Abstract
Stressful life events are causally linked with alcohol use disorders (AUDs), providing support for a hypothesis that alcohol consumption is aimed at stress reduction. We have previously shown that expression of relaxin-3 mRNA in rat brain correlates with alcohol intake and that central antagonism of relaxin-3 receptors (RXFP3) prevents stress-induced reinstatement of alcohol-seeking. Therefore the objectives of these studies were to investigate the impact of Rxfp3 gene deletion in C57BL/6J mice on baseline and stress-related alcohol consumption. Male wild-type (WT) and Rxfp3 knockout (KO) (C57/B6JRXFP3TM1/DGen) littermate mice were tested for baseline saccharin and alcohol consumption and preference over water in a continuous access two-bottle free-choice paradigm. Another cohort of mice was subjected to repeated restraint followed by swim stress to examine stress-related alcohol preference. Hepatic alcohol and aldehyde dehydrogenase activity was assessed in mice following chronic alcohol intake and in naive controls. WT and Rxfp3 KO mice had similar baseline saccharin and alcohol preference, and hepatic alcohol processing. However, Rxfp3 KO mice displayed a stress-induced reduction in alcohol preference that was not observed in WT littermates. Notably, this phenotype, once established, persisted for at least six weeks after cessation of stress exposure. These findings suggest that in mice, relaxin-3/RXFP3 signalling is involved in maintaining high alcohol preference during and after stress, but does not appear to strongly regulate the primary reinforcing effects of alcohol.
Introduction
Alcohol use disorders (AUDs), like other substance use disorders, are characterised in part by recurrent, escalating alcohol use [1], and environmental triggers, including stressful life experiences, can elicit a state of persistent anxiety that promotes the development and maintenance of compulsive alcohol-seeking and consumption. In humans, traumatic events, including life-threatening accidents or severe physical assault, are associated with at-risk drinking and AUDs can emerge following traumatic stress exposure [2]. Rodent models using maternal separation or isolation rearing from weaning also demonstrate that stress exposure has long-lasting effects that can increase first-experience alcohol intake in adult rats [3, 4] and mice [5], consistent with stress-related initiation of alcohol consumption.
Stress is also implicated in the maintenance of alcohol consumption in humans, with progression to compulsive alcohol use thought to be due in part to a shift from positively to negatively reinforced drug-seeking in which alcohol is sought to reduce tension or relieve aversive states such as withdrawal [6]. This hypothesis is supported by research using an acute psychosocial stressor, the Trier Social Stress Test (TSST), which potentiated alcohol drinking in non-treatment seeking alcoholics [7]; and in rodent models of alcohol dependence [8, 9].
Conditional genetic deletion of corticotropin-releasing factor (CRF) receptor-1 (CRF1) in extra-hypothalamic regions of adult mouse brain attenuates stress-induced alcohol intake. In contrast, global CRF1 gene deletion (knockout) enhances stress-induced alcohol intake [10], highlighting the complex, site-specific mechanisms by which neuropeptides regulate stress and alcohol consumption. The orexin system is also involved in mediating primary and conditioned reinforcing effects of alcohol [11–14], and stress-induced reinstatement of alcohol-seeking [15]. Notably, recent studies in rat have identified the relaxin-3/RXFP3 system as highly stress responsive [16, 17] and involved in the regulation of alcohol self-administration and stress-induced reinstatement of alcohol-seeking [18].
Relaxin-3 is the most recently identified relaxin family peptide [19, 20] and is highly conserved [21] across species including zebrafish [22], mouse [23], rat [24, 25], macaque [26] and human [19]. The majority of relaxin-3 expressing neurons are located in the midline pontine nucleus incertus (NI) [20, 27] and innervate widespread forebrain areas [23–25, 28] that express the cognate relaxin-3 receptor, ‘relaxin family peptide 3 receptor’ (RXFP3) [29–31]. CRF1 is expressed by relaxin-3 neurons within rodent NI [17, 25], and these relaxin-3 neurons are activated following exposure to various neurogenic stressors in a CRF-dependent manner [16, 25]. Relaxin-3 positive fibres and/or RXFP3 mRNA/binding sites are also present within the amygdala, bed nucleus of the stria terminalis (BNST), periaqueductal grey (PAG), paraventricular thalamic nucleus (PVT), and paraventricular hypothalamic nucleus (PVN) [23–25]. Intracerebroventricular (icv) infusions of relaxin-3 and selective RXFP3 agonists in rat activate PVN CRF neurons and the HPA axis [32], and modulate anxiety and depressive-like behaviour, respectively [33]. Importantly, both central (icv) and intra-BNST infusion of a selective RXFP3 antagonist attenuates alcohol self-administration and stress-induced reinstatement of alcohol-seeking in rat [18], and relaxin-3 mRNA levels in the NI correlate with alcohol intake [34].
Therefore, we aimed to further characterise the role of RXFP3 signalling in alcohol consumption using a germline Rxfp3 knockout (KO) mouse (C57/B6JRXFP3TM1/DGen), which display normal health and well-being, with no overt deficits in motor coordination or memory [35]. We report that Rxfp3 KO mice markedly reduce preference for a 10% v/v ethanol solution following multiple stress exposures compared to wild-type (WT) littermates, an effect that once established was persistent.
Methods
All animal experiments were performed in accordance with the Prevention of Cruelty to Animals Act, 1986 under the guidelines of the National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Experimental Purposes in Australia and approved by the Animal Ethics Committee of The Florey Institute of Neuroscience and Mental Health.
Animals and Housing
Rxfp3 KO mice (C57/B6JRXFP3TM1/DGen) were generated by Deltagen Inc. (San Carlos, CA, USA) and originally supplied by Janssen Pharmaceutical Companies of Johnson & Johnson PR&D LLC (San Diego, CA, USA). F1 129S5:B6 founders were backcrossed onto a C57BL/6J background. Charles River’s Speed Congenics program (Marker-Assisted Accelerated Backcrossing) was used to screen the background genetics of progeny and select those with the highest percentage of C57BL/6J background to mate to produce successive generations until a congenic (~100%) colony was achieved. For further details see [35].
Cohorts of age-matched Rxfp3 KO and WT littermate mice were produced from heterozygous pairings and genotypes were identified using a Sigma REDExtract-N-Amp Tissue PCR Kit (Sigma-Aldrich, Castle Hill, NSW, Australia). Three primers were used for the PCR reaction: WT forward, 5'-GCTCATAGCAGTGAGGAAGAAGACG-3'; WT reverse, 5'-GCTGGTTCTCTACCTGATGAAGAGC-3' and; KO reverse, 5'-GGGCCAGCT CATTCCTCCCACTCAT-3'. After an initialisation step (94°C for 2 min), tail samples were exposed to 30 cycles of PCR. Each cycle included a denaturation (94°C for 30 s), annealing (temperatures of 63°C, 62°C and 60°C for 30 s (2 cycles each for the first 6 cycles) and 58°C for 30 s for the remaining 24 cycles) and elongation (72°C for 1 min) phase. Samples were finally exposed to an additional 10 min of 72°C. This PCR reaction yields 232 bp and/or 461 bp products in the presence of the WT and/or KO alleles, respectively, which were subsequently separated via gel electrophoresis and visualised under ultra violet (UV) light using Invitrogen SYBR safe DNA stain (Life Technologies, Mulgrave, VIC, Australia).
All mice were weaned after 3 weeks and group-housed (maximum 6 per cage, mixed genotypes) in a temperature (~20°C) and humidity (~50%) controlled room on a 12 h light/dark cycle (lights on 0700 h). For two-bottle choice experiments, mice were individually housed at 8–10 weeks of age until study completion. All mice had food and water available ad libitum.
Cohort 1 (n = 14 WT/11 KO male mice) and Cohort 2 (n = 17 WT/16 KO male mice) were given ad libitum two-bottle choice between 0.1% w/v saccharin and tap water, and alcohol (see below for concentrations) and tap water, respectively. Livers were collected from Cohort 2 and naive control mice to assess endogenous alcohol and aldehyde dehydrogenase activity. Cohort 3 (n = 9 WT/20 KO male mice) was used to investigate the impact of stress on voluntary alcohol consumption in the two-bottle choice paradigm.
Treatments
Saccharin solutions for two-bottle choice experiments (0.1% w/v, sodium salt hydrate, Sigma-Aldrich) were prepared fresh weekly by dissolving saccharin in tap water. Ethanol solutions for two-bottle choice experiments were prepared fresh weekly by diluting anhydrous absolute ethanol in tap water.
Two-Bottle Free-Choice Drinking
Two separate cohorts of individually housed mice were acclimatised to drinking from two freely available bottles of tap water for 1 week, after which time one bottle was replaced with either a 0.1% w/v saccharin solution or a 5% v/v ethanol solution for 4 weeks, depending on treatment assignment. Saccharin intake (mL/kg bodyweight), saccharin preference (0.1% saccharin intake [mL]/total fluid intake [mL] × 100) and total fluid intake (mL/kg bodyweight) were measured daily and mice were weighed weekly. Similar formulas were used to calculate preference and total fluid intake of mice consuming alcohol; however alcohol intake was expressed in g/kg bodyweight (alcohol [mL] × alcohol concentration × 0.79/kg bodyweight). Mice were exposed to progressively increasing concentrations of ethanol (5%, 10%, 15% and 20% v/v) with at least 2 weeks at each concentration. All fluids were presented in 250 mL bottles (Tecniplast, Italy) with ball-bearing free sippers, and bottle position was randomly alternated to control for side preference. Bottles were weighed daily between 1000 h and 1200 h to the nearest 0.1 g.
Liver Preparation
A subset of mice from Cohort 2 (n = 6 WT/6 KO males) matched for alcohol preference and intake (g/kg), and naive controls (n = 5 WT/7 KO males), were used and livers were processed ~1 h after alcohol was removed from the home cages of mice in Cohort 2, as described earlier [36]. Mice were anaesthetised by isoflurane inhalation (IsoFLO; Abbott Laboratories, Melbourne, VIC, Australia) and livers were dissected, weighed wet and frozen over liquid nitrogen for storage (-80°C). Livers were subsequently divided into 4 pieces to increase surface area, incubated for 1 h at 37°C in carbogenated physiological saline solution [(PSS); composition in mM: NaCl, 118.0; KCl, 4.7; NaH2PO4, 1.0; MgCl2, 1.2; CaCl2, 1.3; NaHCO3, 25.0 and; ethylenediaminetetraacetate (EDTA), 0.04] and manually homogenised in 9 mL ice-cold sucrose buffer (0.25 M sucrose, 5 mM Tris, 0.5 mM EDTA, and 0.5 mM dithiothreitol; pH 7.5). Homogenates were centrifuged (4°C) at 700 g for 10 min to remove cellular debris and the resultant supernatant was centrifuged (4°C) at 10,000 g for 30 min. The pellet (mitochondrial fraction) was resuspended in 10 mL pyrophosphate buffer (50 mM sodium pyrophosphate; pH 8.8) and divided into 1.5 mL aliquots for storage (-80°C). The supernatant was centrifuged (4°C) at 48,000 g for 1 h and the resultant supernatant (cytosolic fraction) was also divided into 1.5 mL aliquots for storage (-80°C). Protein concentration of each sample was determined in duplicate using the recommended manufacturers’ protocol of a Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Gladesville, NSW, Australia).
Alcohol Dehydrogenase (ADH) Assay
Endogenous hepatic ADH activity was determined by assessing nicotinamide adenine dinucleotide (NADH)-induced increase in absorbance at 340 nm on a microplate spectrophotometer (Bio-Rad Benchmark Plus; Bio-Rad Laboratories) as described [36]. Briefly, the assay was performed in duplicate at 37°C in a final volume of 1.25 mL glycine buffer (0.1 M glycine; pH 10) containing 2.4 mM of β-NAD+ and a volume of cytosolic fraction equivalent to 1 mg protein. Following 5 min of equilibration, the addition of ethanol (0.1–100 mM) initiated the reaction, which was allowed to incubate for 5 min before increased absorbance was measured relative to blank reactions. A concentration-response curve was constructed to determine the concentration of ethanol (10 mM) that produced maximal ADH activity and the ADH inhibitor pyrazole (10 mM) was subsequently used in triplicate to inhibit the reaction induced by 10 mM ethanol, thus testing the specificity of the assay.
Aldehyde Dehydrogenase (ALDH) Assay
Endogenous hepatic ALDH activity was determined by observing the NADH-induced increase in absorbance at 340 nm on a microplate spectrophotometer (Bio-Rad Benchmark Plus) as described [36]. Briefly, the assay was performed in duplicate at room temperature in a final volume of 1.25 mL pyrophosphate buffer (50 mM sodium pyrophosphate; pH 8.8) containing 1.5 mM β-NAD+; pyrazole (0.1 mM) to block ADH activity; rotenone (2 μM in dimethylsulphoxide: 0.2% final volume) to inhibit mitochondrial NADH oxidase; sodium deoxycholate (0.01% w/v) to increase solution clarity for analysis and release latent activity and; mitochondrial fraction corresponding to 1 mg protein. After 5 min of equilibration, the addition of acetaldehyde (1–1000 mM) initiated the reaction which was then incubated in a sealed 48 well plate for 15 min at room temperature. The increase in absorbance was measured relative to blank reactions. After construction of a concentration-response curve, the effect of disulfiram (0.1 mM in ethanol: 0.2% of final volume), an ALDH inhibitor, was examined in triplicate using the concentration of acetaldehyde (10 mM) that yielded maximal ALDH activity.
Stress Protocol and Voluntary Alcohol Consumption
Mice were individually housed at 10 weeks of age and acclimatised to drinking from two freely available bottles of tap water for 1 week. Subsequently, one bottle was replaced with a 5% v/v ethanol solution for 3 weeks, which was increased to 10% v/v ethanol for the duration of the study. Alcohol/water bottles were randomly repositioned and weighed daily to determine preference and intake and mice were weighed weekly. Starting from the 23rd day of access to 10% v/v ethanol, mice were restrained for 30 min/day between 1700 h and 1800 h for 7 consecutive days in modified 50 mL polypropylene tubes (Corning Inc., Corning, NY, USA) with holes drilled through the bottom and sides to permit breathing and airflow. One week after the last restraint stress, mice received 2 consecutive days of swim stress for 5 min/day between 1700 h and 1830 h in opaque buckets (19 cm diameter × 23 cm high) filled with 27°C tap water to a depth of 18 cm. Mice were maintained for a further 6 weeks until study conclusion.
Statistics
SPSS software (Version 20; IBM Corp., Armonk, NY, USA) was used for all ANOVA statistical comparisons. GraphPad Prism (Version 5; GraphPad Software, Inc., La Jolla, CA, USA) was used for t-test statistical comparisons and to generate graphs. Baseline drinking for saccharin and alcohol studies is displayed as an average of the last 7 days of drinking (for alcohol, the last 7 days at each concentration). Data are expressed as means ± S.E.M.
Results
Rxfp3 KO and WT mice display similar taste perception and motivation to consume a palatable substance
A saccharin continuous access two-bottle choice test was used to evaluate taste perception and motivation to consume a palatable substance in WT (n = 14) and Rxfp3 KO (n = 11) mice. WT and Rxfp3 KO mice displayed similar average amounts of saccharin consumed per day (unpaired, two-tailed t-test, t (23) = 1.50, p = 0.147; Fig 1A ) and similar average daily preference for saccharin (unpaired, two-tailed t-test, t (23) = 1.21, p = 0.238; Fig 1B ). Furthermore, total fluid intake was comparable in both genotypes (unpaired, two-tailed t-test, t (23) = 1.40, p = 0.175; Fig 1C ).
Fig 1. Saccharin Two-Bottle Free-Choice Drinking.
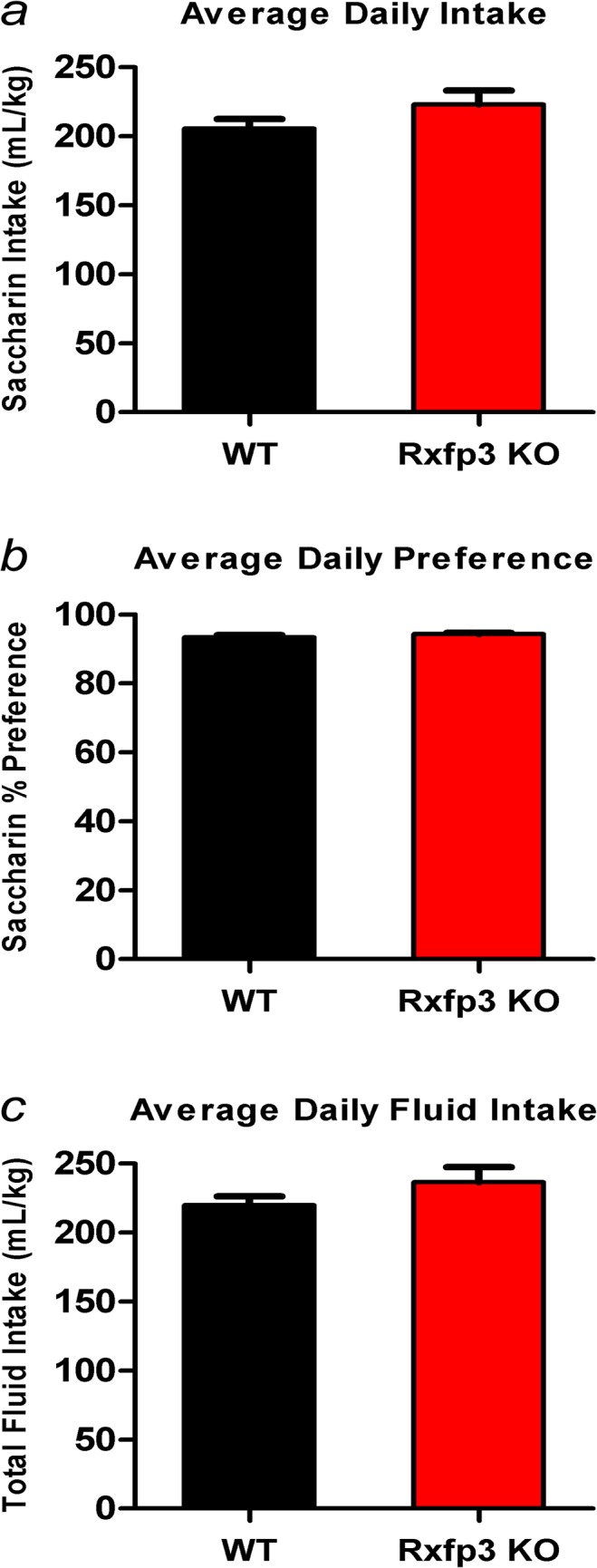
Rxfp3 gene deletion does not alter taste perception or motivation to consume saccharin in mice. (a) Average daily saccharin consumption, (b) saccharin preference, and (c) total fluid intake of Rxfp3 KO mice (n = 11) and WT littermate controls (n = 14) over the final week of saccharin access. Data are presented as mean ± S.E.M.
Rxfp3 KO and WT mice display similar baseline alcohol consumption and preference
Baseline alcohol consumption was measured in WT (n = 17) and Rxfp3 KO (n = 16) mice. In the continuous access two-bottle choice test, Rxfp3 KO mice displayed similar alcohol consumption and preference to WT littermate controls under ‘basal’ conditions, across increasing ethanol concentrations of 5, 10, 15 and 20% v/v. There were no genotype differences, overall or at different ethanol concentrations, in average daily amount of alcohol consumed (2-way RM ANOVA, main effect of genotype F 1,31 = 0.512, p = 0.480; concentration × genotype interaction F 3,93 = 0.988, p = 0.402; Fig 2A ) or in average preference for alcohol (2-way RM ANOVA, main effect of genotype F 1,31 = 0.006, p = 0.938; concentration × genotype interaction F 3,93 = 1.20, p = 0.315; Fig 2B ). However, increasing the concentration of available ethanol from 5 to 20% v/v increased average total amount of alcohol consumed per day from ~5 to 15 g/kg (main effect of concentration F 3,93 = 104.2, p<0.0001; Fig 2A ), and decreased average daily preference for alcohol over water from ~90 to 60% (main effect of concentration F 3,93 = 48.93, p<0.0001; Fig 2B ). Finally, there were no genotype differences overall or at each ethanol concentration in average daily total fluid intake (2-way RM ANOVA, main effect of genotype F 1,31 = 1.553, p = 0.222; concentration × genotype interaction F 3,93 = 2.170, p = 0.097; Fig 2C ); although total fluid intake reduced slightly with increasing concentrations of available ethanol (main effect of concentration F 3,93 = 63.75, p<0.0001; Fig 2C ).
Fig 2. Alcohol Two-Bottle Free-Choice Drinking.
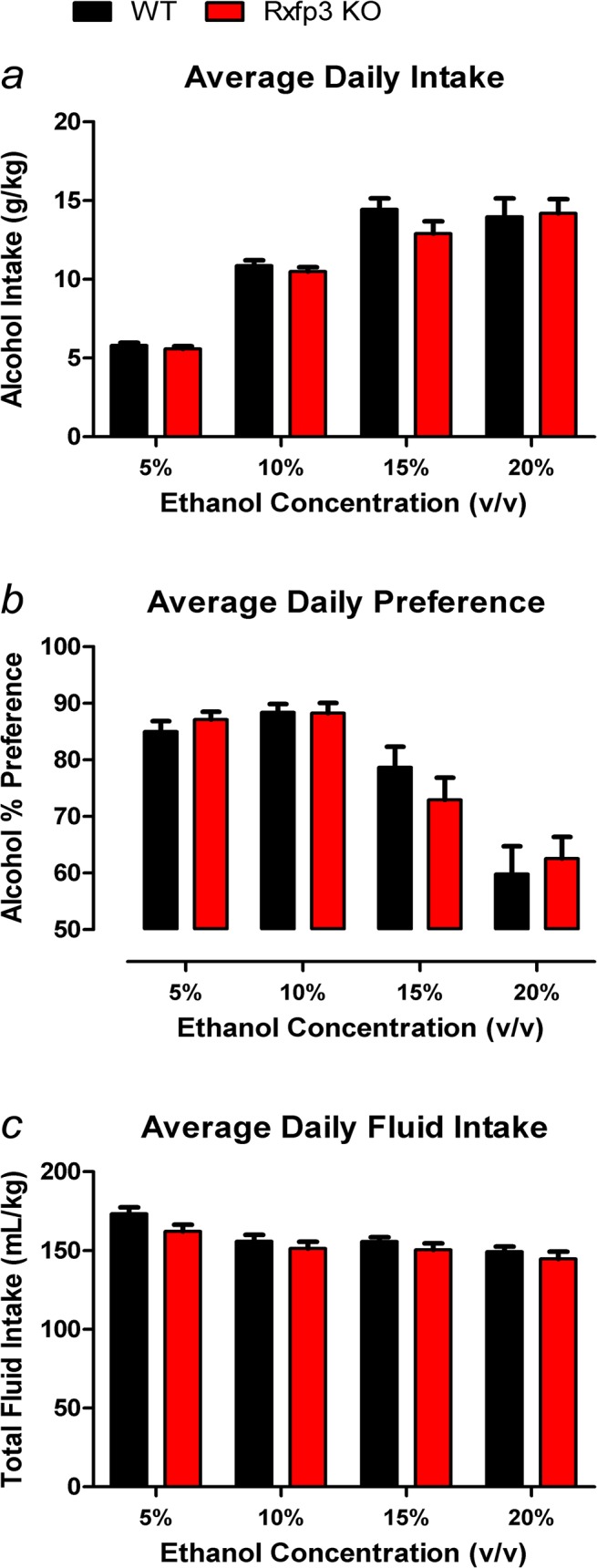
Rxfp3 gene deletion does not impact baseline intake or preference for various ethanol solutions (5%-20% v/v) in the two-bottle choice paradigm. (a) Average daily alcohol consumption, (b) alcohol preference, and (c) total fluid intake of Rxfp3 KO mice (n = 16) and WT littermate controls (n = 17) over the final week of access to each ethanol solution. Data are presented as mean ± S.E.M.
Rxfp3 KO and WT mice display similar hepatic capacity to metabolise alcohol
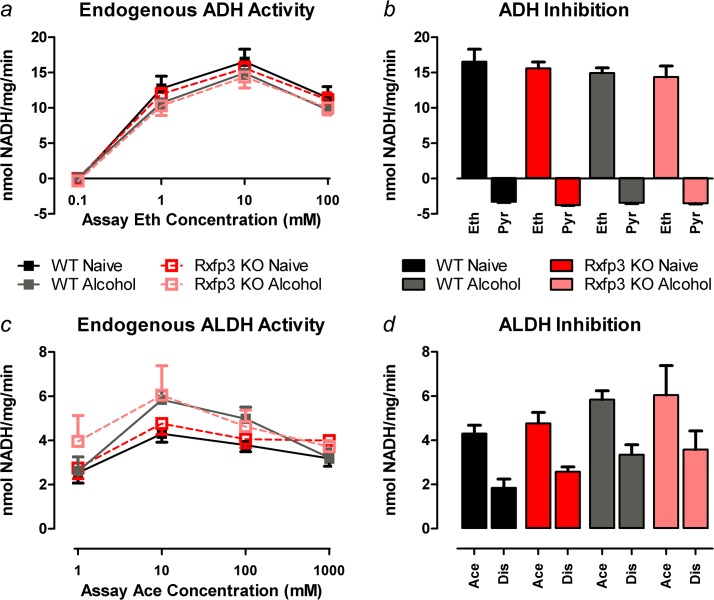
ADH activity was measured from liver samples taken from WT and Rxfp3 KO mice which were culled either naive (n = 5 WT/7 KO), or at the conclusion of the two-bottle choice experiment after chronic access to increasing concentrations (5%-20% v/v) of ethanol for 10 weeks (n = 6 WT/6 KO). No differences in endogenous ADH activity were observed between groups (3-way RM ANOVA, main effect of alcohol pre-treatment F 1,20 = 1.363, p = 0.257; alcohol pre-treatment × genotype interaction F 1,20 = 0.005, p = 0.944; alcohol pre-treatment × assay ethanol concentration interaction F 3,60 = 3.453, p = 0.022; post-tests between alcohol pre-treatments, within assay ethanol concentrations, p>0.05; Fig 3A ). Similarly, no differences were observed between genotypes (main effect of genotype F 1,20 = 0.130, p = 0.722; genotype × assay ethanol concentration interaction F 3,60 = 0.765, p = 0.518; Fig 3A ). Endogenous ADH activity varied with assay ethanol concentration (main effect of assay ethanol concentration F 3,60 = 947.614, p<0.0001; Fig 3A ), with peak NADH absorbance occurring when 10 mM ethanol was used to initiate the reaction. Hence, the 10 mM ethanol concentration was used for a subsequent ADH inhibition assay, whereby pyrazole significantly reduced ethanol-induced ADH activity in both WT and Rxfp3 KO liver preparations (3-way RM ANOVA, main effect of pyrazole treatment F 1,20 = 899.461, p<0.0001; Fig 3B ).
Fig 3. Alcohol and Aldehyde Dehydrogenase Activity.
Endogenous ADH and ALDH activity in naive Rxfp3 KO (n = 7) and WT (n = 5) liver samples and liver preparations from Rxfp3 KO (n = 6) and WT (n = 6) mice that chronically consumed alcohol (5%-20% v/v) for 10 weeks, as measured by substrate-dependent increase in NADH absorbance. Nicotinamide adenine dinucleotide (β-NADH) was produced from the reduction of β-NAD+. (a) Increase in β-NADH as a result of ADH activity in the cytosolic fraction over various assay ethanol (Eth) concentrations. (b) Impact of pyrazole (Pyr; 10 mM), a specific ADH inhibitor, on the increase in β-NADH produced by the addition of 10 mM ethanol, the assay ethanol concentration that elicited maximal ADH activity. (c) Increase in β-NADH as a result of ALDH activity in the mitochondrial fraction over various assay acetaldehyde (Ace) concentrations. (d) Impact of disulfiram (Dis; 0.1 mM), a specific ALDH inhibitor, on the increase in β-NADH produced by the addition of 10 mM acetaldehyde, the assay acetaldehyde concentration that elicited maximal ALDH activity. Data are presented as mean ± S.E.M.
No differences in ALDH activity were detected between groups or genotypes (3-way RM ANOVA, main effect of alcohol pre-treatment F 1,20 = 1.540, p = 0.229; main effect of genotype F 1,20 = 0.583, p = 0.454; alcohol pre-treatment × genotype interaction F 1,20 = 0.0001, p = 0.992; alcohol pre-treatment × assay acetaldehyde concentration interaction F 3,60 = 2.847, p = 0.045; genotype × assay acetaldehyde concentration interaction F 3,60 = 0.944, p = 0.425; post-tests between alcohol pre-treatments and genotypes, within assay acetaldehyde concentrations, p>0.05; Fig 3C ). As expected, endogenous ALDH activity varied with assay acetaldehyde concentration (main effect of assay acetaldehyde concentration F 3,60 = 27.742, p<0.0001; Fig 3C ), with peak NADH absorbance occurring when 10 mM acetaldehyde was used to initiate the reaction. 10 mM acetaldehyde was therefore used for subsequent studies to verify that ALDH activity was a major contributor to NADH absorbance. Disulfiram inhibited acetaldehyde-induced activity of ALDH by ~50% in both WT and Rxfp3 KO liver preparations (3-way RM ANOVA, main effect of disulfiram treatment F 1,20 = 93.331, p<0.0001; Fig 3D ).
Rxfp3 KO mice display a stress-induced reduction in alcohol preference
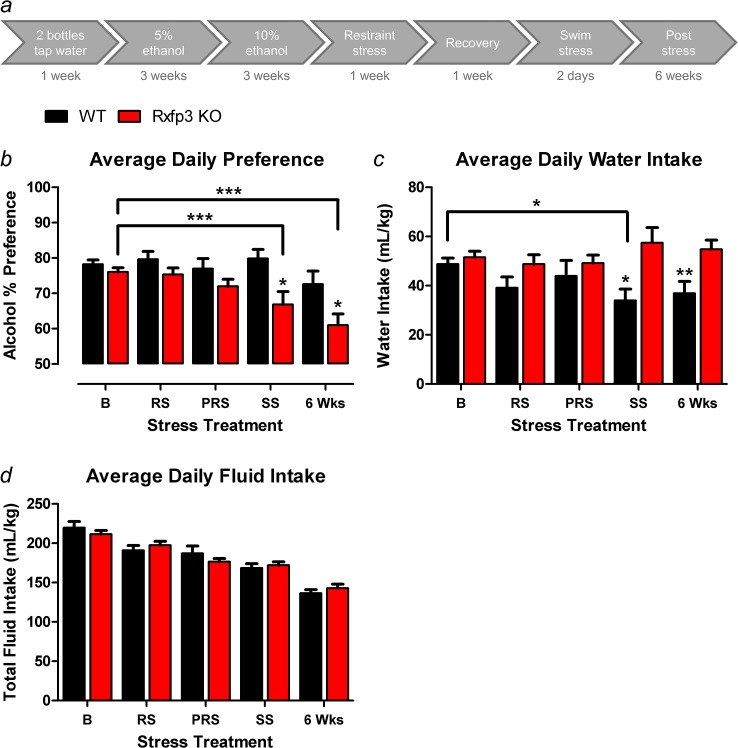
WT (n = 9) and Rxfp3 KO (n = 20) mice from Cohort 3, with access to 10% v/v ethanol, displayed similar alcohol preference and total fluid intake under basal (i.e. pre-stress) conditions, as observed in Cohort 2, indicating these findings are robust and reproducible (Fig 4B and 4D ). Interestingly however, Rxfp3 KO mice exhibited a significant reduction in alcohol preference across stress treatments (2-way RM ANOVA, stress treatment × genotype interaction F 4,108 = 3.463, p = 0.011; Bonferroni post-tests within Rxfp3 KO mice between stress treatments, baseline versus swim stress p<0.001, baseline versus 6 wks post-stress p<0.001; Fig 4B ). This reduction in alcohol preference was not observed in WT mice (Bonferroni post-tests within WT mice between stress treatments, p>0.05; Fig 4B ); and this discrepancy resulted in Rxfp3 KO mice displaying significantly less alcohol preference than WT littermates during swim stress, which persisted for at least 6 weeks after cessation of stress exposure (Bonferroni post-tests between genotypes during swim stress, p = 0.031; 6 wks p = 0.037; Fig 4B ).
Fig 4. Stress Protocol and Stress-Related Alcohol Drinking.
(a) Rxfp3 KO and WT mice were acclimatised to drinking from two bottles of tap water for 1 week. One bottle was subsequently replaced with a 5% v/v ethanol solution for 3 weeks, which was then increased to a 10% v/v ethanol solution for the duration of the experiment. Mice were permitted 3 weeks access to each ethanol solution prior to stress exposure to establish stable baseline drinking. From the 23rd day of access to 10% v/v ethanol, mice were exposed to 7 consecutive days of restraint stress to determine the impact of multiple stress exposures on alcohol preference. After 1 week of recovery, mice were exposed to 2 consecutive days of swim stress as this stressor potently activates relaxin-3 systems and variable stress exposure models the human condition. Alcohol drinking was monitored for 6 weeks following exposure to the final stressor to assess persistent effects. (b) Rxfp3 gene deletion does not impact basal (B) alcohol preference but does result in reduced preference for a 10% v/v ethanol solution following restraint stress (RS), a post-restraint (PRS) period and swim stress (SS). Importantly, this phenotype persists for at least 6 weeks (Wks) following stress exposure. (c) WT (n = 9) mice significantly reduce water consumption during SS compared to B, resulting in less water intake compared to Rxfp3 KO (n = 20) mice, (d) whereas there is no difference in total fluid intake observed between Rxfp3 KO and WT littermate controls. Data are presented as mean ± S.E.M.
WT mice displayed a small but significant reduction in water intake across stress treatments, an effect that was not observed in Rxfp3 KO mice (2-way RM ANOVA, stress treatment × genotype interaction F 4,108 = 3.746, p = 0.007; Bonferroni post-tests within WT mice between stress treatments, baseline versus swim stress p<0.05; Bonferroni post-tests within Rxfp3 KO mice between stress treatments p>0.05; Fig 4C ). This discrepancy resulted in WT mice displaying significantly less water intake than Rxfp3 KO mice during swim stress, which persisted for 6 weeks (Bonferroni post-tests between genotypes during swim stress, p = 0.023; 6 wks p = 0.010; Fig 4C ).
Analysis of total fluid intake (Fig 4D ) revealed that the genotype differences in alcohol preference observed during swim stress and 6 weeks post-swim were not a result of differences in total fluid intake (2-way RM ANOVA, main effect of genotype F 1,27 = 0.003, p = 0.954; stress treatment × genotype interaction F 4,108 = 2.645, p = 0.037; post-tests between genotypes within stress treatments, p>0.05; Fig 4D ). Reductions in total fluid intake were observed in WT and Rxfp3 KO mice across stress treatments (main effect of stress treatment F 4,108 = 123.58, p<0.0001; Fig 4D ).
Discussion
These studies demonstrate that Rxfp3 KO mice exhibit similar baseline alcohol preference to WT littermates, but after repeated exposure to acute stressors (repeated restraint followed by swim stress) Rxfp3 KO mice reduced their alcohol preference, an effect not observed in WT mice. These observations suggest that RXFP3 signalling maintains high levels of alcohol preference during (and after) stress exposure, but does not appear to strongly regulate the primary reinforcing effects of alcohol. These data are in agreement with previous findings that icv and intra-BNST infusion of selective RXFP3 antagonists attenuate stress-induced reinstatement of alcohol-seeking in rat [18]. This phenotype persisted for at least 6 weeks after cessation of stress exposure, analogous to long-lasting alterations in alcohol drinking following stress in which CRF/CRF1 receptor signalling was implicated [37].
Mouse NI neurons express CRF1 [38], and it has been demonstrated in rat that relaxin-3 positive neurons in the NI express CRF1 [17, 25]. Additionally, relaxin-3 mRNA is up-regulated in rat NI following swim stress, which is attenuated by pre-treatment with a CRF1 receptor antagonist, suggesting relaxin-3 neurons are activated by swim stress in a CRF-dependent manner [16]. Importantly, conditional, brain-specific CRF1 receptor knockout (CRF1 NestinCre) mice, which lack extra-hypothalamic CRF1 receptors, also displayed no difference in baseline alcohol consumption relative to WT littermates, but displayed attenuated social defeat stress-induced alcohol intake [10], highlighting the role of extra-hypothalamic CRF1 receptor signalling in stress-induced alcohol consumption, but not in the primary reinforcing properties of alcohol. Notably however, Molander et al. [10] reported an increase in alcohol consumption in CRF1 NestinCre mice and WT littermates following stress exposure, but to a lesser extent in CRF1 NestinCre mice, whereas our data reveal a stress-induced reduction in alcohol preference in Rxfp3 KO mice but unaltered alcohol preference in WT littermates.
The relationship between stress and alcohol intake is complex and the impact of stress on alcohol consumption in rodents is inconsistent in different studies. In fact, stress has been reported to increase, decrease or not change alcohol consumption in mice depending on the type of stressor used and the intensity, chronicity, predictability and controllability of the stressor [39]. Our findings, in agreement with other reports [40, 41], demonstrate that swim stress does not markedly alter alcohol two-bottle free-choice drinking in WT C57BL/6J mice, but suggest that intact relaxin-3/RXFP3 signalling may promote the maintenance of alcohol preference to relieve aversive states associated with acute and persistent anxiety produced by repeated stress exposure. Hence, alcohol preference was persistently attenuated in Rxfp3 KO mice after repeated stress exposure, in stark contrast to WT littermates. Long-lasting, stress-induced adaptations to alcohol drinking behaviour have been reported in mice [37] and relaxin-3/RXFP3 signalling is implicated in the regulation of chronic anxiety associated with prior stress exposure [33]. Importantly, mice in the present studies consumed ~10 g/kg/day of a 10% v/v ethanol solution, which is comparable to, even greater than amounts consumed by C57BL/6J mice in other reports [40, 41].
Central (icv) administration of an RXFP3 antagonist, R3(B1-22)R, significantly attenuated operant responding for alcohol in both alcohol-preferring (iP) and non-alcohol preferring Wistar rats, without impacting sucrose-responding in parallel experiments [18]. Additionally, icv administration of R3(B1-22)R significantly reduced both cue- and stress-induced reinstatement of alcohol-seeking, the effect being most marked for stress-induced relapse-like behaviour. Intra-BNST administration of R3(B1-22)R also significantly attenuated stress-induced reinstatement of alcohol-seeking, suggesting that RXFP3 signalling within the BNST is important for expression of stress-mediated alcohol-seeking behaviour in rat [18]. Importantly, the reduced alcohol preference in Rxfp3 KO mice following stress exposure observed in the present studies is in line with these pharmacological data in rat, and suggests that endogenous relaxin-3/RXFP3 signalling maintains stress-related responding for alcohol.
There were no significant differences between Rxfp3 KO and WT littermate mice in baseline alcohol preference or consumption across a range of ethanol concentrations (5%-20% v/v). Several factors may explain the discrepancy between baseline alcohol consumption in these mice and findings related to self-administration in rat. Firstly, although the relaxin-3/RXFP3 system in mouse is highly homologous to that in rat, there are species differences in distribution and expression levels of relaxin-3 and RXFP3 [23, 24]. Another key difference between these studies was the use of acute RXFP3 antagonist treatments in rat compared to germline Rxfp3 knockout in mice. Germline deletion of genes that encode proteins can induce developmental and other compensatory mechanisms that potentially mask behavioural consequences of the genetic manipulation [42–44]. It would be valuable, therefore, to repeat these studies using a conditional relaxin-3 or Rxfp3 KO model to circumvent any potential compensatory changes in RXFP3-related signalling. Also, there are clear differences between the operant conditioning paradigm used in earlier rat studies and the continuous access two-bottle choice paradigm employed in current studies. Self-administration allows only intermittent access to alcohol and the availability of an alcohol reward is contingent on an instrumental response. The two-bottle choice paradigm allows for continuous access to alcohol and alcohol availability is not response-dependent. Although alcohol preference and operant responding for alcohol likely share some underlying molecular mechanisms, alcohol drinking under two-bottle free-choice conditions may not fully translate to alcohol-reinforced drinking behaviour under operant conditions [45].
Importantly, the saccharin two-bottle free-choice study indicated no differences between Rxfp3 KO and WT mice in taste perception or baseline motivation to consume a palatable substance, as both genotypes displayed similar intake and preference for saccharin. Additionally, Rxfp3 KO and WT mice exhibited similar intake of tap water during acclimatisation to the paradigm and did not differ in total fluid intake under two-bottle choice conditions, suggesting similar fluid homeostasis. To our knowledge, RXFP3 is not expressed in mouse liver, but to assess hepatic liver enzyme activity, ADH and ALDH function was analysed in both naive mice and mice that consumed alcohol chronically for 10 weeks. Results indicated no differences in ADH or ALDH activity between any of the groups, suggesting that enzymatic pathways responsible for alcohol metabolism are intact in Rxfp3 KO mice. Consequently, the reduction in alcohol preference observed in Rxfp3 KO mice is likely due to a specific effect of RXFP3 signalling on stress responsiveness and the ability of stressors to regulate alcohol-seeking, rather than a secondary metabolic effect.
Alcohol has well established anxiolytic effects in humans and rodents, providing support for a ‘tension reduction’ hypothesis of alcohol use in certain circumstances [46–51]. The stress regimen employed in this study incorporated repeated exposure to acute restraint stress followed by two swim stress sessions. As canvassed above, acute, sub-chronic stress exposure during alcohol access has equivocal effects on alcohol consumption in rodents that depend on several characteristics of the stressor and animal strain [39]. Alcohol preference was significantly reduced in Rxfp3 KO mice towards the end of the stress exposure period and ongoing thereafter, so this effect might be due to a specific interaction of relaxin-3/RXFP3 signalling with swim stress or due to a cumulative stress load. Restraint and swim stress are both typically categorised as psychological stressors that, apart from obvious differences in locomotor activation, produce similar patterns of neuronal activation in the brain [52], recruit limbic nuclei, including the BNST [53, 54], and have a similar impact on HPA axis activation, as reflected by corticosterone release and immediate early gene activation [52, 55]. Consequently, it seems unlikely that swim stress would interact with relaxin-3/RXFP3 signalling more robustly than restraint stress to unmask the reduction in alcohol preference observed in Rxfp3 KO mice, particularly as both stressors engage the BNST, an area where RXFP3 signalling contributes to stress-induced reinstatement of alcohol-seeking [18]. The downward trend of preference during restraint stress that became significant during swim stress and thereafter, rather suggests cumulative stress load more likely contributes to the reduction in alcohol preference observed in Rxfp3 KO compared to WT mice. Exposure to variable, unpredictable stressors can alter future responses to stress as well as responsiveness to alcohol in rodents [56, 57]. Additionally, cumulative, variable stress exposure is relevant to alcohol dependence in the human condition as the number of stressful life events is predictive of first onset alcohol dependence [58], and variable stressors (e.g. family, work, health) are unavoidable in life.
We conclude that stress-reactive relaxin-3/RXFP3 signalling is likely involved in maintaining negatively reinforced alcohol preference to relieve persistent aversive states associated with exposure to variable stressors. Future studies are warranted to elucidate the circuit and molecular mechanisms that govern the interaction of relaxin-3/RXFP3 signalling with other systems to regulate complex behaviour.
Acknowledgments
The authors thank Dr Timothy Lovenberg (Neuroscience Drug Discovery, Janssen Pharmaceutical Companies of Johnson & Johnson, San Diego, USA) for commissioning the production of the Rxfp3 KO strain and providing The Florey Institute of Neuroscience and Mental Health with an original breeding colony.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by a National Health and Medical Research Council (NHMRC) of Australia project grant (1021227 to ALG and AJL) and NHMRC (Australia) Research Fellowships (1005985 and 1020737) to ALG and AJL; a grant from the Pratt and Besen Foundations (ALG and AJL); and the Victorian Government's Operational Infrastructure Support Program. AWW was the recipient of a University of Melbourne International Postgraduate Research Scholarship (IPRS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2. McFarlane AC. Epidemiological evidence about the relationship between PTSD and alcohol abuse: the nature of the association. Addict Behav. 1998; 23: 813–825. [DOI] [PubMed] [Google Scholar]
- 3. Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl). 2001; 158: 366–373. [DOI] [PubMed] [Google Scholar]
- 4. Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience. 2003; 117: 243–247. [DOI] [PubMed] [Google Scholar]
- 5. Cruz FC, Quadros IM, Planeta C da S, Miczek KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl). 2008; 201: 459–468. 10.1007/s00213-008-1307-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999; 34: 197–222. [DOI] [PubMed] [Google Scholar]
- 7. Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology (Berl). 2011; 218: 19–28. 10.1007/s00213-010-2163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006; 26: 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002; 72: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology. 2012; 37: 1047–1056. 10.1038/npp.2011.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jupp B, Krivdic B, Krstew EV, Lawrence AJ. The orexin1 receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011; 1391: 54–59. 10.1016/j.brainres.2011.03.045 [DOI] [PubMed] [Google Scholar]
- 12. Jupp B, Krstew EV, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin1 receptors. Br J Pharmacol. 2011; 162: 880–889. 10.1111/j.1476-5381.2010.01088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006; 148: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol-preferring Sprague-Dawley rats. Alcohol. 2009; 43: 379–386. 10.1016/j.alcohol.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl). 2008; 199: 109–117. 10.1007/s00213-008-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banerjee A, Shen PJ, Ma S, Bathgate RAD, Gundlach AL. Swim stress excitation of nucleus incertus and rapid induction of relaxin-3 expression via CRF1 activation. Neuropharmacology. 2010; 58: 145–155. 10.1016/j.neuropharm.2009.06.019 [DOI] [PubMed] [Google Scholar]
- 17. Ma S, Blasiak A, Olucha-Bordonau FE, Verberne AJ, Gundlach AL. Heterogeneous responses of nucleus incertus neurons to corticotrophin-releasing factor and coherent activity with hippocampal theta rhythm in the rat. J Physiol. 2013; 591: 3981–4001. 10.1113/jphysiol.2013.254300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryan PJ, Kastman HE, Krstew EV, Rosengren KJ, Hossain MA, Churilov L, et al. Relaxin-3/RXFP3 system regulates alcohol-seeking. Proc Natl Acad Sci U S A. 2013; 110: 20789–20794. 10.1073/pnas.1317807110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bathgate RAD, Samuel CS, Burazin TCD, Layfield S, Claasz AA, Reytomas IG, et al. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene: Novel members of the relaxin peptide family. J Biol Chem. 2002; 277: 1148–1157. [DOI] [PubMed] [Google Scholar]
- 20. Burazin TCD, Bathgate RAD, Macris M, Layfield S, Gundlach AL, Tregear GW. Restricted, but abundant, expression of the novel rat gene-3 (R3) relaxin in the dorsal tegmental region of brain. J Neurochem. 2002; 82: 1553–1557. [DOI] [PubMed] [Google Scholar]
- 21. Wilkinson TN, Speed TP, Tregear GW, Bathgate RAD. Evolution of the relaxin-like peptide family. BMC Evol Biol. 2005; 5: 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donizetti A, Grossi M, Pariante P, D'Aniello E, Izzo G, Minucci S, et al. Two neuron clusters in the stem of postembryonic zebrafish brain specifically express relaxin-3 gene: first evidence of nucleus incertus in fish. Dev Dyn. 2008; 237: 3864–3869. 10.1002/dvdy.21786 [DOI] [PubMed] [Google Scholar]
- 23. Smith CM, Shen PJ, Banerjee A, Bonaventure P, Ma S, Bathgate RAD, et al. Distribution of relaxin-3 and RXFP3 within arousal, stress, affective, and cognitive circuits of mouse brain. J Comp Neurol. 2010; 518: 4016–4045. 10.1002/cne.22442 [DOI] [PubMed] [Google Scholar]
- 24. Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TCD, Bathgate RAD, et al. Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience. 2007; 144: 165–190. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H, et al. Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci. 2005; 21: 1659–1670. [DOI] [PubMed] [Google Scholar]
- 26. Ma S, Sang Q, Lanciego JL, Gundlach AL. Localization of relaxin-3 in brain of Macaca fascicularis: identification of a nucleus incertus in primate. J Comp Neurol. 2009; 517: 856–872. 10.1002/cne.22197 [DOI] [PubMed] [Google Scholar]
- 27. Ryan PJ, Ma S, Olucha-Bordonau FE, Gundlach AL. Nucleus incertus—an emerging modulatory role in arousal, stress and memory. Neurosci Biobehav Rev. 2011; 35: 1326–1341. 10.1016/j.neubiorev.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 28. Ma S, Shen PJ, Sang Q, Lanciego JL, Gundlach AL. Distribution of relaxin-3 mRNA and immunoreactivity and RXFP3-binding sites in the brain of the macaque, Macaca fascicularis . Ann N Y Acad Sci. 2009; 1160: 256–258. 10.1111/j.1749-6632.2009.03954.x [DOI] [PubMed] [Google Scholar]
- 29. Bathgate RAD, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev. 2006; 58: 7–31. [DOI] [PubMed] [Google Scholar]
- 30. Bathgate RAD, Oh MH, Ling WJ, Kaas Q, Hossain MA, Gooley PR, et al. Elucidation of relaxin-3 binding interactions in the extracellular loops of RXFP3. Front Endocrinol (Lausanne). 2013; 4: 13 10.3389/fendo.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu C, Eriste E, Sutton SW, Chen J, Roland B, Kuei C, et al. Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J Biol Chem. 2003; 278: 50754–50764. [DOI] [PubMed] [Google Scholar]
- 32. Watanabe Y, Miyamoto Y, Matsuda T, Tanaka M. Relaxin-3/INSL7 regulates the stress-response system in the rat hypothalamus. J Mol Neurosci. 2011; 43: 169–174. 10.1007/s12031-010-9468-0 [DOI] [PubMed] [Google Scholar]
- 33. Ryan PJ, Buchler E, Shabanpoor F, Hossain MA, Wade JD, Lawrence AJ, et al. Central relaxin-3 receptor (RXFP3) activation decreases anxiety- and depressive-like behaviours in the rat. Behav Brain Res. 2013; 244: 142–151. 10.1016/j.bbr.2013.01.034 [DOI] [PubMed] [Google Scholar]
- 34. Ryan PJ, Krstew EV, Sarwar M, Gundlach AL, Lawrence AJ. Relaxin-3 mRNA levels in nucleus incertus correlate with alcohol and sucrose intake in rats. Drug Alcohol Depend. 2014; 140: 8–16. 10.1016/j.drugalcdep.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 35. Hosken IT, Sutton SW, Smith CM, Gundlach AL. Relaxin-3 receptor (Rxfp3) gene knockout mice display reduced running wheel activity: Implications for role of relaxin-3/RXFP3 signalling in sustained arousal. Behav Brain Res. 2015; 278: 167–175. [DOI] [PubMed] [Google Scholar]
- 36. Bird MK, Kirchhoff J, Djouma E, Lawrence AJ. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol. 2008; 11: 765–774. 10.1017/S1461145708008572 [DOI] [PubMed] [Google Scholar]
- 37. Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, et al. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002; 296: 931–933. [DOI] [PubMed] [Google Scholar]
- 38. Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000; 428: 191–212. [DOI] [PubMed] [Google Scholar]
- 39. Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl). 2011; 218: 131–156. 10.1007/s00213-011-2443-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007; 91: 77–86. [DOI] [PubMed] [Google Scholar]
- 41. Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res. 2008; 186: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dahlhoff M, Schafer M, Wolf E, Schneider MR. Genetic deletion of the EGFR ligand epigen does not affect mouse embryonic development and tissue homeostasis. Exp Cell Res. 2013; 319: 529–535. 10.1016/j.yexcr.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 43. Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996; 274: 1377–1379. [DOI] [PubMed] [Google Scholar]
- 44. Misawa H, Kawasaki Y, Mellor J, Sweeney N, Jo K, Nicoll RA, et al. Contrasting localizations of MALS/LIN-7 PDZ proteins in brain and molecular compensation in knockout mice. J Biol Chem. 2001; 276: 9264–9272. [DOI] [PubMed] [Google Scholar]
- 45. Ritz MC, George FR, Meisch RA. Ethanol self-administration in ALKO rats: I. Effects of selection and concentration. Alcohol. 1989; 6: 227–233. [DOI] [PubMed] [Google Scholar]
- 46. Correia D, Ribeiro AF, Godard ALB, Boerngen-Lacerda R. Trait anxiety and ethanol: anxiolysis in high-anxiety mice and no relation to intake behavior in an addiction model. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33: 880–888. 10.1016/j.pnpbp.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 47. Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003; 27: 232–243. [DOI] [PubMed] [Google Scholar]
- 48. Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res. 2009; 33: 2154–2161. 10.1111/j.1530-0277.2009.01053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, et al. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008; 88: 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC. Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol. 2014; 17: 2057–2067. 10.1017/S1461145714001047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Skelly MJ, Weiner JL. Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Behav. 2014; 4: 468–483. 10.1002/brb3.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001; 14: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 53. Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007; 27: 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crestani CC, Alves FH, Correa FM, Guimaraes FS, Joca SR. Acute reversible inactivation of the bed nucleus of stria terminalis induces antidepressant-like effect in the rat forced swimming test. Behav Brain Funct. 2010; 6: 30 10.1186/1744-9081-6-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mercier S, Canini F, Buguet A, Cespuglio R, Martin S, Bourdon L. Behavioural changes after an acute stress: stressor and test types influences. Behav Brain Res. 2003; 139: 167–175. [DOI] [PubMed] [Google Scholar]
- 56. Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011; 45: 355–364. 10.1016/j.alcohol.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ostrander MM, Ulrich-Lai YM, Choi DC, Flak JN, Richtand NM, Herman JP. Chronic stress produces enduring decreases in novel stress-evoked c-fos mRNA expression in discrete brain regions of the rat. Stress. 2009; 12: 469–477. 10.3109/10253890802641966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Slopen N, Williams DR, Fitzmaurice GM, Gilman SE. Sex, stressful life events, and adult onset depression and alcohol dependence: are men and women equally vulnerable? Soc Sci Med. 2011; 73: 615–622. 10.1016/j.socscimed.2011.06.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.