Abstract
Suppression of root-knot nematodes is crucially important for maintaining the worldwide development of the banana industry. Growing concerns about human and environmental safety have led to the withdrawal of commonly used nematicides and soil fumigants, thus motivating the development of alternative nematode management strategies. In this study, Meloidogyne javanica was isolated, and the nematicidal effect of Camellia seed cake on this pest was investigated. The results showed that in dish experiments, Camellia seed cake extracts under low concentration (2 g/L) showed a strong nematicidal effect. After treatment for 72 h, the eggs of M. javanica were gradually dissolved, and the intestine of the juveniles gradually became indistinct. Nematicidal compounds, including saponins identified by HPLC-ESI-MS and 8 types of volatile compounds identified by GC-MS, exhibited effective nematicidal activities, especially 4-methylphenol. The pot experiments demonstrated that the application of Camellia seed cake suppressed M. javanica, and promoted the banana plant growth. This study explored an effective nematicidal agent for application in soil and revealed its potential mechanism of nematode suppression.
Introduction
Bananas (Musa spp.) are among the most important crops in the world as a staple food, and they are also the main source of income for local farmers in many developing countries [1]. However, the production of bananas is hampered by many diseases and pests [2]. Among the most damaging banana pests are the widespread plant-parasitic nematodes. In particular, the root-knot nematodes, Meloidogyne spp., are economically important soil-borne pathogens that are reported to infect almost all of the world’s major crop plants [3]. Infection with root-knot nematodes causes root damage that not only leads to severe crop losses in commercial banana plantations for export but also seriously limits the production and viability of other banana types [4]. M. javanica, one species of the root-knot nematodes, attacks banana plants at an early stage in the field [5] and induces the root cells into giant cells. Through these giant cells, the root-knot nematode sucks up nutrients and prevents the plant growth, thereby results the symptom of stunting and yellowing. Thus, it is both necessary and urgent to find a better way to suppress this harmful nematode and maintain worldwide development of the banana industry.
Nematode management is traditionally achieved by application of nematicides. However, growing concerns about human and environmental safety have led to the withdrawal of several commonly used nematicides and soil fumigants [6]. In a previous study, 55 banana accessions were evaluated for resistance to nematode species [7], but no source of resistance was found. Soil fumigation as a pre-plant treatment is reported to be effective in suppressing the nematode population in some plants [8]; however, the effect is short-lived compared to the life of a banana crop. Moreover, plant-parasitic nematodes tend to repopulate an area fairly quickly after fumigation, and some fumigants are potential environmental contaminants [9]. These findings motivate the development of alternative nematode management strategies.
Effective control strategies for M. javanica may include the use of plant-origin nematicidal agents [10]. Camellia seed cake is an organic substance made of the residual from Camellia oleifera Abel seeds after oil extraction. The saponin released from this by-product can kill muciferous mollusks [11] and could therefore be used to prevent the damage caused by root-knot nematodes. Oil cakes in combination with Bradyrhizobium sp. and Paecilomyces lilacinus have already been studied for the control of mungbean root knot nematode [12]. Tea-oil Camellia seed cake extracted with methanol or water was tested for its effects on controlling Bursaphelenchus xylophilus [13]. Because of the high nutritional value and essential elements together with mammalians safety, the tea-oil Camellia seed cake using as a non-conventional fertilizer is increasingly gaining priority [14–15]. However, to our knowledge, the nematicidal effects of Camellia seed cake and its potential mechanisms have not yet been studied. In particular, no reports have described the effects of using Camellia seed cake to amend banana-planting soil for the suppression of M. javanica.
In this study, the nematicidal ability of Camellia seed cake was investigated in detail to explore a green strategy for managing M. javanica in banana plantings. In addition, the nematicidal substance extracted from tea-oil Camellia seed cake by ultrapure water was identified using HPLC-ESI-MS, and the nematicidal ability of 8 VOCs (volatile organic compounds) detected using GC-MS were confirmed. This paper highlights how tea-oil Camellia seed cake can be used to manage M. javanica to reduce its harmful effects on banana plants and examines some of the potential mechanisms involved in the control of M. javanica by tea-oil Camellia seed cake.
Materials and Methods
Ethics statement
Our study was carried out on the farmers' land (18°23′ N, 108°44′ E) with property rights in China (1996–2035) and farmer Yusheng Li should be contacted for future permissions. No specific permits were required for the described field studies and the locations are not protected. The field studied did not involve endangered or protected species.
Isolation and identification of M. javanica
Individual seedlings of Musa AAA Cavendish cv. Brazil were taken from the field in Ledong, Hainan province, China. Tweezers were used to collect all visible suspect Meloidogyne spp. eggmasses after cleaning the roots with deionized water. Then, all the eggmasses (18 in total) were put into plates (one in each plate, numbered C1 to C18) with 5 mL water and hatched at 25°C for 72 h to obtain second-stage juveniles (J2s). Then, the J2s (10 from each plate) were taken for molecular identification via species-specific primer pairs [16]. DNA samples of J2s were prepared according to Li et al. 2008 [17]. Primers Far/Rar, Finc/Rinc, JMV1/JMV2 and Fjav/Rjav used for PCR amplification were synthesized by Invitrogen (Shanghai, China).
The J2s of other suspect Meloidogyne spp. from each plate were inoculated onto banana roots to produce disease. Banana seedlings were cultured in a seedling matrix that was sterilized at 121°C for 30 min prior to use so that no nematodes were observed in the plant roots. Pot experiments were carried out in a greenhouse located at Hainan Wan Zhong Co., Ltd., Hainan, China. The temperature and relative humidity were in accordance with the environment, and the season was suitable for banana planting. Each pot containing 500 g sterilized soil and banana roots were inoculated with 100 J2s. Banana plants grown in soil with no inoculation were treated as the control (CK).
After 6 months, disease symptoms of plants were observed, the root knots were sampled, and the nematode eggmasses and females were harvested for identification. Nematodes inside banana roots were stained using the sodium hypochlorite acid fuchsin method [18] and observed using an Olympus ZX10 stereo microscope. The selected females of Meloidogyne spp. were determined by observation of perineal patterns using an Olympus BX51 microscope (Japan, CCD DP72). Meanwhile, the J2 from one of Meloidogyne spp. in plate C11 was selected for further molecular identification. PCR products were cloned into the pMD19-T vector (TaKaRa) and transformed into competent Escherichia coli DH5α cells. The 18S rRNA gene sequence was sequenced, and the molecular phylogenetic analysis was performed using MEGA version 4.0 [17].
Nematicidal effect of Camellia seed cake extracts
Camellia seed cake was soaked in sterile ultrapure water in different concentrations (1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 50, 100 g/L) for 72 h. After filtration, 10 mL of extracts with different concentrations were transferred to 6 cm petri dishes (300 individual freshly hatched J2s of M. javanica per dish) in order to evaluate their nematicidal effects. An equal volume of deionized water was used as a control. The dishes were allowed to incubate for 36 h, and then the nematodes were observed with an Olympus ZX10 stereo microscope in order to calculate the corrected mortality. Nematodes were considered alive if they moved or appeared as a winding shape and were considered dead if they did not move when probed with a fine needle [19]. Then, the nematodes in each treatment were transferred to distilled water for 48 h to ascertain whether the dead nematodes regained mobility or not. The corrected mortality was calculated according to the following formula: mortality (%) = (mortality of treatment-mortality of CK) / (1-mortality of CK) × 100. Morphological variations of M. javanica juveniles during 72 h treatment with 5 g/L extract were observed using an Olympus BX51 microscope.
To evaluate the effects of Camellia seed cake extract on the relative juveniles hatching ratio, eggmasses contained total about 500 eggs were inoculated in dishes with 5 g/L, 2 g/L and 1 g/L extract and hatched at 25°C for 120 h. Each extract has 5 replicates. Hatching J2s were observed with an Olympus ZX10 stereo microscope. The hatching ratio was calculated at 24 h intervals as (%) = (incubation of treatment-incubation of CK) / (1-incubation of CK) × 100. Morphological variations of eggs treated with 5 g/L extract for 72 h were observed using an Olympus BX51 microscope.
Nematicidal effect of the extracted camellia saponin
The camellia saponin was extracted according to the method of Zhong et al. [20]. Briefly, 13.25 g of Camellia seed cake was ground, wrapped in a filter paper package and placed in a drying oven maintained at 70°C for 2 h to remove water. Then, extraction proceeded using 200 mL methanol (HPLC grade) in a soxhlet extractor. Reflux extraction was performed at 5 min per cycle for 4 h. The extract was then evaporated under reduced pressure using a centrifugal evaporator at room temperature. Residual organic material was re-dissolved in a total of 2 mL of methanol. The re-dissolved extract was diluted to different concentrations (6, 7, 8, 9, 10, 13, 15, 20, 30, 50, 70, 100 g/L). Effects of the extracts on the M. javanica J2s were tested as previously described.
The molecular weight of the saponin was detected by HPLC-ESI-MS (HPLC: 1200 series; ESI-MS: 6410 Triple Quad LC/MS, Agilent, USA) using a C18 column (250 mm × 4.6 mm, 5 μm) as a chromatographic column at a flow-rate of 0.5 mL/min. The column temperature was 20°C, injection volume was 10 μL and wave length for all detections was 280 nm. Mobile phase A was water with 0.1% acetic acid, and mobile phase B was acetonitrile. An elution gradient from 20% to 15% mobile phase A volume fraction, over a gradient time of 0 to 20 min, was used. MS analysis was performed by electrospray ionization in the positive ion mode.
Nematicidal effect of the volatile compounds of Camellia seed cake
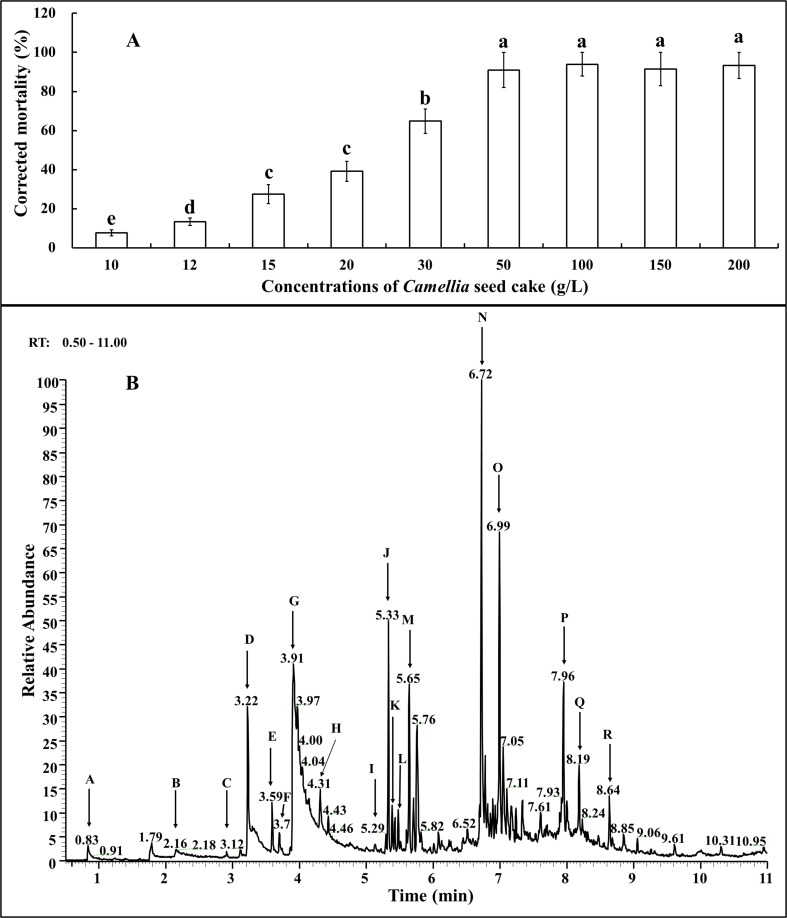
The split plates (Petri dish with vents, Greiner company, Germany) were used to test the nematicidal activity of the volatiles produced by the Camellia seed cake. One side of the split plate was tiled with 5 mL sterile ultrapure water with different concentrations of Camellia seed cake (10, 12, 15, 20, 30, 50, 100, 150, 200 g/L), and the other side held 5 mL of ultrapure water with 300 individuals of freshly hatched juveniles of M. javanica (S1 Fig.). An equal volume of ultrapure water was used as a control. Corrected mortality was determined after the plates stood for 72 h.
The volatiles produced by the Camellia seed cake extract were collected using the solid-phase micro-extraction (SPME) technique [21], which detects volatiles produced at the headspace of the serum bottle. The SPME syringe, equipped with fiber material (50/30 DVB/Carboxen on PDMS stable flex fiber) [22], was then inserted into the center of the parafilm covering the serum bottle. Fiber material was exposed to the volatiles in the headspace to entrap the volatiles. Three days later, the syringe containing the volatiles was inserted into a Gas Chromatography-Mass Spectrometer (GC-MS). GC-MS analysis was performed in electron ionization (EI) mode (70 eV) using a Finnigan gas chromatograph equipped with an MS detector. A Finnigan capillary column (15 m length × 0.5 mm id × 0.25 mm film thickness) was first used, with the following temperature program: the temperature was held at 35°C for 3 min, then increased to 180°C at 10°C/min; this temperature was held for 1 min, and finally increased to 240°C at 40°C min-1 and held at 240°C for 10 min. Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. The samples were analyzed in split mode (1: 20) with an injection and EI source temperature of 220°C and then scanned in the mass range from 30 m/z to 650 m/z. The compounds produced by Camellia seed cake were identified using the NIST (National Institute of Standards and Technology) database on the mass spectrometer.
The nematicidal activity of the various VOCs identified by GC-MS was examined using pure standard substances and split plates. All the selected substances were dissolved with ethanol and later diluted with the sterile ultrapure water. The concentration of diluted solvents was 3000, 300, 30 and 3 mg/L, respectively. Nematicidal activity was tested by placing sterile filter paper discs with 100 μL of diluted solvent in one side of the split plates and 5 mL water with 300 freshly hatched J2s was in another side. The 100 μL of water diluted ethanol was as the control. The corrected mortality of each treatment was determined after standing for 72 h.
Pot experiments
Pot experiments were performed in a greenhouse located at Hainan Wan Zhong Co., Ltd., Hainan, China, during three banana growing seasons from May to July 2012, September to October 2012 and May to July 2013. Banana seedlings (Musa AAA Cavendish cv. Brazil) without nematodes in their roots were used in this experiment. The soil for the pot experiments was collected from a field with serious root-knot nematode-wilt disease in Hainan province, China. The soil had a pH value of 6.52, an organic matter content of 10.12 g/kg, and available N, P, K contents of 38.39, 202.19, and 174.22 mg/kg, respectively.
In each season of pot experiments, four treatments were designed as follows. In the control, CK, no Camellia seed cake was added to the soil. In treatment A, 5 g/kg of Camellia seed cake was added to the soil. In treatment B, 2 g/kg of Camellia seed cake was added to the soil. In treatment C, 1 g/kg of Camellia seed cake was added to the soil. Each treatment was supplemented with identical nutrient content. Each treatment had three blocks; each block contained nine pots.
In the first-season of pot experiments, the soil for all pots (3 kg soil for each pot) was first sterilized. Then, juveniles of M. javanica were added to each pot (600 individuals per 1 kg soil). Then, Camellia seed cake was added to the pot. Seven days later, one banana seedling free of nematodes was transplanted to the pot. All pots were kept in greenhouse under room temperature for 60 days and the seedlings were removed for the measurement of agronomic characteristics and biomasses. The number of eggmasses on roots was also calculated.
In the second- and third-season of pot experiments, the crude soil with nematodes was put into pot and Camellia seed cake was added. Again seven days later, one banana seedling free of nematodes was transplanted to the pot. The conditions for keeping seedlings were the same as described above. Sixty days later, the seedlings were removed from the pots and the agronomic characteristics and biomasses were measured. The roots of each seedling were macerated in a blender and root-knot nematodes were extracted by the Baermann funnel and calculated using an Olympus ZX10 stereo microscope. The total nematodes in soil of each pot were extracted by the Baermann funnel and calculated. The nematodes species were determined using morphological and feeding-habit based classifications. The number of culturable microorganisms including bacteria, fungi and actinomycetes in soil of each pot was determined [23].
Statistical analysis
Differences among treatments were assessed using a one-way ANOVA analysis. All means were calculated from the values of five replicates and subjected to Duncan’s multiple range tests at P = 0.05 and the statistical analysis was carried out using software SPSS version 17.0 (SPSS Inc., Chicago, IL). Different letters indicated in figures means the statistically significant differences at the 0.05 probability level according to the Duncan test.
Results
Isolation and identification of M. javanica
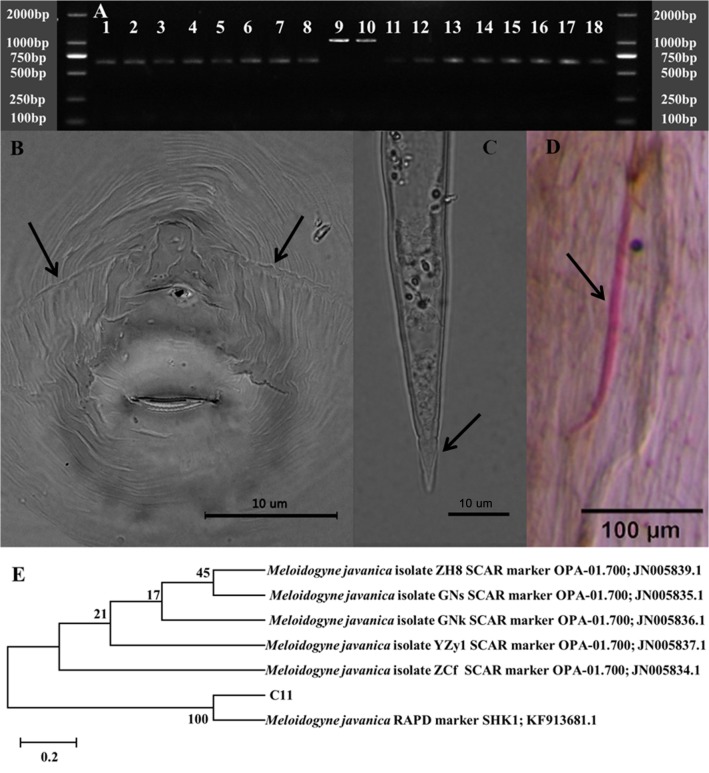
To isolate M. javanica, 18 individual suspect eggmasses with or without females were first isolated and numbered C1 to C18. All the eggmasses were then hatched into Meloidogyne spp. J2s for molecular identification. No PCR products were amplified from all DNA templates using the primers Far/Rar and JMV1/JMV2 (data no shown). Two bands at approximately 1200 bp were observed using the primer Finc/Rinc for C9 and C10; these nematodes were therefore preliminarily identified as M. incognita [16]. Approximately 670 bp band was detected in the rest of other J2s using the primer Fjav/Rjav (Fig. 1A); these nematodes were therefore preliminarily identified as M. javanica [16], which confirmed to be the dominant species on banana.
Fig 1. Molecular identification of M. javanica detected by primers Fjav/Rjav and M. incognita by Finc/Rinc (A); the female perineal pattern of M. javanica showing two obvious lateral lines (B); the hyaline tail observed in J2 of M. javanica (C); the infection of J2s from C11 of M. javanica in banana roots (D); phylogenetic tree of the sequence of nematode C11 (E).
Bars: B, C 10 μm; D 100 μm.
After identification, all the hatched juveniles of M. javanica were inoculated into the banana plants for disease production. Six months after transplanting, the plants were dwarfed and the leaves were pale, and significant root knots were observed in the treatment plants. The root knots were sampled, and white females were harvested. Two obvious lateral lines were observed in the perineal patterns (Fig. 1B) of the females, and the hyaline tail of juvenile was shown in Fig. 1C. The disease symptoms increased with increasing numbers of inoculated juveniles, and no root knots were observed in the control. The infection of C11 juvenile to banana root is shown in Fig. 1D. Due to C11 has the highest activities for juveniles hatching and root infection than others, therefore, it was further selected for molecular identification. An approximately 670 bp band from primer Fjav/Rjav was amplified and sequenced. The phylogenetic tree of the sequence showed that nematode C11 was related to the M. javanica lineage and closely clustered with similar species (Fig. 1E). Based on the above phenotypic characteristics and phylogenetic analysis, nematode C11 was confirmed to be M. javanica. The combination of these results verified that the isolated M. javanica C11 is from the previous inoculation; and was selected for further study.
The nematicidal activity of the Camellia seed cake extracts
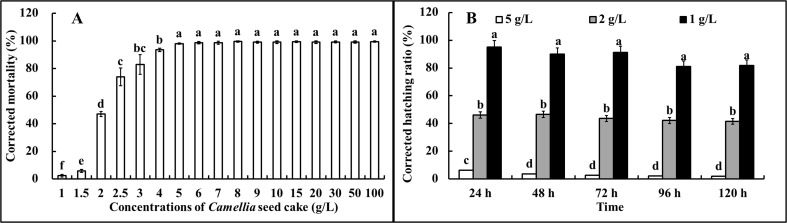
A juvenile mortality of approximately 99% was observed for 100 g/L to 5 g/L of Camellia seed cake extracts. The rate showed a continual decline from 5 g/L to 1 g/L and tended to be lowest for 1 g/L, at approximately 2.4%. Moreover, the nematicidal rate was 57.11% for 2 g/L of Camellia seed cake extract (Fig. 2A). Therefore, concentrations of 5 g/L, 2 g/L and 1 g/L were selected for further study.
Fig 2. Effects of different concentrations of Camellia seed cake extract on the corrected mortality of M. javanica J2s (A); and the corrected hatching ratio of eggs (B).
Approximately no juveniles were hatched from 0 to 72 h at 5 g/L. At 2 g/L, the hatching ratio was nearly 50% from 24 to 120 h, and at 1 g/L, no inhibition effect was observed compared to the control (Fig. 2B).
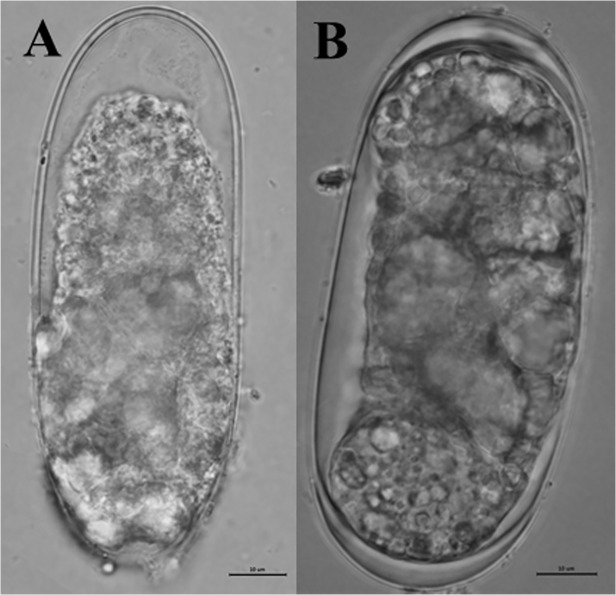
The juvenile had a smooth cuticle and brighter content at 0 h, while the cuticle was crimpled and the intestine was obviously dissolved at 72 h (Fig. 3A). No apparent changes were observed on the anterior part of the body (Fig. 3B) and tail region (Fig. 3C) of J2s. However, the junction region of esophagus (Fig. 3D) and intestine part (Fig. 3E) of J2s was gradually became destroyed as the treatment time increasing. No obvious morphological changes were observed on J2s in water control. The eggs treated with the extracts for 72 h showed the content apparently dissolved and the eggshell layers obviously reduced (Fig. 4A), however, the eggs in water control had a smooth and bright surface and a clear content (Fig. 4B).
Fig 3. Morphological variations of M. javanica J2s (the whole body (A), anterior part of the body (B), tail region (C), junction region of esophagus and intestine (D) and intestine (E)) after treatment with 5 g/L Camellia seed cake extract for different times.
Bars: 10 μm.
Fig 4. Morphological variations of M. javanica eggs after treatment with 5 g/L Camellia seed cake extract.
The egg treated with 5 g/L extract at 72 h (A) and the egg treated with water control (B). Bars: 10 μm.
Nematicidal effect of camellia saponin and its molecular weight
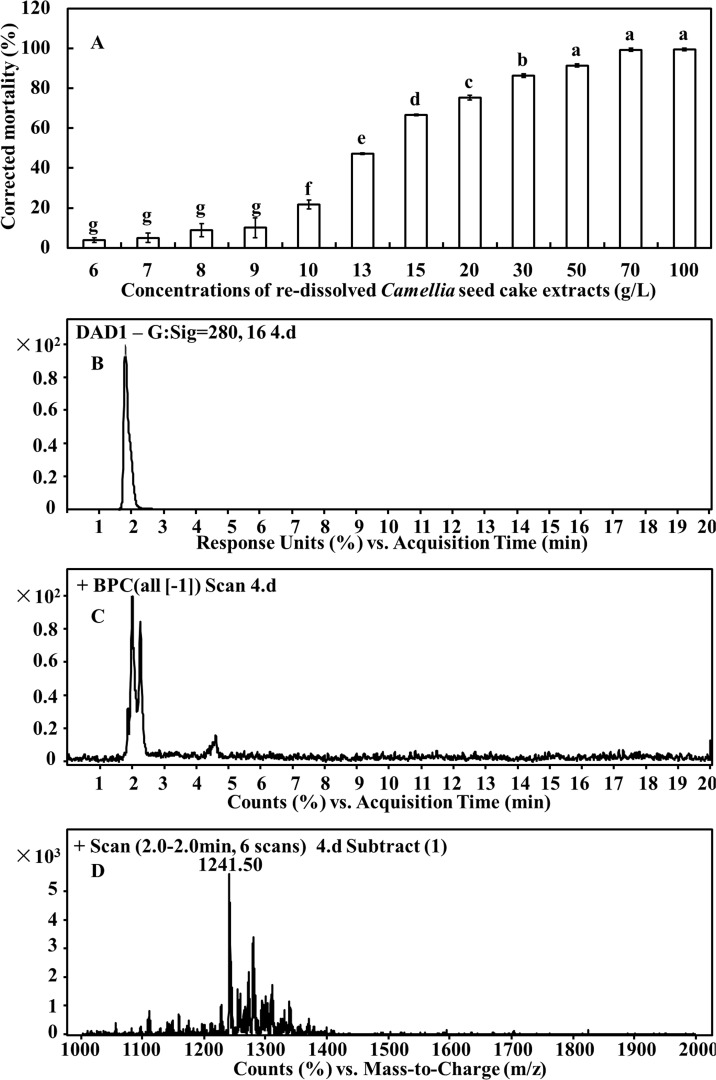
In Fig. 5A, nearly 100% nematicidal rates were observed from 100 g/L to 50 g/L. From 50 g/L to 6 g/L, the nematicidal rate showed a continual decline to approximately 3.88%.
Fig 5. The effects of the re-dissolved extracts on the number of M. javanica J2s (A) and saponin detected by HPLC-MS (include HPLC profile (B), total ion chromatogram (C) and ESI-MS identification (D)).
DAD: Diode-Array Detector; BPC: Base Peak Chromatogram.
One chromatographic peak with retention time of 2.0 min was obtained after HPLC analysis (Fig. 5B). Mass spectrometry analysis detected three major compounds [Rt (min) = 1.85, 2.0, 2.22] in the total ion chromatogram of the mixture ions peak (Fig. 5C). The first and third compound has the same molecular mass as 1001.6 Da [M+H]+, however, no evidences were found to support them as saponin homologues (S2 and S3 Figs.). The second compound has a molecular mass of 1241.50 Da [M+H]+ (Fig. 5D) in the positive-ion mode, showing that the molecular weight of the compound was 1240.50, which was conjectured as saponin homologues [24–26].
Nematicidal activity of the volatile compounds in the plate
The effects of VOCs from Camellia seed cake extract on the juvenile population of M. javanica are presented in Fig. 6A. Nearly all the nematodes were killed from 50 g/L to 200 g/L. From 50 g/L to 10 g/L, the nematicidal rate showed a continual decline to approximately 7.56%. In addition, the nematicidal rate was nearly 50% at 20 g/L and 7.56% at 10 g/L (Fig. 6A).
Fig 6. Effects of volatile components (VOCs) from Camellia seed cake extract on the M. javanica J2s (A) and the GC profiles for VOCs produced by the Camellia seed cake (B).
The compounds corresponding to the retention times 0.83, 2.16, 3.12, 3.22, 3.59, 3.7, 3.91, 4.31, 5.29, 5.33, 5.39, 5.48, 5.65, 6.72, 6.99, 7.96, 8.19, 8.64 were designated as products A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P, Q and R, respectively.
VOCs were further identified by GC-MS. The retention times of the products are summarized in Table 1. According to the NIST library, products A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P, Q and R shown in Fig. 6B were identified as 1-butanol, 2-methyl-butanoic acid, 1-octen-3-ol, Butylesterbutanoic acid, 2-methylbutanebutyl, 3,3-dimethyloctane, 4-methylphenol, 2-methyl-2-butenoate butyl, 2-bromo dodecane, 2,7,10-trimelthyldodecane, Bcosane, 2,6,10,15-tetramethylheptadecane, 2,6,11-trimethyldodecane, Heptacosane, 2-methyltetradecane, 2,6,10,15-tetramethylheptadecane, 2-methylnanadecane and 3,7,11,15-tetramethyl-2-hexadecen-1-ol, respectively (Table 1). We acquired 8 of the 18 pure compounds; the other 10 were not available for purchase. The effects of the 8 pure compounds on the juveniles of M. javanica are shown in Table 2. The highest efficiency was observed for 4-methylphenol, which yielded a corrected mortality of 9.50% with an added amount of 3 mg/L.
Table 1. Compounds from Camellia seed cake identified by GC-MS.
| Metabolite | Rt(min) | Chemical name |
|---|---|---|
| A | 0.83 | 1-butanol |
| B | 2.16 | 2-methyl-butanoic acid |
| C | 3.12 | 1-octen-3-ol |
| D | 3.22 | Butylester butanoic acid |
| E | 3.59 | 2-methylbutanebutyl |
| F | 3.7 | 3,3-dimethyloctane |
| G | 3.91 | 4-methylphenol |
| H | 4.31 | 2-methyl-2-butenoate butyl |
| I | 5.29 | 2-bromo dodecane |
| J | 5.33 | 2,7,10-trimelthyldodecane |
| K | 5.39 | Bcosane |
| L | 5.48 | 2,6,10,15-tetramethylheptadecane |
| M | 5.65 | 2,6,11-trimethyldodecane |
| N | 6.72 | Heptacosane |
| O | 6.99 | 2-methyltetradecane |
| P | 7.96 | 2,6,10,15-tetramethylheptadecane |
| Q | 8.19 | 2-methylnanadecane |
| R | 8.64 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol |
Table 2. Nematicidal compounds from Camellia seed cake identified by GC-MS.
| Metabolite | Rt(min) | Chemical name | 3000 mg/L | 300 mg/L | 30 mg/L | 3 mg/L |
|---|---|---|---|---|---|---|
| A | 0.83 | 1-butanol | 24.26±0.37g | 21.50±0.64g | 7.18±1.31g | 0g |
| B | 2.16 | 2-methyl-butanoic acid | 39.39±0.87e | 33.97±0.91d | 36.07±5.56c | 4.10±0.32b |
| C | 3.12 | 1-octen-3-ol | 100a | 45.58±0.18c | 40.75±1.07b | 4.08±0.11b |
| D | 3.22 | Butylester butanoic acid | 64.58±2.95b | 44.64±0.74c | 26.01±1.43d | 2.54±0.06de |
| E | 3.59 | 2-methylbutanebutyl | 39.39±0.87e | 33.97±1.29d | 26.28±0.42d | 2.65±0.05d |
| F | 3.7 | 3,3-dimethyloctane | 66.55±0.61b | 52.44±1.43b | 32.21±0.82c | 3.15±0.07c |
| G | 3.91 | 4-methylphenol | 100a | 100a | 100a | 9.50±0.23a |
| H | 5.29 | 2-bromo dodecane | 33.99±2.70f | 23.88±1.14f | 22.08±1.43de | 2.26±0.03e |
All values are the mean of five replicates. Numbers following “±” represent the standard errors (SE). Different letters in the same column indicate statistically significant differences at the 0.05 probability level according to the Duncan test.
Pot experiments
For the first-season, sixty days after transplanting the banana seedlings, the outcomes of each treatment were compared to the control (CK). Treatment A showed the significant differences from CK by increasing the plant height, stem diameter, and the fresh weights of shoots and roots with 51.74%, 31.72%, 36.77% and 18.37%, respectively (Table 3). Treatment A also had the significant differences from CK by decreasing the eggmasses per plant and the eggmasses per gram root with 42.57% and 82.07%, respectively, compared with CK. Treatment B showed the significant differences from CK by increasing the plant height and stem diameter with 27.64% and 24.27%, respectively and decreasing the eggmasses per plant and the eggmasses per gram root with 31.08% and 57.30%, respectively. No significant differences were observed for other growth parameters in Treatment B. Treatment C only increased the stem diameter by 17.19% and decreased the eggmasses per plant by 33.27%. These results show that the application of 5 g/kg of Camellia seed cake to the soil effectively promoted banana growth and inhibited M. javanica infection.
Table 3. Effects of application of Camellia seed cake on biomass and egg masses densities of banana plants 60 days after transplantation in the pot experiments (first-season).
| Treatment | Plant height (cm) | Stem diameter (mm) | Fresh weight of shoots (g) | Fresh weight of roots (g) | Eggmasses per plant | Eggmasses per gram root |
|---|---|---|---|---|---|---|
| CK | 18.67±1.53c | 25.13±0.26d | 158.95±11.95b | 113.42±5.50bc | 197.33±13.05a | 147.61±36.94a |
| A | 28.33±1.53a | 33.1±0.2a | 217.47±12.90a | 134.25±10.46a | 113.33±26.31b | 26.46±4.34c |
| B | 23.83±3.40ab | 31.23±0.15b | 161.17±10.92b | 122.32±4.75b | 136±9.85b | 63.03±21.44bc |
| C | 20.4±3.44c | 29.45±0.39c | 160.82±13.56b | 109.24±6.32c | 131.67±7.02b | 111.29±28.06ab |
All values are the mean of five replicates. Numbers following “±” represent the standard errors (SE). Different letters in the same column indicate statistically significant differences at the 0.05 probability level according to the Duncan test.
For the second-season, sixty days after transplanting the banana seedlings, the outcomes of each treatment were compared to the control (CK). Treatment A showed the significant differences from CK by increasing the plant height, stem diameter, the fresh and dry weights of shoots with 26.17%, 20.71%, 52.59% and 66.35%, respectively. Treatment A also had significant differences from CK by increasing the nematode density in soil with 60.15% and decreasing the nematode density in the roots with 28.23%. Treatment B increased the plant height, stem diameter and the dry weight of shoots by 13.73%, 16.79% and 15.48%, respectively. Treatment B also increased the nematode density in soil by 14.18% and decreased the nematode density in roots by 14.29%. Treatment C increased the stem diameter and the dry weight of shoots by 12.49% and 18.93%, respectively, and decreased the nematode density in roots by 9.52% (Table 4). In addition, after identifying the nematodes in the soil using morphology and feeding-habit based classification, the numbers of plant parasites in treatments A and B were significantly lower than in the control, while the numbers of fungivores and bacterivores were greater in treatments A and B than in the control (S1 Table). For the culturable microbes, the numbers of bacteria, fungi and actinomycetes in the three treatments were all significantly higher than in the control (S2 Table).
Table 4. Effects of application of Camellia seed cake on biomass and nematode densities of banana plants 60 days after transplantation in the pot experiments (second-season).
| Treatment | Plant height (cm) | Stem diameter (mm) | Fresh weight of shoots (g) | Dry weight of shoots (g) | Fresh weight of roots (g) | Nematode density in roots (individuals 10 g−1 root biomass) | Nematode density in soil (individuals 100 g−1 dry soil) |
|---|---|---|---|---|---|---|---|
| CK | 20.9±1.55c | 28.83±0.75d | 160.71±20.69b | 14.74±0.42c | 118.91±14.12a | 980±65.57a | 870±52.92c |
| A | 26.37±1.02a | 34.80±0.36a | 245.22±4.92a | 24.52±0.49a | 145.04±29.90a | 703.33±65.06c | 1393.33±30.55a |
| B | 23.77±1.19b | 33.67±0.45b | 185.10±12.67b | 18.51±1.27b | 137.32±19.10a | 840±26.46b | 993.33±75.72b |
| C | 21.5±0.61c | 32.43±0.12c | 174.27±5.78b | 17.53±0.61b | 117.41±18.15a | 886.67±20.82b | 923.33±41.63bc |
All values are the mean of five replicates. Numbers following “±” represent the standard errors (SE). Different letters in the same column indicate statistically significant differences at the 0.05 probability level according to the Duncan test.
For the third-season, similar results for the growth parameters were observed as for the second-season. When compared with the control (CK), treatment A decreased the nematode density in roots by 31.84%. Moreover, treatment A increased the nematode density in soil by 73.30%, and treatment B by 21.87% (Table 5).
Table 5. Effects of application of Camellia seed cake on biomass and nematode densities of banana plants 60 days after transplantation in the pot experiments (third-season).
| Treatment | Plant height (cm) | Stem diameter (mm) | Fresh weight of shoots (g) | Dry weight of shoots (g) | Fresh weight of roots (g) | Nematode density in roots (individuals 10 g−1 root biomass) | Nematode density in soil (individuals 100 g−1 dry soil) |
|---|---|---|---|---|---|---|---|
| CK | 20.53±0.49c | 27.53±0.7d | 158.76±21.63b | 14.9±0.24c | 115.79±13.51a | 975.67±48.02a | 831.83±46.47c |
| A | 27.77±0.45a | 34.6±1.63a | 240.18±14.33a | 25.01±0.45a | 135.67±13.83a | 665±36.26c | 1441.6±56.26a |
| B | 24.87±1.24b | 32.27±0.50b | 175.27±6.50b | 17.80±0.92b | 127.58±13.87a | 820±43.44b | 1013.8±64.50b |
| C | 20.80±0.72c | 29.73±0.55c | 163.28±8.31b | 17.36±0.57b | 118.83±18.14a | 871±22.61b | 918.62±43.38bc |
All values are the mean of five replicates. Numbers following “±” represent the standard errors (SE). Different letters in the same column indicate statistically significant differences at the 0.05 probability level according to the Duncan test.
Discussion
Root knot nematodes on bananas seriously damage its production and induce the complex diseases caused by the other plant pathogens, therefore exploring a high efficient nematicide with environmental security is urgently needed. In this study, the effects of Camellia seed cake on controlling M. javanica were evaluated and its possible mechanisms for nematicidal activities were elucidated. M. javanica has already been reported to be the dominant banana pathogen in Pakistan [27]. It has also been reported to be the dominant pathogen for almost all of the world’s major crop plants, including papaya, potato, peanut, tomato, and rootstocks [28–31]. The populations isolated from banana roots in Hainan Province, China were mainly identified as M. javanica based on morphological and molecular characteristics, which was confirmed to be the dominant species of banana root-knot nematodes in our research.
Camellia seed cake acts as a non-conventional fertilizer and has been reported to effectively suppress plant-parasitic nematodes [32]. In this study, more than 50% of M. javanica J2s were killed at Camellia seed cake extract concentrations above 2 g/L, and at these concentrations, half of the M. javanica eggs could not be hatched. The results proved the potential nematicidal activity of Camellia seed cake, which is similar to those of Wang et al. [33], who reported that Camellia plant extracts had strong nematicidal activity on B. xylophilus and M. incognita. After treatment with Camellia seed cake extracts, the eggs of M. javanica were gradually dissolved, and the intestine of the juveniles gradually became indistinct in our observation. Similar symptoms have been reported that J2s and eggs of M. incognita were damaged after treatment with Bacillus cereus X5 [34] or 2, 4-diacetylphloroglucinol, the secondary metabolite of Pseudomonas fluorescens CHA0 [35]. These in vitro tests indicated the eggs and J2s of M. javanica could be controlled by the Camellia seed cake extract.
Several papers have reported that Camellia seed cake contained 15% camellia saponin. Therefore, the nematicidal effect and molecular weight of camellia saponin were investigated in this study. Saponins are steroid or triterpenoid glycosides, which are commonly observed in many plants [36]. Saponins from Pulsatilla koreana root or from Medicago sativa could control plant-parasitic nematodes [37]. Our results also confirmed that camellia saponin possesses a distinct nematicidal effect. After detection by HPLC-ESI-MS, a compound with a molecular weight of 1240.50 Da was observed. In previous reports, the molecular weight of triterpene saponin from Lysimachia capillipes is 1240.61 Da [24], from the roots of Panax notoginseng, is 1240.65 Da [25–26]. Therefore, we propose that compound detected in this study was confirmed as saponin.
After soaking, volatile gases produced by Camellia seed cake were observed to kill most of the root-knot nematodes. Therefore, the volatile gases produced by Camellia seed cake were collected using headspace-SPME and identified by GC-MS. The headspace-SPME represents an excellent, solventless analysis technique that has been applied to identify VOCs, e.g., in blood, viscera samples, urine, and food [38–40]. Through GC-MS, 18 compounds were identified, of which 8 standard compounds were reported for the first time to show nematicidal activities. Among them, 4-methylphenol showed the best efficiency. Our results are similar to those of Li et al. [41], who reported that the volatile compound methyl thiobutyrate has inhibiting effects on Caenorhabditis elegans and M. incognita, and can be further applied to prepare a vermifuge from its precursor substance. Most likely, the nematicidal effect of Camellia seed cake is due to the production of both volatile and non-volatile inhibitory compounds.
In our three seasons of pot experiments using sterilized or crude soils, the application of Camellia seed cake significantly suppressed M. javanica and promoted plant growth. We speculate that the growth promotion may be attributable to the suppression of the harmful nematode M. javanica and the nutrient content of the Camellia seed cake. Our results agree with previous reports of effective suppression of harmful nematodes by nematicidal agents from plant sources in pot and field experiments. Four medicinal plants, Azadirachta indica, Calotropis procera, Datura stramonium and Tagetes erecta had nematicidal effects against M. incognita [42], while the aqueous and ethanol extracts from leaves, stem, bark and fruit of Eucalyptus sp., against M. javanica [43]. The extracts of Eucalyptus sp not only reduced egg hatching and increased J2s mortality as exposure time increased, but also significantly increased the shoot length, shoot weight, root length and root weight [43], which showed the similar nematicidal activities and plant promotion as that of the Camellia seed cake.
After morphological observation at the end of the second-season pot experiment, the number of plant parasites in the Camellia seed cake treatments was lower than in the control, while the density of total nematodes and other functional groups showed the opposite tendency. This result may be caused by the fact that Camellia seed cake application increased the number of bacteria, fungi and actinomycetes, which in turn increased the number of total nematodes, especially bacterivores, fungivores and omnivores. Our results are similar to those of González [44], who showed that situ ingestion rates of fluorescently labeled bacteria could estimate the active bacterivores in natural aquatic systems. Remén [45] showed that ectomycorrhizal fungi constituted an important food source for fungivorous soil fauna and may be a factor regulating these faunal communities. Moreover, promotion of plant growth by bacterial-feeding nematodes has been previously reported [46–47]. The effects of bacterial-feeding nematodes may be another explanation of the growth-promotion effect observed in the pot experiments. Therefore, we proposed that the alteration of nematode populations and different functional groups is a new mechanism for promoting plant growth by the application of Camellia seed cake. However, further studies need to be provided to support this hypothesis.
Conclusions
The harmful root-knot nematode M. javanica was identified in this study and confirmed to be the dominant species on banana. The Camellia seed cake extracts effectively killed J2s of M. javanica and suppressed the hatching rate of eggs. Nematicidal compounds produced by Camellia seed cake, including saponins and VOCs, effectively killed J2s. In pot experiments of banana seedlings, application of Camellia seed cake not only suppressed M. javanica but also promoted plant growth. The direct biocontrol efficiency of Camellia seed cake in field experiments requires further study.
Supporting Information
(TIF)
(TIF)
(TIF)
(DOC)
(DOC)
Acknowledgments
We gratefully acknowledge Dr. Manqiang Liu of Nanjing Agricultural University for excellent assistance in nematode community analysis and thank Hainan Wanzhong Co., Ltd for huge help to us in banana planting.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Key Basic Research Program of China (2015CB150506), the National Natural Science Foundation of China (31372142), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), 111 project (B12009), the National Key Technology R&D Program of the Ministry of Science and Technology (2011BAD11B03), and the Innovative Research Team Development Plan of the Ministry of Education of China (IRT1256). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang B, Yuan J, Zhang J, Shen Z, Zhang M, Li R, et al. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol Fertil Soils. 2013;49: 435–446. [Google Scholar]
- 2. Jaizme-Vega MC, Tenoury P, Pinochet J, Jaumot M. Interactions between the root-knot nematode Meloidogyne incognita and Glomus mosseae in banana. Plant Soil. 1997;196: 27–35. [Google Scholar]
- 3. Oka Y, Koltai H, Bar-Eyal M, Mor M, Sharon E, Chet I, et al. New strategies for the control of plant-parasitic nematodes. Pest Manag Sci. 2000;56: 983–88. [Google Scholar]
- 4. Gowen S, Quénéhervé P. Nematode parasites of bananas, plantains and abaca In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, ed. Luc M, Sikora RA and Bridge J. CAB International, Institute of Parasitology, Wallingford, UK; 1990. Pp. 431–460. [Google Scholar]
- 5. Sikora RA, Schlosser E. Nematodes and fungi associated with root systems of bananas in a state of decline in Lebanon. Plant Dis Rep. 1973;57 (7): 615–618. [Google Scholar]
- 6. Oka Y, Shuker S, Tkachi N. Influence of soil environments on nematicidal activity of fluensulfone against Meloidogyne javanica . Pest Manag Sci. 2013;69: 1225–1234. 10.1002/ps.3487 [DOI] [PubMed] [Google Scholar]
- 7. Quénéhervé P, Valette C, Topart P, Montcel HT, Salmon F. Nematode resistance in bananas: screening results on some wild and cultivated accessions of Musa spp. Euphytica. 2009;165: 123–136. [Google Scholar]
- 8. Mogtahedi H, Santo GS, Hang AN, Wilson JH. Suppression of root-knot nematode populations with selected rapeseed cultivars as green manure. J Nematol. 1991;23 (2): 170–174. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang KH, Hooks CRR. Survey of nematodes on banana in Hawai’i, and methods used for their control. Plant Disease. 2009;PD-69.
- 10. Chitwood DJ. Phytochemical based strategies for nematode control. Annu Rev Phytopathol. 2002;40: 221–249. [DOI] [PubMed] [Google Scholar]
- 11. Chen RS, Wang KL, Wu CY. Assessment of the camellia seed meal impact on loaches in paddy fields. Paddy Water Environ. 2012;10: 291–300. [Google Scholar]
- 12. Mansoor F, Sultana V, Ehteshamul-Haque S. Enhancement of biocontrol potential of Pseudomonas aeruginose and Paecilomyces lilacinus against root knot of mungbean by a medicinal plant Launaea nudicalis L. Pak J Bot. 2007;39(6), 2113–2119. [Google Scholar]
- 13. Long J, Cao F, Peng J. Effect of plant extracts on nematicidal activity of Bursaphelenchus xylophilus. North China Horticulture. 2010;22: 148–150. [Google Scholar]
- 14. Njuguna DG, Wanyoko JK, Kinyanjui T, Wachira FN. Mineral elements in the Kenyan tea seed oil cake. Int J Res Chem Environ. 2013;3 (1): 253–261. [Google Scholar]
- 15. Lee CP, Yen GC. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) Oil. J Agric Food Chem. 2006;54 (3): 779–784. [DOI] [PubMed] [Google Scholar]
- 16. Adam MAM, Phillips MS, Blok VC. Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.). Plant Pathol. 2007;56: 190–197. [Google Scholar]
- 17. Li H, Trinh PQ, Waeyenberge L, Moens M. Bursaphelenchus chengi sp. n. (Nematoda: Parasitaphelenchidae) isolated at Nanjing, China, in packaging wood from Taiwan. Nematology. 2008;10 (3): 335–346. [Google Scholar]
- 18. Bybd DW, Kirkpatrick T, Barker KR. An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol. 1983;15 (1): 142–143. [PMC free article] [PubMed] [Google Scholar]
- 19. El-Rokiek KG, El-Nagdi WM. Dual effects of leaf extracts of Eucalyptus citriodora on controlling purslane and root-knot nematode in sunflower. J Plant Prot Res. 2011;51: 121–129. [Google Scholar]
- 20. Zhong S, He G, Zhang C, Hua H, Yan Q. RP-HPLC determination of tea saponin in tea-seed cake. PT CA (Part B: Chem Anal). 2008;44 (12): 1169–1175. [Google Scholar]
- 21. Strobel GA, Dirkse E, Sears J, Markworth C. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology. 2001;147: 2943–2950. [DOI] [PubMed] [Google Scholar]
- 22. Farag MA, Ryu CM, Sumner LW, Pare PW. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry. 2006;67: 2262–2268. [DOI] [PubMed] [Google Scholar]
- 23. Shen Z, Zhong S, Wang Y, Wang B, Mei X, Li R, et al. Induced soil microbial suppression of banana fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur J Soil Biol. 2013;57: 1–8. [Google Scholar]
- 24. Tian JK, Xu LZ, Zou ZM, Yang SL. Three novel triterpenoid saponins from Lysimachia capillipes and their cytotoxic activities. Chem Pharm Bull. 2006;54 (4): 567–569. [DOI] [PubMed] [Google Scholar]
- 25. Yoshikawa M, Murakami T, Ueno T, Yashiro K, Hirokawa N, Murakami N, et al. Bioactive saponins and glycosides. VIII. Notoginseng (1): new dammarane-type triterpene oligoglycosides, notoginsenosides-A,-B,-C, and-D, from the dried root of Panax notoginseng (Burk.) F.H. Chen. Chem Pharm Bull (Tokyo). 1997;45(6):1039–1045. [DOI] [PubMed] [Google Scholar]
- 26. Sun HX, Qin F, Ye YP. Relationship between haemolytic and adjuvant activity and structure of protopanaxadiol-type saponins from the roots of Panax notoginseng . Vaccine. 2005;23: 5533–5542. [DOI] [PubMed] [Google Scholar]
- 27. Jabeen S, Bilqees FM, Khan A, Khatoon N. Pathogenicity of Meloidogyne javanica on banana in Pakistan. Proc Parasitol. 1996;21: 11–76. [Google Scholar]
- 28. Pradhan MAA, Rahaman MM, Paul SK, Ahamad MU, Goswami BK. Effect of BAU-biofungicide, neem oil and a nematicide on the root-knot (Meloidogyne javanica) of papaya (Carica papaya). Bangladesh J Agril Res. 2012;37 (2): 271–277. [Google Scholar]
- 29. Vovlas N, Mifsud D, Landa BB, Castillo P. Pathogenicity of the root-knot nematode Meloidogyne javanica on potato. Plant Pathol 2005;54: 657–664. [Google Scholar]
- 30. Tomaszewski EK, Khalil MAM, El-Deeb AA, Powers TO, Starr JL. Meloidogyne javanica parasitic on peanut. J Nematol. 1994;26 (4): 436–441. [PMC free article] [PubMed] [Google Scholar]
- 31. Rumbos CI, Khah EM, Sabir N. Response of local and commercial tomato cultivars and rootstocks to Meloidogyne javanica infestation. Aust J Crop Sci. 2011;5(11): 1388–1395. [Google Scholar]
- 32. Huang Y, Yang M, Li Y, Lin W, Hong S. Effects of Camellia cake organic fertilizer on growth of turf grass Cynodon spp. Rich. J Cent South Univ Forestry and Technol. 2013;4 (33): 1–6. [Google Scholar]
- 33. Wang L, Wan X, Hou R, Xu J, Wu H. Control of Camellia plant extracts and its preparation against plant parasitic nematodes. J Anhui Agri Univ. 2013;40 (4): 642–648. [Google Scholar]
- 34. Xiao TJ, Tan SY, Shen QR, Ran W. Bacillus cereus X5 suppresses root-knot nematode of tomato by colonizing in roots and soil. Afr J Microbiol Res. 2012;6 (10): 2321–2327. [Google Scholar]
- 35. Siddiqui IA, Shaukat SS. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: importance of bacterial secondary metabolite, 2, 4-diacetylphloroglucinol. Soil Biol Biochem. 2003;35: 1615–1623. [Google Scholar]
- 36. Dinda B, Debnath S, Mohanta BC, Harigaya Y. Naturally occurring triterpenoid saponins. Chem Biodivers. 2010;7: 2327–2580. 10.1002/cbdv.200800070 [DOI] [PubMed] [Google Scholar]
- 37. D’Addabbo T, Carbonara T, Leonetti P, Radicci V, Tava A, Avato P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa . Phytochem Rev. 2011;10: 503–519. [Google Scholar]
- 38. Tranthim-Fryer DJ, Hansson RC, Norman KW. Headspace/solid-phase microextraction/gas chromatography-mass spectrometry: a screening technique for the recovery and identification of volatile organic compounds (VOC's) in postmortem blood and viscera samples. J Forensic Sci. 2001;46 (4): 934–946. [PubMed] [Google Scholar]
- 39. Silva CL, Passos M, Câmara JS. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br J Cancer. 2011;105 (12): 1894–1904. 10.1038/bjc.2011.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wardencki W, Michulec M, Curyło J. A review of theoretical and practical aspects of solid-phase microextraction in food analysis. Int J Food Sci Technol. 2004;39: 703–717. [Google Scholar]
- 41.Li G, Lu H, Zhang K. Volatile compound methyl thiobutyrate and application Faming Zhuanli Shenqing Gongkai Shuomingshu, CN103439426A. 2013. (in Chinese)
- 42. Hussain MA, Mukhtar T, Kayani MZ. Efficacy evaluation of Azadirachta indica, Calotropis procera, Datura stramonium and Tagetes erecta against root-knot nematodes Meloidogyne incognita. Pak J Bot. 2011;43: 197–204. [Google Scholar]
- 43. Dawar S, Younus SM, Zaki MJ. Use of Eucalyptus sp., in the control of Meloidogyne javanica root knot nematode. Pak J Bot. 2007;39 (6): 2209–2214. [Google Scholar]
- 44. González JM. Bacterivory rate estimates and fraction of active bacterivores in natural protist assemblages from aquatic systems. Appl Environ Microbiol. 1999;65 (4): 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Remén C, Fransson P, Persson T. Population responses of oribatids and enchytraeids to ectomycorrhizal and saprotrophic fungi in plant-soil microcosms. Soil Biol Biochem. 2010;42:978–985. [Google Scholar]
- 46. Jiang Y, Wu Y, Xu W, Cheng Y, Chen J, Xu L, et al. IAA-producing bacteria and bacterial-feeding nematodes promote Arabidopsis thaliana root growth in natural soil. Eur J Soil Biol. 2012;52: 20–26. [Google Scholar]
- 47. Irshad U, Villenave C, Brauman A, Plassard C. Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol Biochem. 2011;43: 2121–2126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.