Abstract
Important roles for reactive oxygen species (ROS) in physiology and pathophysiology have been increasingly recognized. Under normal conditions, ROS serve as signaling molecules in the regulation of cellular functions. However, enhanced ROS production as a result of the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase contributes significantly to the pathogeneses of vascular diseases. Although it has become evident that increased ROS is associated with erectile dysfunction (ED), the sources of ROS in the penis remain largely unknown. In recent years, emergent evidence suggests the possible role of NADPH oxidase in inducing ED. In this review, we examine the relationship between ROS and ED in different disease models and discuss the current evidence basis for NADPH oxidase-derived ROS in ED.
Keywords: reactive oxygen species, erectile function, superoxide, penis, nitric oxide
1 Introduction
Erectile dysfunction (ED) affects millions of men worldwide and reduces quality of life [1]. Although ED may result from psychological, neurological, and hormonal defects, vascular impairment accounts for a major portion of male ED [2, 3]. ED is often associated with chronic vascular diseases such as atherosclerosis, hypertension, and heart disease. Elucidation of erectile mechanisms has led to the discoveries of therapeutic targets, among which nitric oxide (NO) has been recognized as a critical molecule in erection physiology. Sexual stimuli induce the release of NO from penile nerve endings and endothelial cells, which in turn relaxes corpus cavernosal smooth muscle and increases blood flow to penis. Studies have shown that increased reactive oxygen species (ROS) reduce NO production or bioavailability, leading to impaired endothelial function and erectile function [4–7].
ROS include free radicals such as superoxide and hydroxyl radicals and non-radicals such as hydrogen peroxide. Abundant evidence has demonstrated the importance of ROS in physiologic functions and the pathogeneses of various diseases. ROS are produced either through non-enzymatic ways or through enzymatic systems such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, un-coupled endothelial NO synthase, cytochrome P450, and the mitochondria respiratory chain. Under normal conditions, cells are capable of self-protecting against the continuous formation of low levels of ROS via major intracellular antioxidant enzymes including superoxide dismutase (SOD), catalase, glutathione peroxidase, and glutathione reductase. For example, studies have shown that cavernous endothelial cells are able to detoxify ROS within a well-defined range using these enzymes [8]. These antioxidant enzymes are usually up-regulated as a compensatory mechanism when ROS production is increased [5, 9]. However, sustained high levels of ROS generation will diminish antioxidant enzyme activities and increase oxidative stress, leading to cell damage.
The effects of ROS on erectile function have been investigated with most of the emphasis placed on the activities of antioxidant enzymes. However, the sources of ROS remain largely unknown. It has become evident that NADPH oxidase is a major source for ROS formation in the vascular wall. In recent years, emergent evidence also suggests the possible role of NADPH oxidase in inducing ED. In the first part of this review, we will briefly examine the relationship between ROS and ED in different disease models. In the second part, we will focus on the basic molecular actions of NADPH oxidase and discuss the current knowledge of NADPH oxidase-derived ROS in ED.
2 ROS and ED
2.1 Hypertension
Most forms of hypertension are characterized by impaired endothelium-dependent vasodilation, which is partly due to an increase in ROS production. Results from clinical studies indicate the high prevalence of ED in hypertensive patients when compared to the general population [2, 10]. In experimental hypertensive animal models, ED has been demonstrated in both spontaneously hypertensive rats (SHR) and deoxycorticosterone acetate-salt hypertensive rats [11, 12]. The levels of thiobarbituric acid reactive substances (TBARS), an indicator for oxidative stress, were increased in SHR penes, leading to reduced NO-dependent relaxations of isolated cavernosal smooth muscle [6]. Diminished SOD activity was observed in SHR rat penes when compared to that of Wistar-Kyoto rats, which may be one of the causes for this increase in ROS production in SHR.
2.2 Diabetes
Diabetes mellitus is the most common risk factor for ED [13]. The risk of ED is three-fold greater for diabetic men and occurs at an earlier age than non-diabetic men. The link between ROS and diabetes-associated ED has been investigated. One possible source of ROS is from circulating monocytes. Morano et al. [14] reported that ROS generated by monocytes were significantly increased in patients with ED when compared to those of patients without ED. In streptozotocin (STZ)-induced type I diabetic rats, endothelial NO synthase and neuronal NO synthase-mediated relaxations of cavernosal muscle strips are reduced [4]. Treatment with the antioxidant, α-lipoic acid, partially restores the relaxations, suggesting increased ROS production in diabetic penes. Another group studied the effects of ROS during the progression of diabetes. They found that increased ROS formation, indicated by increased TBARS levels and reduced glutathione levels, were associated with impaired ED in STZ-induced diabetic rats. More interestingly, oxidative stress is further increased in rats with long-term diabetes which is correlated with more severe ED when compared to rats with short-term diabetes [15]. Protein kinase C (PKC) is an important molecule that regulates a variety of cellular functions such as smooth muscle contraction [16]. Hyperglycemia increases ROS production and protein expression of PKC isoforms in cultured rat cavernosal smooth muscle cells [17]. The up-regulation of PKC is dependent on ROS because exposure of these cells with the antioxidant, vitamin E, prevents this increase. Taken together, these data suggest that ROS contribute significantly to diabetes-associated ED. This conclusion is further supported by the evidence that gene transfer of extracellular-SOD reduces superoxide production and restores erectile function in STZ-induced diabetic rats [18]. Data are lacking at present to suggest that ROS production contributes to ED in type 2 diabetes.
2.3 Hypercholesterolemia and hyperlipidemia
High levels of ROS were detected in cavernosal strips from hypercholesterolemic rabbits which is correlated with impaired endothelium-dependent relaxation but not endothelium-independent relaxation [5]. Increased ROS production may increase oxidized low density lipoprotein, leading to enhanced contractility of cavernosal strips [19]. Although it is not clear whether hypercholesterolemic rabbits have impaired erectile function in vivo, these data provide some evidence to support the clinical observations that hypercholesterolemia, hyperlipidemia and subsequently developed atherosclerosis are risk factors for ED [20].
2.4 Hyperhomocysteinemia
A high plasma concentration of homocysteine is an independent risk factor for the development of cardiovascular diseases such as atherosclerosis. Homocysteine-induced endothelial injury is associated with elevated ROS production [21–23]. The adverse effects of homocysteine were demonstrated in isolated rabbit cavernosal strips. Pre-incubation of cavernosal strips isolated from normal rabbits with homocysteine decreases endothelium-dependent relaxation responses, which are reversed by SOD or catalase [24]. Similarly, cavernosal strips isolated from hyperhomocysteinemic rabbits also display impaired endothelium-dependent relaxation along with increased ROS formation [25]. Copper exaggerated the ability of homocysteine to produce ROS through the Fenton reaction. Hyperhomocysteinemic rabbits having received penicillamine, a copper-chelator, have reduced superoxide generation in penes and better endothelium-dependent relaxation [25].
2.5 Aging
The prevalence of ED is significantly increased from 5% in men aged between 20 to 39 years to more than 70% in men aged 70 years and above [26]. Strong evidence has supported the concept that ROS are the key players of the aging process. Increased ROS levels were detected in aged Brown-Norway rat penes by lucigenin-enhanced chemiluminescence and dihydroethidium (DHE) [27]. Moreover, increased oxidative stress is implicated with a decreased ratio of the reduced form of glutathione versus oxidized glutathione in aged penes [28]. It is associated with decreased SOD protein expression in penes from aged rats when compared to that of young rats [28]. In vivo adenoviral gene transfer of extracellular-SOD restores erectile responses to cavernous nerve and agonist-stimulation to the levels observed in young rats [27]. These data suggest that ROS play significant roles in the development of aging-associated ED.
2.6 Nerve injury
Nerve injury-related ED is common after radical prostatectomy. Using a unilateral cavernous nerve injury rat model, our group demonstrated that nerve injury induces oxidative stress in rat penile tissue indicated by increased nitrotyrosine staining [9]. The injury induces a rapid compensatory response in penile tissue by increasing glutathione peroxidase protein expression. However, this effect is not sustained in saline-treated nerve injured rats. Treatment with FK506, a nerve protecting agent, maintains the high levels of glutathione peroxidase expression and improves erectile function [9]. Consistently, studies from another group showed that increased oxidative stress and ED occurred within three weeks in the rats that had unilateral nerve dissection [29]. Resection of the cavernous nerve causes more severe injury, which leads to worse erectile function and greater ROS formation when compared to that of rats underwent nerve dissection [29].
3 NADPH oxidase
3.1 NADPH oxidase subunits
NADPH oxidase, a multicomponent enzyme, catalyzes the production of superoxide using an electron derived from NADPH. Superoxide is converted to hydrogen peroxide by SOD which subsequently leads to the formation of other ROS such as hydroxyl radicals.
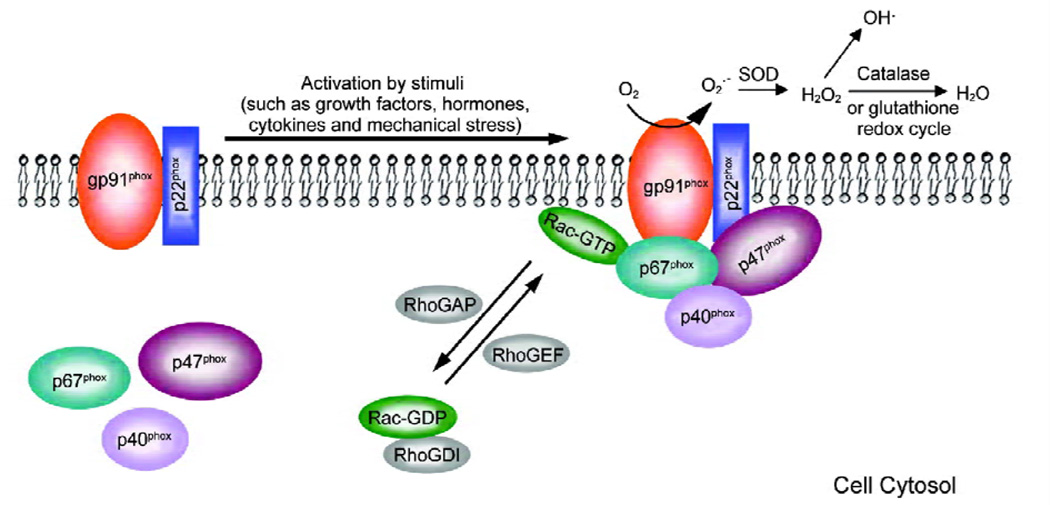
NADPH oxidase was first identified in phagocytes. It consists of the catalytic subunit gp91phox (phox, phagocyte oxidase) and regulatory subunits p22phox, p47phox, p67phox, and p40phox (Figure 1) [30, 31]. gp91phox contains a flavin adenine dinucleotide (FAD) and two heme groups and provides binding sites for NADPH and oxygen molecule. It exists together with p22phox as a stabilized membrane-bound complex called cytochrome b558 [32]. p47phox, p67phox, and p40phox are located in the cytosol in the resting state. In addition, a small GTPase protein, Rac, participates in the assembly of the NADPH oxidase complex and is required for NADPH oxidase activation, Once stimulated, the cytosolic subunits migrate to the membrane, where they assemble with gp91phox and p22phox [33]. The active oxidase generates superoxide by transferring the electron from NADPH to oxygen.
Figure 1.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation. In the resting state, p47phox, p67phox, and p40phox are located in the cytosol while p22phox and gp91phox form a cytochrome b558 complex on the membrane. Rac-GDP is stabilized by GDI in the cytosol. Following stimulation, the cytosolic subunits migrate to the membrane, where they assemble with gp91phox and p22phox. After RhoGEF facilitates the exchange of GTP for GDP, Rac-GTP binds to p67phox, helping to activate the NADPH oxidase and generate superoxide (O2·−). O2·− is catalyzed to hydrogen peroxide (H2O2) by superoxide dismutase (SOD), and H2O2 subsequently either is converted to H2O by catalase or becomes hydroxyl radicals. In addition, H2O2 is degraded via glutathione redox cycle catalyzed by glutathione peroxidase and glutathione reductase. RhoGDI, Rho GDP-dissociation inhibitor; RhoGEF, Rho guanine exchange factor; RhoGAP, Rho GTPase activating protein.
3.2 Activation of NADPH oxidase
NADPH oxidase is activated by endothelin-1, angiotensin II (Ang II), thrombin, platelet-derived growth factor, tumor necrosis factor-α (TNF-α) and cytokines through receptor-mediated mechanisms [34–37]. Mechanical stress and phorbol-12-myristate-13-acetate (PMA) can also directly activate NADPH oxidase [38–40]. Phosphorylation of p47phox on several serine residues is a crucial step to initiate the translocation of cytosolic components to the membrane and activate NADPH oxidase [41], although some data suggest that only Ser379 is essential for oxidase activity and membrane association [42]. The phosphorylation causes a conformational change of p47phox and enables the two SH3 (Src homology 3) domains of p47phox to bind to the prolinerich region on the carboxyl terminus of p22phox [43]. In addition, p47phox has a Phox-homology (PX) domain which binds to phosphoinositides on the membrane, contributing to membrane anchoring of p47phox. p47phox facilitates the recruitment of p67phox to the membrane through tail to tail interactions between its proline-rich region and the SH3 domain of p67phox. Therefore, p47phox functions as an organizer. p67phox is considered an activator because its main function is to transfer the electron from NADPH to FAD [44, 45]. This is a rate-limiting step in superoxide generation. The function of p40phox is less known and controversial. Some studies suggest that p40phox is not required for high level superoxide production or even down-regulates NADPH oxidase activity [46, 47]. In contrast, other reports indicate that p40phox enhances superoxide production through improving the efficiency of p67phox in the activation of NADPH oxidase [48, 49].
Activation of NADPH oxidase also requires a small GTP-binding protein-Rac. Rac is a member of the Rho family, and three isoforms of Rac have been identified: Rac1, Rac2 and Rac3. Rac1 and Rac3 are universally expressed except that Rac3 is not expressed in neutrophils. Rac2 is only expressed in hematopoietic cells. Rac functions as a molecular switch cycling between the GDP-bound inactive form and the GTP-bound active form. This process is tightly regulated by three proteins: GDP-dissociation inhibitor (GDI), Rho guanine exchange factor (RhoGEF), and GTPase activating protein (GAP). GDP-bound Rac is stabilized by GDI in the cytosol of the resting cells. During activation, RhoGEF facilitates the exchange of GTP for GDP, leading to the migration of Rac to the membrane along with the core cytosolic NADPH oxidase subunits. GAP inactivates Rac by increasing the intrinsic GTP hydrolytic activity of Rac. Studies have shown that p67phox has a tetratricopeptide repeat domain serving as a docking site specific for Rac [50].
3.3 Homologues of NADPH oxidase
Recently, gp91phox and its novel homologues were found in various types of cells other than phagocytes. These homologues are termed NOX (NADPH oxidase). NOX1, gp91phox (or NOX2), NOX3, NOX4 and NOX5 are expressed in smooth muscle cells, endothelial cells, fibroblasts and cancer cells [51, 52]. NOX1 through NOX4 have very similar structures. However, studies have shown that NOX4 activation requires only the presence of p22phox and not any cytosolic subunits [53]. Because NOX5 has an extra unique domain containing four binding sites for calcium, the regulation of NOX5 is dependent on intracellular calcium concentration and does not require any other subunits [54, 55].
In addition, homologues of p47phox, NOX organizer 1 (NOXO1) and p67phox, NOX activator 1 (NOXA1) were discovered in colon epithelial cells [56, 57]. Co-expression of NOXO1 and NOXA1 greatly increase the ability of NOX1 to generate superoxide.
3.4 Localization of NADPH oxidase
3.4.1 Endothelial cells
NOX2 and its regulators p22phox, p47phox, p67phox and Rac1 were all found in endothelial cells isolated from animals as well as human coronary artery and umbilical vein [58, 59]. In addition, NOX1, NOX3, and NOX4 were demonstrated in mouse lung endothelial cells [60, 61]. Recently, NOX5 variants NOX5β, NOX5δ and NOX5S were identified in human microvascular endothelial cells [62].
3.4.2 Smooth muscle cells
In addition to NOX2, p22phox, p47phox, p67phox, Rac1, and novel NOX family members were demonstrated in human and animal smooth muscle cells [63]. mRNA and protein expressions of NOX1 and NOX4 were detected in mouse lung smooth muscle cells, rat aortic smooth muscle cells and human aortic smooth muscle cells [64–66]. It is not known whether NOX3 and NOX5 are expressed in smooth muscle cells.
3.4.3 Fibroblast
NOX2, p22phox, p47phox, and p67phox were detected in rat and rabbit aortic adventitial fibroblasts [67, 68]. NOX4 and NOX5 were predominantly expressed in human cardiac fibroblasts while NOX1 and NOX2 were barely detectable [69].
4 Physiologic role of NADPH oxidase
Phagocytes generate high levels of ROS to kill bacteria as a self-defense mechanism through activation of NADPH oxidase. In non-phagocytic cells, a small amount of NADPH oxidase-derived ROS is generated at physiologic levels, functioning as second messengers in response to cellular stimuli. ROS are involved in the regulation of cell growth and proliferation, cell migration, angiogenesis and apoptosis through activation of various signaling pathways. The actions of ROS on smooth muscle cell growth and proliferation in response to growth factors and hormones are mainly mediated by mitogen-activated protein kinases (MAPK) such as ERK1/2, p38MAPK, c-Jun N-terminal kinase [70, 71]. Protein kinase B (Akt) is another downstream molecular target of ROS. Hingtgen et al. [72] reported that ROS generated by NADPH oxidase activates Akt, which play a key role in Ang II-induced cardiomyocyte hypertrophy. NADPH oxidase also participates in cytoskeletal reorganization through interaction with the orphan adaptor TRAF4 and focal contact scaffold Hic-5 in lamellipodia at the leading edge of the cells, which is essential for cell migration [73, 74]. During the process of angiogenesis, NADPH-derived ROS not only up-regulate vascular endothelial growth factor (VEGF) expression, but also mediate VEGF induced phosphatidylinositol 3-kinase (PI3K)/ERK1/2 signaling cascade via VEGF receptor-2 [61, 75, 76]. In addition, NADPH oxidase participates in the control of cell cycle arrest and apoptosis via activation of cell cycle inhibitors p21cip1 and p53 [77].
5 Pathophysiologic role of NADPH oxidase in ED
Lower levels of ROS are important in the regulation of physiologic function. However, excessive production of ROS may overwhelm the cellular antioxidant defense mechanisms, leading to pathologic changes observed in vascular diseases. ROS cause vascular damages by promoting smooth muscle cell growth and proliferation, increasing extracellular matrix protein deposition, activating matrix metalloproteinases, inducing endothelial dysfunction, and altering vascular tone. It has been well established that enhanced NADPH oxidase activity contributes to the development of atherosclerosis, hypertension, diabetes and hypercholesterolemia. The role of NADPH oxidase in ED is vastly understudied compared to other vascular diseases.
Although increased ROS production is linked to ED in different disease models, the sources of ROS have never been investigated in vivo. Some initial in vitro experiments provide indirect evidence for the possible involvement of NADPH oxidase in the development of ED. Greater superoxide production is detected in penile tissue isolated from hypercholesterolemic rabbits than that in control rabbit penes [78]. NADPH oxidase inhibitors apocynin and diphenyleneiodonium chloride (DPI) significantly reduce not only the basal production of superoxide in control rat penes but also that in hypercholesterolemic penes. Inhibitors of xanthine oxidase, NO synthase and the mitochondrial electron transport chain have no effect on superoxide formation. Apocynin and DPI also partially restore the impaired endothelium-dependent smooth muscle relaxation. These data suggest that NADPH oxidase-derived ROS are produced at low levels in normal penes and enhanced NADPH oxidase activity is responsible for ED in hypercholesterolemic rabbits. The phosphodiesterase 5 (PDE-5) inhibitor NCX 911 recovers cavernosal relaxation partly through inhibition of NADPH oxidase activity.
Another study demonstrated that the NADPH oxidase subunit p47phox is expressed in rabbit cavernosal smooth muscle cells [79]. Incubation with U46619, a thromboxane mimetic, induces ROS production along with increased p47phox protein expression. The PDE-5 inhibitor sildenafil reduces ROS in smooth cells by reducing NADPH oxidase subunit p47phox expression. Teixeira et al. [80] recently examined the role of NADPH oxidase in mouse penes. NADPH oxidase-dependent superoxide generation is significantly increased after incubating mouse cavernosal tissue with U46619 for 1 h or 8 h. This effect is reversed by the soluble guanylyl cyclase activator, BAY 41-2272, through decreasing protein expression of p22phox and gp91phox. On the other hand, NADPH oxidase-derived ROS may regulate PDE-5 protein expression. TNF-α and nicotine stimulate ROS production and increase PDE5 protein expression in rabbit cavernosal smooth cells [81]. Apocynin, SOD, catalase as well as sildenafil reduce PDE-5 expression through inhibition of ROS formation [81]. These data suggest that NO signaling inhibits NADPH oxidase activity. Conversely, ROS generated from NADPH oxidase negatively regulates the NO pathway.
Although these in vitro studies suggest that NADPH oxidase activity is increased in disease states or can be stimulated by agonists, it remains unclear whether and to what extent NADPH oxidase functions in vivo in the penis or whether it plays an important role in the pathogenesis of ED. We recently performed a series of experiments designed to investigate the role of NADPH oxidase in the development of ED in hypertension [82]. Consistent with other studies showing that hypertensive rats exhibit ED [11, 12], in our Ang II-infused hypertensive rats (4 weeks infusion of subpressor doses of Ang II), erectile function was significantly impaired. ROS generation was significantly increased in penes isolated from Ang II-infused hypertensive rats accompanied by increased NADPH oxidase subunit p47phox protein expression. Chronic treatment with apocynin reduced NADPH oxidase protein expression and ROS levels in Ang II-infused hypertensive rat penes. Correspondingly, preserved erectile function in hypertensive rats by apocynin treatment provides further evidence that elevated NADPH oxidase activity is an important mechanism for hypertension-associated ED. Furthermore, we investigated the downstream targets of ROS. We found that increased ROS formation in Ang II-infused hypertensive rat penes led to up-regulation of RhoA protein expression, a key component in the RhoA/Rho-kinase pathway of contractile mechanism in the penis. ROS also decreased endothelial NO synthase expression in hypertensive rat penes. Apocynin treatment reversed these changes to the levels similar to that of control penes (unpublished data, Jin et al.). This is the first study to our knowledge that demonstrated a relationship between NADPH oxidase-derived ROS and ED at an in vivo level.
6 Conclusion
Accumulating evidence has demonstrated the importance of NADPH oxidase-derived ROS in both physiologic and pathophysiologic processes. There is a dynamic balance between antioxidant systems and ROS generating systems within cells. Cells may be protected against the unfavorable effects caused by moderate increases in ROS production through compensatory mechanisms of up-regulated antioxidant enzyme activity. However, excessive production of ROS in response to pathogenic stimuli destroys these antioxidant mechanisms and causes cell damage. In recent years, the link between elevated ROS levels and ED has been established and therapeutic strategies have centered on restoring the antioxidant ability of cells in the penis. However, another important aspect is blockage of sources of ROS formation since ROS forms are extremely active and interchangeable. It may not be completely effective to simply scavenge one of the ROS. Therefore, it is crucial to investigate the mechanisms of ROS generation. Ours and other studies have demonstrated that NADPH oxidase represents a major source for ROS in the development of ED. Yet, further characterization of NADPH oxidase and molecular targets of ROS in penile tissue are essential to obtain a better understanding of the pathogenesis of ED and develop pharmacological and molecular interventions.
Acknowledgement
We acknowledge the support from the National Institute of Health (DK073531 to Liming Jin and DK67223 to Arthur L. Burnett) and American Heart Association (0530007N to Liming Jin).
References
- 1.Aytac IA, Mckinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 2.Jensen J, Lendorf A, Stimpel H, Frost J, Ibsen H, Rosenkilde P. The prevalence and etiology of impotence in 101 male hypertensive outpatients. Am J Hypertens. 1999;12:271–275. doi: 10.1016/s0895-7061(98)00225-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser FE, Viosca SP, Morley JE, Mooradian AD, Davis SS, Korenman SG. Impotence and aging: clinical and hormonal factors. J Am Geriatr Soc. 1988;36:511–519. doi: 10.1111/j.1532-5415.1988.tb04021.x. [DOI] [PubMed] [Google Scholar]
- 4.Keegan A, Cotter MA, Cameron NE. Effects of diabetes and treatment with the antioxidant alpha-lipoic acid on endothelial and neurogenic responses of corpus cavernosum in rats. Diabetologia. 1999;42:343–350. doi: 10.1007/s001250051161. [DOI] [PubMed] [Google Scholar]
- 5.Kim SC, Kim IK, Seo KK, Baek KJ, Lee MY. Involvement of superoxide radical in the impaired endothelium-dependent relaxation of cavernous smooth muscle in hypercholesterolemic rabbits. Urol Res. 1997;25:341–346. doi: 10.1007/BF01294663. [DOI] [PubMed] [Google Scholar]
- 6.Ushiyama M, Morita T, Kuramochi T, Yagi S, Katayama S. Erectile dysfunction in hypertensive rats results from impairment of the relaxation evoked by neurogenic carbon monoxide and nitric oxide. Hypertens Res. 2004;27:253–261. doi: 10.1291/hypres.27.253. [DOI] [PubMed] [Google Scholar]
- 7.Burnett AL, Musicki B, Jin L, Bivalacqua TJ. Nitric oxide/redox-based signalling as a therapeutic target for penile disorders. Expert Opin Ther Targets. 2006;10:445–457. doi: 10.1517/14728222.10.3.445. [DOI] [PubMed] [Google Scholar]
- 8.Dobrina A, Patriarca P. Neutrophil-endothelial cell interaction. Evidence for and mechanisms of the self-protection of bovine microvascular endothelial cells from hydrogen peroxide-induced oxidative stress. J Clin Invest. 1986;78:462–471. doi: 10.1172/JCI112598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. Fk506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4:908–916. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M. The multinational men’s attitudes to life events and sexuality (males) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607–617. doi: 10.1185/030079904125003467. [DOI] [PubMed] [Google Scholar]
- 11.Behr-Roussel D, Chamiot-Clerc P, Bernabe J, Mevel K, Alexandre L, Safar ME, et al. Erectile dysfunction in spontaneously hypertensive rats: pathophysiological mechanisms. Am J Physiol Regul Integr Comp Physiol. 2003;284:R682–R688. doi: 10.1152/ajpregu.00349.2002. [DOI] [PubMed] [Google Scholar]
- 12.Chitaley K, Webb RC, Dorrance AM, Mills TM. Decreased penile erection in doca-salt and stroke prone-spontaneously hypertensive rats. Int J Impot Res. 2001;13(Suppl 5):S16–S20. doi: 10.1038/sj.ijir.3900773. [DOI] [PubMed] [Google Scholar]
- 13.Moore CR, Wang R. Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl. 2006;8:675–684. doi: 10.1111/j.1745-7262.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 14.Morano S, Gatti A, Mandosi E, Tiberti C, Fallarino M, Cipriani R, et al. Circulating monocyte oxidative activity is increased in patients with type 2 diabetes and erectile dysfunction. J Urol. 2007;177:655–659. doi: 10.1016/j.juro.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Ryu JK, Kim DJ, Lee T, Kang YS, Yoon SM, Suh JK. The role of free radical in the pathogenesis of impotence in streptozotocin-induced diabetic rats. Yonsei Med J. 2003;44:236–241. doi: 10.3349/ymj.2003.44.2.236. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen H, Takuwa Y, Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987;1:177–185. [PubMed] [Google Scholar]
- 17.Ganz MB, Seftel A. Glucose-induced changes in protein kinase C and nitric oxide are prevented by vitamin E. Am J Physiol Endocrinol Metab. 2000;278:E146–E152. doi: 10.1152/ajpendo.2000.278.1.E146. [DOI] [PubMed] [Google Scholar]
- 18.Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, et al. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–197. doi: 10.1111/j.1743-6109.2005.20228_1.x. discussion 97–8. [DOI] [PubMed] [Google Scholar]
- 19.Ahn TY, Gomez-Coronado D, Martinez V, Cuevas P, Goldstein I, Saenz de Tejada I. Enhanced contractility of rabbit corpus cavernosum smooth muscle by oxidized low density lipoproteins. Int J Impot Res. 1999;11:9–14. doi: 10.1038/sj.ijir.3900378. [DOI] [PubMed] [Google Scholar]
- 20.Kim SC. Hyperlipidemia and erectile dysfunction. Asian J Androl. 2000;2:161–166. [PubMed] [Google Scholar]
- 21.Jin L, Caldwell RB, Li-Masters T, Caldwell RW. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J Physiol Pharmacol. 2007;58:191–206. [PubMed] [Google Scholar]
- 22.Handy DE, Zhang Y, Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (gpx1) by decreasing translation. J Biol Chem. 2005;280:15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- 23.Becker JS, Adler A, Schneeberger A, Huang H, Wang Z, Walsh E, et al. Hyperhomocysteinemia, a cardiac metabolic disease: role of nitric oxide and the p22phox subunit of NADPH oxidase. Circulation. 2005;111:2112–2118. doi: 10.1161/01.CIR.0000162506.61443.15. [DOI] [PubMed] [Google Scholar]
- 24.Khan MA, Thompson CS, Emsley AM, Mumtaz FH, Mikhailidis DP, Angelini GD, et al. The interaction of homocysteine and copper markedly inhibits the relaxation of rabbit corpus cavernosum: New risk factors for angiopathic erectile dysfunction? BJU Int. 1999;84:720–724. doi: 10.1046/j.1464-410x.1999.00253.x. [DOI] [PubMed] [Google Scholar]
- 25.Koupparis AJ, Jeremy J, Angelini G, Persad RAJ, Shukla N. Penicillamine administration reverses the inhibitory effect of hyperhomocysteinaemia on endothelium-dependent relaxation in the corpus cavernosum in the rabbit. BJU Int. 2006;98:440–444. doi: 10.1111/j.1464-410X.2006.06212.x. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Bivalacqua TJ, Armstrong JS, Biggerstaff J, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, et al. Gene transfer of extracellular sod to the penis reduces superoxide and improves erectile function in aged rats. Am J Physiol Heart Circ Physiol. 2003;284:H1408–H1421. doi: 10.1152/ajpheart.00770.2002. [DOI] [PubMed] [Google Scholar]
- 28.Ferrini MG, Davila HH, Valente EGA, Gonzalez-Cadavid NF, Rajfer J. Aging-related induction of inducible nitric oxide synthase is vasculo-protective to the arterial media. Cardiovasc Res. 2004;61:796–805. doi: 10.1016/j.cardiores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Ozkara H, Alan C, Atukeren P, Uyaner I, Demirci C, Gumustas MK, et al. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manuplation in rat penis. Chin J Physiol. 2006;49:160–166. [PubMed] [Google Scholar]
- 30.Bellavite P. The superoxide-forming enzymatic system of phagocytes. Free Radic Biol Med. 1988;4:225–261. doi: 10.1016/0891-5849(88)90044-5. [DOI] [PubMed] [Google Scholar]
- 31.Leto TL, Lomax KJ, Volpp BD, Nunoi H, Sechler JM, Nauseef WM, et al. Cloning of a 67-kd neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science. 1990;248:727–730. doi: 10.1126/science.1692159. [DOI] [PubMed] [Google Scholar]
- 32.Parkos CA, Allen RA, Cochrane CG, Jesaitis AJ. The quaternary structure of the plasma membrane b-type cytochrome of human granulocytes. Biochim Biophys Acta. 1988;932:71–83. doi: 10.1016/0005-2728(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 33.Park JW, Ma M, Ruedi JM, Smith RM, Babior BM. The cytosolic components of the respiratory burst oxidase exist as a m(r) approximately 240,000 complex that acquires a membrane-binding site during activation of the oxidase in a cell-free system. J Biol Chem. 1992;267:17327–17332. [PubMed] [Google Scholar]
- 34.Catarzi S, Biagioni C, Giannoni E, Favilli F, Marcucci T, Iantomasi T, et al. Redox regulation of platelet-derived-growth-factor-receptor: role of NADPH-oxidase and c-src tyrosine kinase. Biochim Biophys Acta. 2005;1745:166–175. doi: 10.1016/j.bbamcr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, et al. Tumor necrosis factor-{alpha} induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 36.Laplante MA, de Champlain J. The interrelation of the angiotensin and endothelin systems on the modulation of NAD(P)H oxidase. Can J Physiol Pharmacol. 2006;84:21–28. doi: 10.1139/Y05-146. [DOI] [PubMed] [Google Scholar]
- 37.Vendrov AE, Madamanchi NR, Hakim ZS, Rojas M, Runge MS. Thrombin and NAD(P)H oxidase-mediated regulation of cd44 and bmp4-id pathway in vsmc, restenosis, and atherosclerosis. Circ Res. 2006;98:1254–1263. doi: 10.1161/01.RES.0000221214.37803.79. [DOI] [PubMed] [Google Scholar]
- 38.Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41:699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- 39.Suzuma I, Murakami T, Suzuma K, Kaneto H, Watanabe D, Ojima T, et al. Cyclic stretch-induced reactive oxygen species generation enhances apoptosis in retinal pericytes through c-jun NH2-terminal kinase activation. Hypertension. 2007;49:347–354. doi: 10.1161/01.HYP.0000253535.26659.2f. [DOI] [PubMed] [Google Scholar]
- 40.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol. 2007;292:F993–F998. doi: 10.1152/ajprenal.00383.2006. [DOI] [PubMed] [Google Scholar]
- 41.Rotrosen D, Leto TL. Phosphorylation of neutrophil 47-kda cytosolic oxidase factor. Translocation to membrane is associated with distinct phosphorylation events. J Biol Chem. 1990;265:19910–19915. [PubMed] [Google Scholar]
- 42.Faust LR, el Benna J, Babior BM, Chanock SJ. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest. 1995;96:1499–1505. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, et al. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci USA. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 45.Nisimoto Y, Motalebi S, Han CH, Lambeth JD. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b(558) J Biol Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 46.Lopes LR, Dagher MC, Gutierrez A, Young B, Bouin AP, Fuchs A, et al. Phosphorylated p40phox as a negative regulator of NADPH oxidase. Biochemistry. 2004;43:3723–3730. doi: 10.1021/bi035636s. [DOI] [PubMed] [Google Scholar]
- 47.Sathyamoorthy M, de Mendez I, Adams AG, Leto TL. P40phox down-regulates NADPH oxidase activity through interactions with its SH3 domain. J Biol Chem. 1997;272:9141–9146. doi: 10.1074/jbc.272.14.9141. [DOI] [PubMed] [Google Scholar]
- 48.Tamura M, Shiozaki I, Ono S, Miyano K, Kunihiro S, Sasaki T. p40phox as an alternative organizer to p47phox in Nox2 activation: a new mechanism involving an interaction with p22phox. FEBS Lett. 2007;581:4533–4538. doi: 10.1016/j.febslet.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 49.Tsunawaki S, Kagara S, Yoshikawa K, Yoshida LS, Kuratsuji T, Namiki H. Involvement of p40phox in activation of phagocyte NADPH oxidase through association of its carboxyl-terminal, but not its amino-terminal, with p67phox. J Exp Med. 1996;184:893–902. doi: 10.1084/jem.184.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, Sumimoto H. Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J Biol Chem. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- 51.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 52.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: Cloning and tissue expression of nox3, nox4, and nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 53.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 54.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of nox1- and nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 55.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, et al. Mechanism of Ca2+ activation of the NADPH oxidase 5 (nox5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 56.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD (P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 57.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, et al. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 58.Ago T, Kitazono T, Kuroda J, Kumai Y, Kamouchi M, Ooboshi H, et al. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- 59.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of nox2 and nox4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 60.Babior BM. The NADPH oxidase of endothelial cells. IUBMB Life. 2000;50:267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- 61.Datla SR, Peshavariya H, Dusting GJ, Jiang F. Important role of nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. Epub 2007 Aug 23. [DOI] [PubMed] [Google Scholar]
- 62.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, et al. Nox5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 63.Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, et al. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Moe KT, Aulia S, Jiang F, Chua YL, Koh TH, Wong MC, et al. Differential upregulation of nox homologues of NADPH oxidase by tumor necrosis factor-alpha in human aortic smooth muscle and embryonic kidney cells. J Cell Mol Med. 2006;10:231–239. doi: 10.1111/j.1582-4934.2006.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, et al. Novel gp91phox homologues in vascular smooth muscle cells: Nox1 mediates angiotensin ii-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 66.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 67.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 68.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: Enhancement by angiotensin ii. Proc Natl Acad Sci U S A. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-{beta}1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 70.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 71.Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin ii and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22:1141–1149. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, et al. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin ii-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 73.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 74.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, et al. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/kdr. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 76.Chen JX, Zeng H, Tuo QH, Yu H, Meyrick B, Aschner JL. NADPH oxidase modulates myocardial Akt, Erk1/2 activation, and angiogenesis after hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2007;292:H1664–H1674. doi: 10.1152/ajpheart.01138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li JM, Fan LM, George VT, Brooks G. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic Biol Med. 2007;43:976–986. doi: 10.1016/j.freeradbiomed.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol. 2005;517:224–231. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 79.Koupparis AJ, Jeremy JY, Muzaffar S, Persad R, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47phox NAD[P]H oxidase induced by the thromboxane a2 mimetic, u46619, in corpus cavernosal smooth muscle cells. BJU Int. 2005;96:423–427. doi: 10.1111/j.1464-410X.2005.05643.x. [DOI] [PubMed] [Google Scholar]
- 80.Teixeira CE, Priviero FB, Webb RC. Effects of 5-cyclopropyl- 2-[1-(2-fluoro-benzyl)-1h-pyrazolo[3,4-b]pyridine-3-yl] pyrimidin-4-ylamine (bay 41-2272) on smooth muscle tone, soluble guanylyl cyclase activity, and NADPH oxidase activity/expression in corpus cavernosum from wild-type, neuronal, and endothelial nitric-oxide synthase null mice. J Pharmacol Exp Ther. 2007;322:1093–1102. doi: 10.1124/jpet.107.124594. [DOI] [PubMed] [Google Scholar]
- 81.Hotston MR, Jeremy JY, Bloor J, Koupparis A, Persad R, Shukla N. Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-alpha in cavernosal vascular smooth muscle cells: mediation by superoxide. BJU Int. 2007;99:612–618. doi: 10.1111/j.1464-410X.2006.06618.x. [DOI] [PubMed] [Google Scholar]
- 82.Jin L, Lagoda G, Leite R, Webb RC, Burnett AL. NADPH oxidase activation: A mechanism of hypertension-associated erectile dysfunction. J Sex Med. 2007 doi: 10.1111/j.1743-6109.2007.00733.x. (in press). [DOI] [PubMed] [Google Scholar]