk for developing a second primary breast cancer (3–6). Germline mutations in BRCA1 are responsible for approximately half of hereditary breast cancer cases (7). BRCA1-mutated breast cancers are particularly aggressive and exhibit poor outcomes compared to other subtypes of breast cancer. They frequently contain mutations in TP53 and tend to be ‘triple-negative’ due to their lack of expression of estrogen receptor, progesterone receptor and HER2/NEU oncogene, thereby rendering them relatively resistant to existing anti-cancer strategies that rely on p53-dependent apoptosis or utilize endocrine-based or HER2-directed therapy (8–11, 2). Notably, the selective estrogen receptor modifiers (SERMs) tamoxifen and raloxifene are the only FDA-approved chemoprevention agents for breast cancer. Tamoxifen has displayed mixed results in studies that evaluated its preventive benefit in carriers of BRCA1 mutations (12–16), however, due to the lack of expression of estrogen and progesterone receptors in BRCA1-mutated tumors, SERMs are thought to be relatively ineffective in this setting. Furthermore, raloxifene is not currently FDA-approved for the chemoprevention of breast cancer in pre-menopausal women, and carriers of BRCA1 mutations tend to develop breast cancer prior to menopause (17–18). Therefore, BRCA1-associated malignancies are prevalent, aggressive, and in need of an effective chemoprevention strategy.
Cellular characteristics that contribute to tumorigenesis, such as defects in the defense mechanisms against oxidative DNA damage (ODD), are important for the discovery of cancer prevention strategies. ODD is generated by reactive oxygen species due to normal metabolism and other biological processes. A single human cell experiences approximately 104 oxidative lesions per day (19), making ODD the most common form of DNA damage. ODD is typically repaired by the base-excision DNA repair (BER) pathway. When left unrepaired, ODD leads to mutagenesis, genetic instability, and ultimately the initiation and progression of breast and other cancers (20–22). BRCA1 has been shown to play a role in BER of oxidative DNA damage (23–24), and BRCA1-mutated breast cancers displayed a compromised ability for BER of oxidative DNA damage (23). Therefore, in pursuit of a targeted chemoprevention strategy for BRCA1-associated malignancies, we sought to identify small molecules that enhanced the repair of ODD in the presence of mutant BRCA1, which in turn, may prevent tumorigenesis.
Materials and Methods
Cell Lines
Human breast cell lines included those that represent mutant BRCA1 breast cancer (SUM149, SUM1315, and HCC1937), wild-type BRCA1 breast cancer (BT474, MCF7), and the normal breast (MCF10A). SUM149 and SUM1315 were purchased and characterized by Asterand® plc using short tandem repeat polymorphism analysis. All other cell lines were purchased and characterized by ATCC® using short tandem repeat DNA profiling. All cell lines tested negative for mycoplasma, were passaged for fewer than 6 months after receipt or resuscitation from frozen stocks created within two weeks of purchase, and were then cultured as recommended by the manufacturer. Human breast cancer cell lines isogenic for BRCA1 included MCF7-shCTRL and MCF7-shBRCA1. Non-targeting control shRNA (5′-GGAGATCAGCCATTAATAT-3′) and BRCA1 shRNA (5′-TGCCAAAGTAGCTAATGTA-3′) were cloned into pSUPER.retro.puro (Oligoengine) according to the manufacturer’s instructions, and then transduced into MCF7 cells. Stable selection was carried out with puromycin.
High-throughput Chemical Screen
The reagents, compound library, screening protocol, and post-screen analysis as depicted in Fig. 1 are further described in Supplementary Methods.
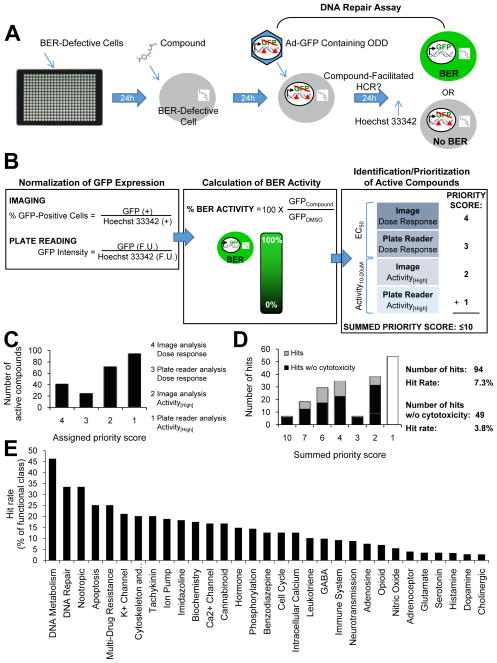
Fig. 1. A high-throughput chemical screen identified DNA repair-activating agents.
(A) The screening protocol consisted of 4 basic steps: (1) plating of BER-defective cells, (2) addition of compounds/controls, (3) the BER assay, and (4) addition of Hoechst 33342 live-cell dye (to allow for normalization to cell number) prior to fluorescent detection by image analysis and plate reading. (B) Post-screen analysis included normalization of GFP expression, calculation of the ‘% BER activity’ relative to the negative control for each method of detection, and identification/prioritization of active compounds. Active compounds were defined as having produced a dose-response increase in GFP expression (EC50) or having increased GFP expression >10% over the negative control at either of the two highest concentrations tested (Activity[High]). For each compound, up to four ‘priority scores’ were assigned (to reflect the significance of the method of detection and calculation) and added to generate a ‘summed priority score’ ≤10. (C) Each bar represents the number of active compounds assigned to each ‘priority score’. (D) Hits were defined as active compounds with a ‘summed priority score’ >1. The graph depicts the number of hits with and without potential cytotoxicity for each ‘summed priority score’. Higher ‘summed priority scores’ predict BER activity with greater significance. (E) The compound library was sorted by functional class (as defined by the manufacturer), and the percentage of hits within each class was calculated.
Drugs
Benserazide, acetohexamide, tamoxifen, and carbidopa were purchased from Sigma-Aldrich and prepared as described by the manufacturer. Treatment with drug occurred for 24 hours unless specified otherwise.
BER Assay
Adenovirus coding GFP (ad-GFP, Clontech) was incubated with methylene blue and exposed to visible light for zero (undamaged control) or two minutes to induce ODD. Cells were then infected with undamaged or damaged ad-GFP, and after 24 hours, analyzed for GFP expression. % Repair is the percentage of GFP expression (normalized to cell number) from damaged virus relative to undamaged virus.
DNA Damage
Detection of 8oxoG was measured by flow cytometry using the OxyFLOW™ kit (HemoGenix®) according to the manufacturer’s instructions. Detection of H202-induced ODD was measured by the alkaline comet assay modified for detection of oxidative damage. Cells were treated with 25μM H202 for 10 minutes at 4°C and then allowed to repair for 4 hours under normal culture conditions. Cells were then subjected to the alkaline comet assay using the CometAssay™ HT Reagent Kit (Trevigen) as described by the manufacturer with the following modifications. Prior to denaturation, DNA was treated ±FPG enzyme (NE Biolabs) at 37°C and 5%CO2 for 45 minutes to nick DNA at sites of ODD. Comet heads indicate undamaged DNA and comet tails indicate DNA damage, therefore, comets were analyzed for the ‘% DNA in tail’ using CometScore™ software (TriTek Corporation). ODD was calculated by subtracting ‘%DNA in tail’ in FPG-minus samples from ‘%DNA in tail’ in FPG-plus samples. Detection of DNA strand breaks was also carried out by the alkaline comet assay using the CometAssay™ HT Reagent Kit (Trevigen) as described above, however, DNA strand breaks were calculated as the ‘%DNA in tail’ in FPG-minus samples. Detection of serum levels of 8oxodG was carried out using the HT 8-oxo-dG ELISA Kit (Trevigen) according to the manufacturer’s instructions.
Anchorage-Independent Growth
Single cell suspensions were cultured in soft agar supplemented with medium containing drug or vehicle control for up to 14 days. Colonies were counted and expressed as a percentage of the control.
Trypan Blue Exclusion Assay
Single cell suspensions were cultured in regular growth media with drug or vehicle control. After 2 days, cells were washed and subjected to trypan blue dye. The number of blue/non-viable and white/viable cells were then counted to calculate the percentage of viable cells.
Tumor Progression
Animal experiments were carried out in compliance with AAALAC Accreditation guidelines and were approved by the Institutional Animal Care and Use Committee at Stanford University. NOD/SCID female mice 4–6 weeks of age were obtained from Jackson Laboratories and were maintained under specific pathogen-free conditions. Mice were inoculated by subcutaneous injection with 3×106 HCC1937 human breast cancer cells confirmed to be pathogen-free in 100ul of PBS and matrigel (1:1). Subcutaneous injection into the mammary fat pad occurred under 0.5–3% isoflurane for animal comfort. One day prior to tumor inoculation, mice were divided into three groups for study: vehicle control (saline), 50 mg/kg benserazide, or 250mg/kg benserazide. Mice were administered treatment in 0.05ml saline daily by i.p. injection until procedure endpoint (60 days). Palpable tumor burden was determined weekly beginning at day 30. At the study endpoint, blood was collected for analysis of oxidative DNA damage, tumor was excised and weighed, and anatomy was analyzed for metastasis. To decipher between treatment-mediated effects and the lack of tumor uptake, mice without a palpable tumor were monitored for late tumor formation for six additional weeks without treatment after the study endpoint. Late tumor formation indicated that the lack of tumor at the study endpoint was due to treatment-mediated effects rather than a lack of tumor uptake; tumor and metastasis data collected at the study endpoint was then included in the analyses. Conversely, the absence of late tumor formation suggested the lack of tumor uptake, and data was thereby excluded from the analysis.
Statistics
All statistical analyses were carried out using the student’s t-test with the following exceptions. The relationship between the average serum levels of 8oxodG and tumor weights was determined using the Pearson correlation coefficient (r). P-values for in vivo data over time were relative to the vehicle control and determined by ANOVA.
Results
High-throughput screening for small molecules that activate BER
We first established a high-throughput screening protocol to identify small molecules that activate repair of ODD. Our protocol consisted of screening a compound library using a DNA repair assay that evaluated BER of ODD while maintaining an intact cellular environment (23). Briefly, cells are delivered an ODD-containing GFP reporter gene via adenoviral-mediated gene transfer, allowed sufficient time to carry out repair, and then analyzed for fluorescence. In this system, ODD interferes with the transcription and subsequent expression of GFP. Therefore, cells with defective BER do not fluoresce. However, small molecules that facilitate repair of the ODD-containing GFP reporter gene may enable the expression of GFP in cells with defective BER, and thereby produce a fluorescent signal. We utilized the BRCA1-mutant SUM149 human breast cancer cell line due to its susceptibility to adenoviral infectivity (required for delivery of the ODD reporter gene) and its inability to effectively repair ODD (required for detection of compound-facilitated BER) (Supp. Fig. 1 and (23)). We chose to screen the LOPAC1280™ library (Sigma-Aldrich®), which consisted of 1280 compounds with known pharmacological activity, in quantitative high-throughput (qHT) format (25) starting at a 20μM final concentration of compound in each well (0.2% DMSO) followed by up to six (two-fold) dilutions. DMSO was the negative control and 18μM BrdU was the positive control. We identified BrdU in a preliminary screen (Supp. Fig. 2) due to the lack of an existing but necessary small-molecule positive control.
We next adapted and validated our protocol for high-throughput screening (Supp. Figs. 3–4). The protocol, as depicted in Figure 1A, produced a Z′-factor= 0.6, CV=7%, S/B=10, and no significant edge or drift effects (Supp. Fig. 4C).
Finally, we carried out the validated protocol in 384-well qHT-format. BRCA1-mutant SUM149PT cells were treated with up to seven doses of compound (or control), subjected to the DNA repair assay, and assessed for GFP expression by two different means (i.e. imaging and plate reading). Greater GFP expression indicated greater enhancement of repair.
We then developed a scoring system to identify and prioritize active compounds based on their ability to enhance BER of ODD. The scoring system consisted of assigning ‘priority scores’ based on the level of significance of each method of GFP detection (imaging > plate reading) and definition of an active compound (EC50 > Activity[High]), and then calculating a ‘summed priority score’ that ranged from 1–10 for each molecule; higher scores indicated higher priority (Fig. 1B). Figs. 1C and 1D depict the number of molecules for each assigned ‘priority score’ and ‘summed priority score’, respectively. In general, the number of molecules increased as the score decreased. The high prevalence and low priority of molecules with a ‘summed priority score’ = 1 resulted in their elimination; remaining molecules were defined as hits.
We identified 94 molecules that potentially enhanced BER of ODD (hit rate = 7.3%; Fig. 1D). Evaluation of the functional classes of these compounds revealed the greatest percentage of hits as having activity in DNA metabolism or DNA repair (Fig. 1E). Given that cytotoxic compounds may be undesirable for chemoprevention, we used our screening data acquired by imaging to identify compounds that decreased the number of Hoechst 33342-positive cells (i.e. living cells) by ≥20% compared to the negative control. Elimination of these potentially cytotoxic compounds resulted in a subset of 49 hits (hit rate = 3.8%; Fig. 1D). Detailed results for all 94 hits have been provided in Supplemental Table 1, including the EC50 and % BER activity at high concentrations (for each method of GFP detection), the ‘summed priority score’, % Hoechst-positive cells, and functional class. These molecules have been classified as ‘DNA repair-activating agents’.
Functional activity of DNA repair-activating agents
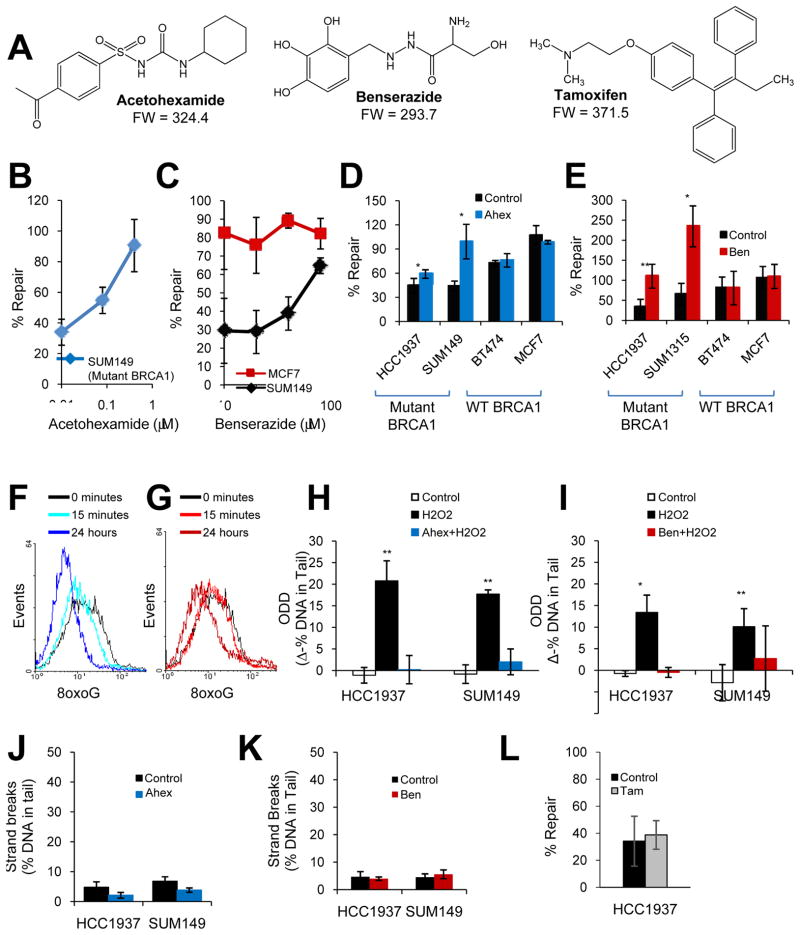
We further analyzed two hits, acetohexamide and benserazide (Fig. 2), because they carried the highest ‘summed priority score’ with the least amount of cytotoxicity (based on screening data). Both acetohexamide and benserazide carried ‘summed priority scores’ of 7 and resulted in 87.8% and 85.9% of cells staining positive for Hoechst 33342-live cell dye, respectively. To validate activity, acetohexamide and benserazide were re-ordered from the original vendor and analyzed for BER activity and ODD levels. Acetohexamide produced a dose-response increase in repair of an oxidatively-damaged GFP reporter gene in the mutant BRCA1-containing cell line SUM149PT (Fig. 2B). Likewise, benserazide produced a dose-response increase in repair of an oxidatively-damaged GFP reporter gene in SUM149PT cells but not in wild-type BRCA1 MCF7 breast cancer cells. Additional breast cancer cell lines displayed similar results (Fig. 2D–E) using the same assay. Treatment of mutant BRCA1-containing cell lines with 20μM acetohexamide, i.e. the highest concentration used in the HT-screen, produced a statistically significant increase in BER (HCC1937, p=0.01; SUM149, p=0.007), whereas the same treatment in wild-type BRCA1-containing cell lines cells exhibited no effect on BER (BT474, p=0.4; MCF7, p=0.1) (Fig. 2D). Similarly, treatment of mutant BRCA1-containing cell lines with 20μM benserazide produced a statistically significant increase in BER (HCC1937, p=0.007; SUM1315, p=0.01), whereas the same treatment in wild-type BRCA1-containing cell lines cells resulted in no effect on BER (BT474, p=0.3; MCF7, p=0.5) (Fig. 2E). Acetohexamide and benserazide also decreased basal levels of 8oxoG lesions, i.e. the most common form of ODD, in a time-dependent manner in SUM149 cells (Fig. 2F–G), and decreased ODD induced by 25μM H2O2 to levels similar to those of the undamaged control in two breast cancer cell lines with mutant BRCA1 (Fig. 2H–I). Taken together, these data suggest that acetohexamide and benserazide are two DNA repair-activating agents that function by enhancing BER of ODD in the presence of mutant, but not wild-type BRCA1.
Fig. 2. DNA repair-activating agents enhance BER of ODD in the presence of mutant BRCA1.
(A) Chemical structures are shown for DNA repair-activating agents, acetohexamide and benserazide, and the FDA-approved chemoprevention agent for breast cancer, tamoxifen. (B–C) The graphs depict the effect of increasing concentrations of acetohexamide (B) or benserazide (C) on BER in SUM149 (mutant BRCA1) or MCF7 (wild-type BRCA1) cells. (D–E) The graphs illustrate the effect of vehicle control, 20μM acetohexamide (D), or 20μM benserazide (E) on BER in cell lines with mutant or wild-type BRCA1. (F–G) Basal levels of 8oxoG lesions in SUM149 cells treated with 20μM acetohexamide (F) or 20μM benserazide (G) for the specified time were determined by flow cytometry. A leftward shift in histogram peak indicates decreased 8oxoG levels. Data represent two independent experiments. (H–I) Levels of H2O2-induced ODD following pretreatment with vehicle control, 0.4μM acetohexamide (H) or 20μM benserazide (I) for 24 hours were compared to undamaged control for mutant BRCA1 cell lines using the alkaline comet assay modified for detection of oxidative lesions. Each bar represents the average of three experiments ± s.e.m. (J–K) DNA strand breaks in mutant BRCA1 cell lines treated with vehicle control, 0.4μM acetohexamide (J) or 20μM benserazide (K) for 28 hours were measured by the alkaline comet assay. (L) The bar graph indicates the effect of vehicle control or 1μM tamoxifen on BER in SUM149 cells. Unless otherwise indicated, all data are means and represent at least three independent experiments, error bars denote s.d., and p-values are relative to the control. *, p<0.05; **, p<0.01. Ahex, acetohexamide; Ben, benserazide; Tam, tamoxifen; 8oxoG, 8-oxoguanine adducts.
Under normal conditions, DNA damage activates mechanisms of DNA repair (26); therefore, we tested whether or not acetohexamide or benserazide functioned by inducing DNA damage. When mutant BRCA1-containing cell lines were treated with 0.4μM acetohexamide or 20μM benserazide and then analyzed for DNA strand breaks using the alkaline comet assay, we found that neither drug induced DNA strand breaks relative to the vehicle control (Fig. 2J–K). Therefore, DNA repair-activating agents, acetohexamide and benserazide, increase BER directly, rather than indirectly through induction of DNA damage.
For purposes of comparison, we also tested the effect of tamoxifen on BER (due to it being the current FDA-approved chemoprevention agent for breast cancer). We used the highest concentration of tamoxifen that did not produce a statistically significant decrease in cell viability in breast cancer cell lines under our experimental conditions (i.e. 1μM). Unlike DNA repair-activating agents, treatment with tamoxifen did not significantly increase repair of an oxidatively-damaged GFP reporter gene in SUM149 cells (p=0.28; Fig. 2L).
Effect of a DNA repair-activating agent on anchorage-independent growth
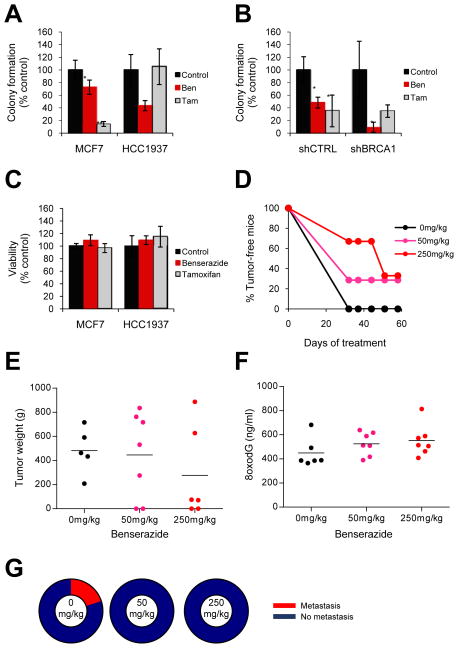
Anchorage independence is a characteristic of transformed cells that often indicates tumorigenic potential. We asked whether or not DNA repair-activating agents could reduce anchorage-independent growth. Therefore, we treated breast cancer cells with benserazide or vehicle control and measured colony formation in soft agar. Benserazide significantly decreased colony formation in a mutant BRCA1-containing cell line (HCC1937, p=0.0003) as well as a wild-type BRCA1-containing cell line (MCF7, p=0.03), with the effect being significantly greater for the mutant BRCA1-containing cell line compared to the wild-type cell line (p=0.001) (Fig. 3A). Similarly, benserazide reduced colony formation in MCF7 cells stably transduced with shRNA to BRCA1 (p=0.03) or a non-targeting control (p=0.02), with the effect being significantly greater in those cells containing shRNA to BRCA1 compared to the control (p=0.005) (Fig. 3B). We next assessed whether the reduction in colony formation was due to a decrease in anchorage-independent growth or a decrease in cell viability. Therefore, we carried out the soft agar colony formation assay in parallel to the trypan blue exclusion assay for the wild-type and mutant BRCA1-containing cell lines, and found that benserazide did not significantly affect cell viability in either cell line (MCF7, p=0.2; HCC1937, p=0.4) (Fig. 3C).
Fig. 3. Benserazide decreases malignant properties associated with tumorigenesis.
(A) Anchorage-independent growth was measured by colony formation in soft agar. Colony formation is displayed for MCF7 (wild-type BRCA1) and HCC1937 (mutant BRCA1) cells treated with vehicle control, 20μM benserazide, or 1μM tamoxifen. Tamoxifen is the current FDA-approved chemoprevention agent for breast cancer. Each bar represents the average of at least three experiments ± s.e.m. (B) Colony formation is displayed for MCF7 cells stably transduced with shRNA to BRCA1 (shBRCA1) or a non-targeting control (shCTRL) as described in (A). (C) Cells in (A) were simultaneously subjected to the trypan blue exclusion assay to assess viability. (D) Tumor progression was measured using a xenograft mouse model. NOD/SCID mice were treated daily with vehicle control or benserazide as described. Twenty-four hours after the initial treatment, mice were inoculated with HCC1937 cells. The graph depicts the percentage of tumor-free mice for up to 60 days of treatment. (E–G) At the study endpoint, tumor weight (E), serum levels of 8oxodG (F), and the percentage of mice with and without metastasis (G) was determined for each treatment group. Closed circles represent individual tumors; black lines indicate mean levels. Ben, benserazide; Tam, tamoxifen. P-values are relative to the vehicle control. *, p<0.05; **, p<0.01.
To compare benserazide to the existing FDA-approved chemoprevention agent for breast cancer, we also examined the effect of tamoxifen on anchorage-independent growth using the soft agar colony formation assay. Tamoxifen showed no effect on colony formation in mutant BRCA1-containing cells (HCC1937, p=0.7), but significantly decreased colony formation in wild-type BRCA1-containing cells (MCF7, p=0.0003) (Fig. 3A). Likewise, tamoxifen did not significantly decrease colony formation in MCF7 cells transduced with shRNA to BRCA1 (p=0.2), but significantly decreased colony formation in cells with the non-targeting control (p=0.03) (Fig. 3B). A parallel experiment using the trypan blue exclusion assay revealed that tamoxifen did not affect cell viability in either mutant (HCC1937, p=0.3) or wild-type (MCF7, p=0.6) BRCA1-containing cells (Fig. 3C), but rather reduced colony formation in wild-type BRCA1-containing cells due to a decrease in anchorage-independent growth.
Taken together, benserazide was more effective than tamoxifen at decreasing the tumorigenic potential of cell lines with mutant or deficient BRCA1 but not wild-type BRCA1.
Effect of a DNA repair-activating agent on tumor progression
Tumor progression is the final step of tumorigenesis that allows cells to develop and maintain malignant properties. Therefore, we carried out an in vivo study to analyze characteristics of tumor progression including tumor formation, growth, and metastasis. We used a xenograft mouse model and began treatment with DNA repair-activating agent 24 hours prior to inoculation of mutant-BRCA1 HCC1937 cells into the mammary fat pad. Treatment with vehicle control, 50mg/kg benserazide, or 250mg/kg benserazide continued daily for up to 60 days while monitoring for tumor formation by palpitation. After the 60 days (i.e. study endpoint), we collected serum for analysis of 8oxodG levels, excised and weighed tumors (if present), and analyzed metastasis by necropsy. If no tumor was present, mice remained under surveillance for tumor formation in the absence of treatment for up to 6 weeks after the study endpoint to distinguish between unsuccessful tumor inoculation and drug-mediated effects. Mice that did not form tumors were eliminated from the data pool presumably due to the lack of tumor uptake following inoculation rather than an anti-tumor effect by the drug. This model system feasibly allowed for completion of an in vivo study that mimicked chemoprevention rather than therapeutic conditions.
We found that benserazide delayed the rate of tumor formation in mice in a dose-response manner (Fig. 3D). Compared to the vehicle control, treatment with 50mg/kg and 250mg/kg benserazide increased the percentage of tumor-free mice over time (p= 0.27 and 0.047, respectively). We also discovered that benserazide reduced tumor growth as evidenced by a dose-response decrease in the average weight of tumors at the study endpoint (Fig. 3E). Interestingly, benserazide simultaneously increased serum levels of 8oxodG (Fig. 3F), a byproduct of DNA repair of oxidative DNA damage. Indeed, the average tumor weight negatively correlated with the average serum levels of 8oxodG among treatment groups (r = −0.82), indicating that the reduction in tumor growth was proportional to benserazide-mediated BER. Finally, unlike the vehicle control, both concentrations of benserazide prevented metastasis (Fig. 3G).
Analysis of the molecular target of benserazide
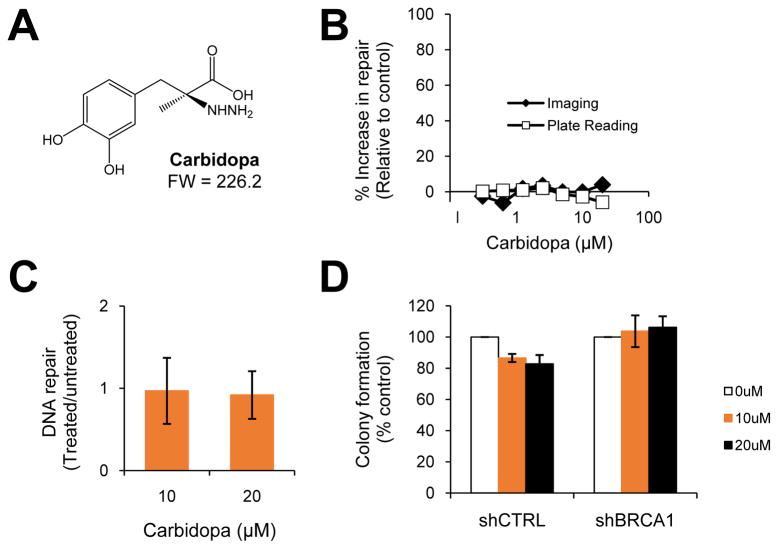
Benserazide and its analog, carbidopa, (Fig. 4A) are both known to function as DOPA decarboxylase inhibitors. Benserazide and carbidopa were also both included among the compounds screened for BER-enhancing activity (Fig. 1). However, our screen did not identify carbidopa as a hit (Fig. 4B). Therefore, we first validated our screening data by subjecting the mutant BRCA1-containing SUM149 cell line to the BER assay following pre-treatment with vehicle control or carbidopa. Given that benserazide is typically administered at approximately half the dose of carbidopa (27), we used carbidopa at the dose equivalent to the effective dose of benserazide (10μM) and at the highest screened concentration or twice the effective dose of benserazide (20μm). Indeed, carbidopa exhibited no effect on repair activity compared to the vehicle control (10μM, p=0.4; 20μM, p=0.3) (Fig. 4C). We next asked whether carbidopa had an effect on tumorigenic potential by measuring anchorage-independent growth. The soft agar colony formation assay revealed that compared to the vehicle control, treatment with 10μM or 20μM carbidopa had no significant effect on colony formation of MCF7 cells transduced with either shRNA to BRCA1 (10μM, p=0.09; 20μM, p=0.1) or a non-targeting control (10μM, p=0.7; 20μM, p=0.4) (Fig. 4D). Taken together, unlike benserazide, its carbidopa analog does not function as a DNA repair-activating agent or decrease anchorage-independent growth. These data suggest that the benserazide-mediated effects on BER of ODD and tumorigenic potential are independent of DOPA decarboxylase inhibition.
Fig. 4. Carbidopa, a benserazide analog, does not enhance repair or prevent anchorage-independent growth.
(A) The chemical structure of carbidopa is shown. (B) Carbidopa was included in the chemical screen described in Fig. 1. The screening data is displayed and includes the percent increase in BER relative to the vehicle control for both methods of detection- imaging and plate reading. (C) The graph depicts the effect of carbidopa on BER activity relative to the untreated vehicle control using a small-scale BER assay for validation of screening results. Data are representative of three experiments; error bars denote s.d. (D) The bar graph displays colony formation for shCTRL- and shBRCA1-containing MCF7 cells (described in Fig. 3B) treated with vehicle control or increasing concentrations of carbidopa. Each bar represents the average of at least three experiments ± s.e.m.
Discussion
BRCA1 mutation or deficiency leads to defective base-excision DNA repair and excessive oxidative DNA damage (23–24), which in turn, leads to tumorigenesis. We have discovered small molecules that enhance BER of ODD and reduce malignant properties associated with tumorigenesis, including anchorage-independent growth and tumor progression. While previous studies have reported small molecules that inhibit DNA repair (28), this study, to our knowledge, reports small molecules that activate repair for the first time. These DNA repair-activating agents may potentially be used for the chemoprevention of BRCA1-associated malignancies.
A high-throughput chemical screen identified a novel class of drugs termed “DNA repair-activating agents”. We pursued two of these agents, acetohexamide and benserazide, because of their predicted activity, lack of potential cytotoxicity, and clinical potential. Both acetohexamide and benserazide enhanced BER (Fig. 2B–E) as well as decreased basal levels and H202-induced levels of ODD (Fig. 2F–I). These effects were observed in a dose-response and/or time-dependent manner and were specific to mutant BRCA1-containing cells. Neither drug induced DNA damage, including single- or double-strand breaks (Fig. 2J–K), suggesting that the ability to enhance DNA repair was due to direct means as opposed to indirect means of damage-induced repair. Both acetohexamide and benserazide also exhibited minimal cytotoxicity at concentrations that enhanced BER of ODD (Fig. 3C and Supp. Fig. 5) and lacked chemical, toxicological, and environmental health concerns according to the U.S. National Library of Medicine TOXNET database. Furthermore, we derived DNA repair-activating agents by screening compounds with known pharmacological activity, thereby facilitating their translation into the clinic. For example, acetohexamide and benserazide have already been through human trials, and have been approved for clinical use in the United States for diabetes and in the United Kingdom and Canada for Parkinson’s disease, respectively. Taken together, DNA repair-activating agents may potentially be repurposed to reverse a defect in DNA repair associated with BRCA1-mediated tumorigenesis.
The DNA repair-activating agent benserazide decreased malignant properties associated with tumorigenesis. Our data showed that compared to vehicle controls, benserazide reduced anchorage-independent growth in vitro as well as delayed tumor formation and decreased the average weight of tumors in vivo when administered prior to tumor implantation (Fig. 3A–D). We observed a statistically significant decrease in the rate of tumor formation for mice treated with the higher dose (250mg/kg) but not the lower dose (50mg/kg) of benserazide. The lack of a statistically significant difference in the latter case may have been due to small sample sizes. Nonetheless, the effects of benserazide on tumor formation and growth occurred in a dose-response manner. Importantly, an increase in levels of a serum marker for repair of ODD (8oxodG) correlated with the decrease in tumor weight in mice treated with benserazide (r = −0.82) (Fig. 3E–F). On the other hand, carbidopa, a benserazide analog, did not exhibit BER activity (Fig. 4B–C) and did not reduce in vitro tumorigenic potential (Fig. 4D). Together, these data provide evidence for a link between enhancing BER and preventing tumorigenesis. Interestingly, treatment with benserazide also prevented metastasis (Fig. 3G), suggesting that DNA repair-activating agents may not only be useful prior to a cancer diagnosis for chemopreventive purposes, but also after a diagnosis for the treatment of BRCA1-mutated breast cancers. Through its role in maintaining genetic stability, BRCA1 has been implicated in both the initiation and progression steps of tumorigenesis (29–30, 21). However, we only captured data for tumor progression because our assays utilized cancer cell lines that have already undergone tumor initiation. It remains possible that benserazide or other DNA repair-activating agents compensate for mutant BRCA1 function at the earlier step of tumor initiation. A model system that utilizes primary cultures with a single BRCA1 mutation or a mouse model with a conditional Brca1 mutation (31) will better serve to answer this question, but unfortunately, these models are limited by uncertainties of tumor formation and long latency periods. Furthermore, we avoided data that was skewed in favor of treatment-mediated effects due to our use of established cell lines by monitoring for tumor formation after the study endpoint and eliminating data obtained from mice that did not successfully experience tumor uptake upon inoculation.
Our study suggests that DNA repair-activating agents may provide a better option than tamoxifen for the chemoprevention of BRCA1-associated breast cancers. While benserazide significantly enhanced BER of ODD (Fig. 2C, E, G, I) and decreased malignant properties associated with tumorigenesis (Fig. 3A–B, 3D–F), tamoxifen did not enhance BER of ODD in BRCA1-mutant cells (Fig. 2L), nor did it effectively reduce tumorigenic potential in BRCA1-mutant or deficient cells (Fig. 3A–B). However, tamoxifen significantly reduced tumorigenic potential in wild-type BRCA1-containing cells (Fig. 3A–B), which is consistent with published studies that examined its effectiveness in breast cancer populations (14). The use of tamoxifen for breast cancer prevention is often avoided by patients because it increases the risk for endometrial carcinoma (32–36). While tamoxifen is a well-established carcinogen, neither acetohexamide nor benserazide have been reported to exhibit carcinogenic potential (37–38). Therefore, DNA repair-activating agents provide an added advantage to carriers of BRCA1 mutations who are naturally susceptible to carcinogen-induced DNA damage.
The molecular target(s) of DNA repair-activating agents that are responsible for enhancing BER remain unknown. Given that the chemical screen used to identify these agents utilized a library of compounds with known pharmacological activity, DNA repair-activating agents have well-established molecular targets. However, the molecular targets responsible for BER-enhancing activity are independent of their established targets because functional analogs of DNA repair-activating agents do not enhance BER or prevent tumorigenic potential. Benserazide (used in the UK and Canada) and carbidopa (the equivalent used in the US) both target DOPA decarboxylase in the periphery. There is no significant difference in the therapeutic effects for Parkinson’s disease between these two drugs (27). However, unlike benserazide (Fig. 2C, E, G, I and Fig. 3), carbidopa did not enhance BER, nor did it reduce tumorigenic potential (Fig. 4). Likewise, the chemical screen used to identify DNA repair-activating agents (Fig. 1A–B) tested six sulfonylureas that stimulate insulin release via ATP-dependent K+ channels, but only two of these anti-diabetic molecules, acetohexamide (strong hit) and glipizide (weak hit), showed BER-enhancing activity (Figs. 2B, D, F, H and Supp. Table 1). Furthermore, if acetohexamide functioned through its known/anti-diabetic mechanism, one would predict all sulfonylureas to reduce the risk for breast cancer, which is not the case (39). Examination of an NCBI GEO dataset for MCF7 cells treated with benserazide revealed an up-regulation of BER genes previously shown to be transcriptionally activated by BRCA1, including OGG1 by 35-fold, NTH1 by 130-fold, and APE1 by 1049-fold (40, 24). Therefore, DNA repair-activating agents may function by increasing the expression of one of these molecules, though it is unlikely to be OGG1 given that we found no significant difference in the effect of benserazide or acetohexamide on BER activity between shOGG1-containing and non-targeting control MCF7 cells (data not shown). Since these data reflect activity in the presence of wild-type BRCA1, extensive studies are underway to reveal the molecular targets of DNA repair-activating agents in mutant BRCA1-containing cells. DNA repair-activating agents are expected to directly or indirectly target a required enzyme(s)/protein for BER rather than an accessory protein because these agents were identified using an endpoint (not rate-determining) assay for BER ((23) and Fig. 1A). They may inhibit a negative regulator of BER, as most drugs function through inhibition, or they may activate any positive regulator of BER. For example, evidence suggests that altering protein-protein interactions or the acetylation state of APEX1 endonuclease may stimulate BER activity (41). Although DNA repair-activating agents were identified using an assay that enriched for molecules that alter BER activity, ODD may convert to single- or double-strand breaks, and thus DNA repair-activating agents may alter other repair processes in which BRCA1 also plays a role, such as homologous recombination, non-homologous end-joining, or nucleotide excision repair (42–46). However, this possibility is unlikely due to our finding that both benserazide and acetohexamide reduced levels of ODD (Fig. 2F–I) and not strand breaks (Fig. 2J–K).
In summary, this study describes a novel class of drugs that target a defect in repair associated with mutations in BRCA1. Our data suggest that these drugs, i.e. DNA repair-activating agents, may be used to prevent or slow the progression of primary or secondary breast tumors or even delay metastasis. Therefore, these findings support further studies to evaluate the use of DNA repair-activating agents for the chemoprevention of BRCA1-mutated cancers. Clinical studies are best suited following the development of better chemoprevention models for BRCA1-mutated cancers and upon a better understanding of the molecular targets of these drugs. However, benserazide is typically administered with L-DOPA and has little effect on its own, and thus is primed for clinical translation. Other DNA repair-activating agents should first undergo structure-activity-relationship studies to identify an appropriate analog that maintains its DNA repair activity without any adverse effects. For example, acetohexamide should undergo modification to eliminate its glucose-lowering activity to minimize the possibility of inducing hypoglycemia in healthy patients being treated for cancer prevention. Finally, these drugs may be effective for other malignancies associated with oxidative stress, including other cancers, degenerative diseases, and sclerotic disorders.
Supplementary Material
Acknowledgments
Financial Support: Support for this study includes a Susan G. Komen for the Cure Postdoctoral Fellowship (E.A.), Mary Kay Ash Charitable Foundation Grant, and U.S. National Institute of Health R21 grant (1R21NS061674-01A2).
We thank Allison Kurian at the Stanford Women’s Cancer Center for helpful discussion on the manuscript and Jason Wu at the Stanford High-Throughput Bioscience Center for technical assistance with the chemical screen. We also thank Shaveta Vinayak and Kedar Hastak for advice and technical assistance with the in vivo protocol.
Footnotes
Conflicts of Interest: E.A. and J.M.F. have a patent pending, and thus potential ownership interest. There are no other conflicts of interest.
References
- 1.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22(12):2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- 5.Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010;28(14):2404–2410. doi: 10.1200/JCO.2009.24.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kolk DM, de Bock GH, Leegte BK, Schaapveld M, Mourits MJ, de Vries J, et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat. 2010;124(3):643–651. doi: 10.1007/s10549-010-0805-3. [DOI] [PubMed] [Google Scholar]
- 7.Lux MP, Fasching PA, Beckmann MW. Hereditary breast and ovarian cancer: review and future perspectives. J Mol Med. 2006;84(1):16–28. doi: 10.1007/s00109-005-0696-7. [DOI] [PubMed] [Google Scholar]
- 8.Crook T, Crossland S, Crompton MR, Osin P, Gusterson BA. p53 mutations in BRCA1-associated familial breast cancer. Lancet. 1997;350(9078):638–639. doi: 10.1016/S0140-6736(05)63327-2. [DOI] [PubMed] [Google Scholar]
- 9.Crook T, Brooks LA, Crossland S, Osin P, Barker KT, Waller J, et al. p53 mutation with frequent novel condons but not a mutator phenotype in BRCA1- and BRCA2-associated breast tumours. Oncogene. 1998;17(13):1681–1689. doi: 10.1038/sj.onc.1202106. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt MS, Chappuis PO, Bond JP, Hamel N, Foulkes WD. TP53 mutations in breast cancer associated with BRCA1 or BRCA2 germ-line mutations: distinctive spectrum and structural distribution. Cancer Res. 2001;61(10):4092–4097. [PubMed] [Google Scholar]
- 11.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009;20(12):1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 13.King MC, Wieand S, Hale K, Lee M, Walsh T, Owens K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. Jama. 2001;286(18):2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 14.Gronwald J, Tung N, Foulkes WD, Offit K, Gershoni R, Daly M, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118(9):2281–2284. doi: 10.1002/ijc.21536. [DOI] [PubMed] [Google Scholar]
- 15.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips KA, Milne RL, Rookus MA, Daly MB, Antoniou AC, Peock S, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31(25):3091–3099. doi: 10.1200/JCO.2012.47.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94(18):1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 18.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 19.Ames BN, Shigenaga MK. Oxidants are a major contributor to aging. Ann N Y Acad Sci. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 20.Malins DC, Polissar NL, Gunselman SJ. Progression of human breast cancers to the metastatic state is linked to hydroxyl radical-induced DNA damage. Proc Natl Acad Sci U S A. 1996;93(6):2557–2563. doi: 10.1073/pnas.93.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477(1–2):7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 22.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 23.Alli E, Sharma VB, Sunderesakumar P, Ford JM. Defective repair of oxidative dna damage in triple-negative breast cancer confers sensitivity to inhibition of poly(ADP-ribose) polymerase. Cancer Res. 2009;69(8):3589–3596. doi: 10.1158/0008-5472.CAN-08-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha T, Rih JK, Roy R, Ballal R, Rosen EM. Transcriptional regulation of the base excision repair pathway by BRCA1. J Biol Chem. 2010;285(25):19092–19105. doi: 10.1074/jbc.M110.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, et al. Quantitative high-throughput screening: A titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103(31):11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenacre JK, Coxon A, Petrie A, Reid JL. Comparison of levodopa with carbidopa or benserazide in parkinsonism. Lancet. 1976;2(7982):381–384. doi: 10.1016/s0140-6736(76)92403-x. [DOI] [PubMed] [Google Scholar]
- 28.Vinayak S, Ford JM. PARP Inhibitors for the Treatment and Prevention of Breast Cancer. Curr Breast Cancer Rep. 2010;2(4):190–197. doi: 10.1007/s12609-010-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinzler KW, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997;386(6627):761, 763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 30.Deng CX, Scott F. Role of the tumor suppressor gene Brca1 in genetic stability and mammary gland tumor formation. Oncogene. 2000;19(8):1059–1064. doi: 10.1038/sj.onc.1203269. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22(1):37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 32.Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfversward C, et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1(8630):117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 33.Magriples U, Naftolin F, Schwartz PE, Carcangiu ML. High-grade endometrial carcinoma in tamoxifen-treated breast cancer patients. J Clin Oncol. 1993;11(3):485–490. doi: 10.1200/JCO.1993.11.3.485. [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86(7):527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 35.van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrere CH, Otter R, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343(8895):448–452. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- 36.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 37.Chemical Carcinogenesis Research Information System [Internet] Bethesda (MD): National Library of Medicine (US), National Cancer Institute; 2011. [cited 2014 June 11]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CCRIS. [Google Scholar]
- 38.NTP. Report on Carcinogens. 12. Research Triangle Park (NC): U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program; 2011. p. 499. [Google Scholar]
- 39.Pasello G, Urso L, Conte P, Favaretto A. Effects of sulfonylureas on tumor growth: a review of the literature. Oncologist. 2013;18(10):1118–1125. doi: 10.1634/theoncologist.2013-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 41.Fishel ML, Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol Aspects Med. 2007;28(3–4):375–395. doi: 10.1016/j.mam.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Hartman AR, Ford JM. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet. 2002;32(1):180–184. doi: 10.1038/ng953. [DOI] [PubMed] [Google Scholar]
- 43.Hartman AR, Ford JM. BRCA1 and p53: compensatory roles in DNA repair. J Mol Med. 2003;81(11):700–707. doi: 10.1007/s00109-003-0477-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res. 2005;3(10):531–539. doi: 10.1158/1541-7786.MCR-05-0192. [DOI] [PubMed] [Google Scholar]
- 45.Hastak K, Alli E, Ford JM. Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly(ADP-Ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010;70(20):7970–7980. doi: 10.1158/0008-5472.CAN-09-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alli E, Sharma VB, Hartman AR, Lin PS, McPherson L, Ford JM. Enhanced sensitivity to cisplatin and gemcitabine in Brca1-deficient murine mammary epithelial cells. BMC Pharmacol. 2011;11:7. doi: 10.1186/1471-2210-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.