Abstract
The greenhouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) is a polyphagous pest in greenhouse crops. The efficacy of two entomopathogenic nematodes (EPN), Steinernema feltiae and Heterorhabditis bacteriophora, as biological control agents against T. vaporariorum was evaluated using two model crops typical of vegetable greenhouse productions: cucumber and pepper. Laboratory tests evaluated adults and second nymphal instars for pest susceptibility to different EPN species at different concentrations of infective juveniles (IJ; 0, 25, 50, 100, 150, 200, and 250 IJ per cm2); subsequent greenhouse trials against second nymphal instars on cucumber and pepper plants evaluated more natural conditions. Concentrations were applied in combination with Triton X-100 (0.1% v/v), an adjuvant for increasing nematode activity. In laboratory studies, both life stages were susceptible to infection by the two nematode species, but S. feltiae recorded a lower LC50 than H. bacteriophora for both insect stages. Similarly, in greenhouse experiments, S. feltiae required lower concentrations of IJ than H. bacteriophora to reach the same mortality in nymphs. In greenhouse trials, a significant difference was observed in the triple interaction among nematode species × concentration × plant. Furthermore, the highest mortality rate of the second nymphal instars of the T. vaporariorum was obtained from the application of S. feltiae concentrated to 250 IJ/cm2 on cucumber (49 ± 1.23%). The general mortality caused by nematodes was significantly higher in cucumber than in pepper. These promising results support further investigation for the optimization of the best EPN species/concentration in combination with insecticides or adjuvants to reach a profitable control of this greenhouse pest.
Keywords: biological control, entomopathogenic nematodes, greenhouse, insect pathology, Trialeurodes vaporariorum
The greenhouse whitefly (GHWF), Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae), is a cosmopolitan, polyphagous, and serious insect pest throughout the world. This whitefly is a destructive and important pest on several greenhouse crops like cucumbers, tomatoes, peppers, and a range of other plants (Hamdi et al., 2011; Choi et al., 2003). More than 250 plant species from different regions of the world are recorded as its host (Quesada-Moraga et al., 2006). Both adults and nymphs feed on phloem sap, causing direct damage by feeding. They also cause indirect damage by excreting a lot of honeydew as desirable medium for the development of saprophyte fungi-like sooty mould (Capnodium spp.), which affects plant photosynthesis. In addition, this hemipteran is a key vector for different plant pathogens like the geminivirus (Manzano and van Lenteren, 2009). The extensive use of pesticides against GHWF has resulted in resistance to chemical insecticides, phytotoxicity, and pesticide residue problems on vegetables (Lucas et al., 2004; Qiu et al., 2008; Hamdi et al., 2011). The demand for a reduction in pesticide use and the requirement for residue-free food have turned focus onto developing alternative control methods, including biological control methods for use in greenhouses (Laznik et al., 2011).
A prevalent method for biocontrol of greenhouse pests is mass release of natural enemies. Biological control agents that are currently commercialized for the control of T. vaporariorum include Encarsia formosa Gahan (Hymenoptera: Aphelinidae), Macrolophus caliginosus Wagner (Heteroptera: Miridae) (Bonato et al., 2006), and Lecanicillium lecanii (Zimmermann) Gams & Zare (Scorsetti et al., 2008). However, exploring other biological control agents and their compatibility with current practices might provide alternatives for their control that can assist producers with different profitable and ecologically friendly ways to control the pest. In this regard, entomopathogenic nematodes (EPN) from the families Steinernematidae and Heterorhabditidae are excellent candidates. They are important biological control agents for a variety of economically important pests (Grewal et al., 2005). They are mutually associated with the bacteria in the genera Xenorhabdus spp. and Photorhabdus spp., respectively. When these bacteria are released into the insect hemocoel, both nematode and bacteria cause the insect death within 48 hr (Kaya and Gaugler, 1993; Boemare, 2002). Some species of Steinernema and Heterorhabditis are proven biocontrol agents and being used to control a wide range of foliar insect pests in various commercial systems (Arthurs et al., 2004; Laznik and Trdan, 2014). Species from both genera are among the important biocontrol agents of the leafminer, Liriomyza huidobrensis (Blanchard) (Diptera: Agromyzidae) (Williams and Walters, 2000), diamondback moth larvae Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) (Schroer and Ehlers, 2005), and western flower thrips Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) under glasshouse condition or the Colorado potato beetle Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) on potato under field conditions (Laznik et al., 2010). Moreover, during the previous studies Steinernema feltiae (Filipjev) has been shown to be an effective agent against the sweet potato whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) and the second nymphal instar was significantly more susceptible than other instars (Cuthbertson et al., 2003a, 2007). To our knowledge, there is a scarcity of data about possible effects of EPN on the GHWF except Laznik et al. (2011) who evaluated efficacy of S. feltiae against adult stage of GHWF and compared the results with those of thiamethoxam on cucumber.
Host-finding behavior of EPN is an important criterion for their selection as a biocontrol agent against a target pest. The foraging behavior of EPN ranges from ambushing to cruising (Lewis et al., 1993). Among EPN species, Steinernema carpocapsae (Weiser) is an ambusher species while Heterorhabditis bacteriophora (Poinar) display a cruiser foraging and are more effective against less mobile insects in soils (Campbell and Gaugler, 1993). However, this description about those two terms in respect of EPN behavior might be more complex than expected in the early studies, and the ecological conditions might interact the foraging behavior. For example, recently it was hypothesized that S. carpocapsae shows different behavior of host finding depending on the host insect and condition (Wilson et al., 2012). Also, some nematodes species display intermediate behaviors, such as, for example, S. feltiae (Lewis, 2002).
In addition to the differential EPN foraging behavior displayed by species and affected by the environment, it is known that host plants affect nematode activity when applied in above-ground systems (Cuthbertson et al., 2003b, 2005). The leaf surface characteristics and trichomes of host plants may either positively or negatively influence the movement ability of nematodes during host finding. Entomopathogenic nematodes need a film of water surrounding them to move; trichomes of the host plant can maintain humidity along the leaf surface (Cuthbertson et al., 2003b; Head et al., 2004; Qiu et al., 2008). To investigate the possible effect of morphology–structure of leaves on the ability of EPN to control an above-ground pest, we investigated how two nematode species (S. feltiae and H. bacteriophora), with potentially two differential foraging behaviors, can control two developmental stages of T. vaporariorum (nymph and adults) in two host plants with variable leaf morphology and structure, cucumber (Cucumis sativus L., Cucurbitaceae) and pepper (Capsicum annum L., Solanaceae). This information will provide the basis of applied studies in search of elaborate new strategies for enhancing pest control and crop health in greenhouse productions.
Materials and Methods
Organisms under study:
T. vaporariorum was obtained from the Biocontrol Laboratory of Ferdowsi University of Mashhad (Razavi Khorasan Province, Iran, 36°18′N 59°36′E). The cultures are refreshed timely with insects collected from the field (36.20° North latitude and 59.35° East longitude on 985 m above sea level ) to preserve a large genetic base in the culture. Insects were maintained in a growth chamber using cucumber (C. sativus L., Cucurbitaceae) and verbena Verbena hybrid L. (Verbenaceae) plants as hosts at 23 ± 1°C, 60 ± 5% RH, and a photoperiod 16:8 hr (light:dark, L:D) (Poprawski et al., 2000) for at least five successive generations before being used in experiments (Qiu et al., 2008).
The species used in the experiments were cucumber (C. sativus L., Cucurbitaceae) and pepper (C. annum L., Solanaceae). These plants were selected as two economical vegetable plants that are commonly infested by T. vaporariorum (Bi et al., 2002). Moreover, the leaves of these plants differ in ways that could affect nematode movement and survival (Qiu et al., 2008). The leaves of the cucumber are hairy, while those of the pepper are glabrous and waxy. Plants were grown in a greenhouse at 23 ± 1°C with a 16:8 hr (L:D) regime and used in tests at the six- to eight-leaf stage.
Two commercial products of EPN including Larvanem (H. bacteriophora) and Entonema (S. feltiae) were provided by Koppert Biological Systems B.V. (The Netherlands). Both EPN species were cultured in parallel on the last instar larvae of the greater wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae) (Kaya and Stock, 1997). The IJ were harvested from White traps (White, 1927) and stored in damp sponges at 12°C (Kaya and Stock, 1997) for 2 weeks until used in experiments.
General procedures:
Approximately 30 adults with a sex ratio of 2:1 females to males of T. vaporariorum were introduced in each cylindrical clip cage (10 cm diameter × 11 cm height) and allowed to mate and lay eggs. The adults were removed after a 48-hr oviposition period, and those plants were maintained within the greenhouse at 25 ± 1°C, 65% RH for 16:8 hr (L:D). The plants were kept in the greenhouse for a further 12 d until the appearance of the second nymphal instar (Cuthbertson et al., 2007). We selected second nymphal instar because most works including Cuthbertson et al. (2003a) and Cuthbertson and Walters (2005) showed the second nymphal stages as the most susceptible to nematode infection.
Laboratory experiments:
The susceptibilities of nymphs and adults to the two EPN species were evaluated under laboratory conditions using 90-mm diameter petri dishes filled with a thin layer of 1.5% agar (Merck, Darmstadt, Germany) as the experimental unit. Nematode suspensions to produce the concentrations 0-control, 25, 50, 100, 150, 200, and 250 IJ/cm2 (Cuthbertson et al., 2003a) were adjusted by volumetric dilutions in distilled water to a final volume of 1 ml (Glazer and Lewis, 2000). All treatments, including the control containing only distilled water, were mixed with Triton X-100 Merck (0.1% v/v). Then, each suspension was sprayed onto the surface of each petri dish until runoff using a handheld sprayer. Each treatment had four replicates, and the whole experiment was conducted twice, with different batches of fresh nematodes, insects, and plants.
The first experiment evaluated the efficiency of the two EPN species against the second nymphal instars of T. vaporariorum. Cucumber leaves infested with T. vaporariorum second nymphal instars were separated from the plant and each leaf was placed into a petri dish (90-mm-diameter). After inoculation of nematodes the plates were covered and incubated at 20 ± 1°C, 85% RH, and 12:12-hr L:D period for 72 hr (Cuthbertson et al., 2008). Each treatment had four replicates, and the whole experiment was conducted two times. The infectivity of S. feltiae and H. bacteriophora against T. vaporariorum nymphs was checked after 72 hr with a stereomicroscope by dissection of an individual nymph.
The second experiment evaluated EPN efficacy against the T. vaporariorum adult. After the inoculation of EPN, 50 adults were transferred into each petri dish, covered with a lid, sealed with parafilm, and incubated under the conditions described above. Following treatment and incubation for 72 hr, mortality rate was recorded and cadavers were dissected under a stereomicroscope.
Greenhouse trial:
To establish the colony, 5 to 6 leaves were randomly selected from each plant (cucumber or pepper), individualized in clip cages, and kept until second instar nymphs appeared. Then, the EPN concentrations and the water-control suspensions with Triton-X100 described above were applied in the same manner to ensure homogeneous distribution with a handheld sprayer (Keshtzarsanat Co., Karaj, Iran). Spraying was performed at greenhouse in the afternoon. Thereafter, plants were maintained at 20 ± 1°C, 85% RH, and a 12:12-hr L:D period for 72 hr (Cuthbertson et al., 2008).
The infectivity of S. feltiae and H. bacteriophora was checked 72 h after treatment. Each treatment had three replicates, and the whole experiment was repeated twice. Cadavers were dissected under a stereomicroscope to confirm that mortality resulted from EPN infection.
Statistical analysis:
Insect mortality was corrected according to the control treatment values using Abbott’s formula (Abbott, 1925). Data corresponding to mortality percentages were arcsine square-root transformed to meet the assumptions of normality and homogeneity of variance. In the laboratory experiments, LC50 was calculated for each EPN species and for both second instar nymphs and adults using probit analysis. A parallelism test was performed to compare the differential effectiveness of each EPN species for each of the insect stages tested (second instar nymph and adult). To assess the effects of host plant type on the efficacy of nematode species in the greenhouse experiment, the data of second nymphal instar mortality was subjected to three-way analysis of variance (nematode species × nematode concentration × host plant type) followed by a least significant difference test. Regression analysis was also used to determine how EPN concentration influenced the mortality rate of the second instar nymph of T. vaporariorum in relation to either host plant or EPN species. SAS software, version 9.1 was used for all statistical analyses (SAS Institute, 2002).
Results
Laboratory experiments:
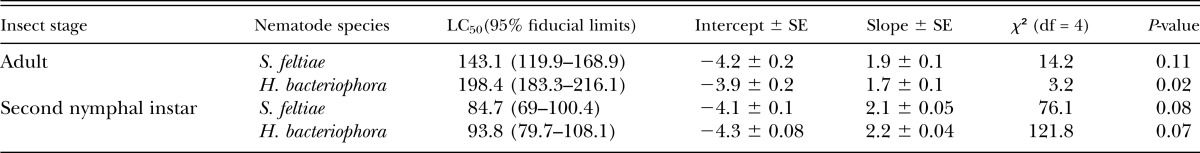
Both EPN species were able to kill second instar nymphs and adults of T. vaporariorum. The LC50 value for S. feltiae was 84 IJ/cm2 for second instar nymph and 143.1 IJ/cm2 for adults. H. bacteriophora required slightly higher numbers for nymphs (93 IJ/cm2) and close to 200 IJ for adults (198 IJ/cm2) (Table 1). There was no significant difference between LC50 values for both EPN on the nymphal stage while those indices showed a significant difference for the adult insects. The parallelism test between the regression lines of nematode species in mortality percentages of second instar nymphs (χ2 = 0.94, df = 1, P > 0.05) and adults (χ2 = 2.65, df = 1, P > 0.05) indicated both qualitative and quantitative differential effects of the nematode species on second instar nymph and adult mortality.
Table 1.
LC50 values for S. feltiae and H. bacteriophora against the second nymphal instar and adult of T. vaporariorum in the petri dish experiments.
Greenhouse trial:
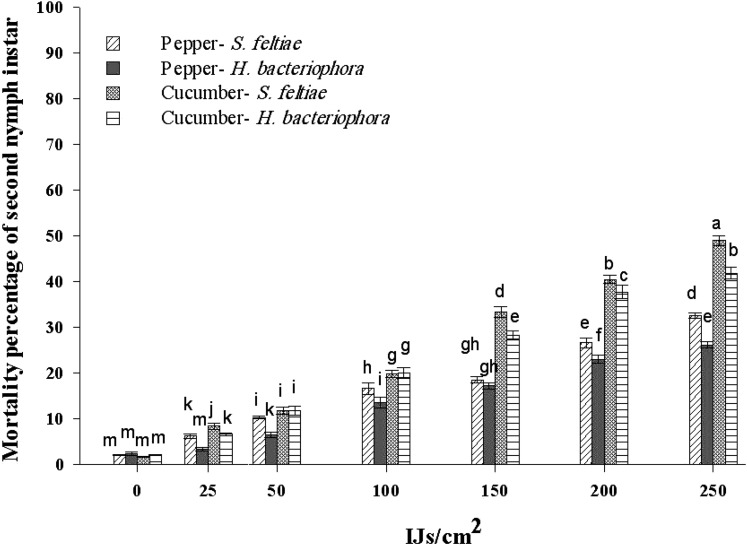
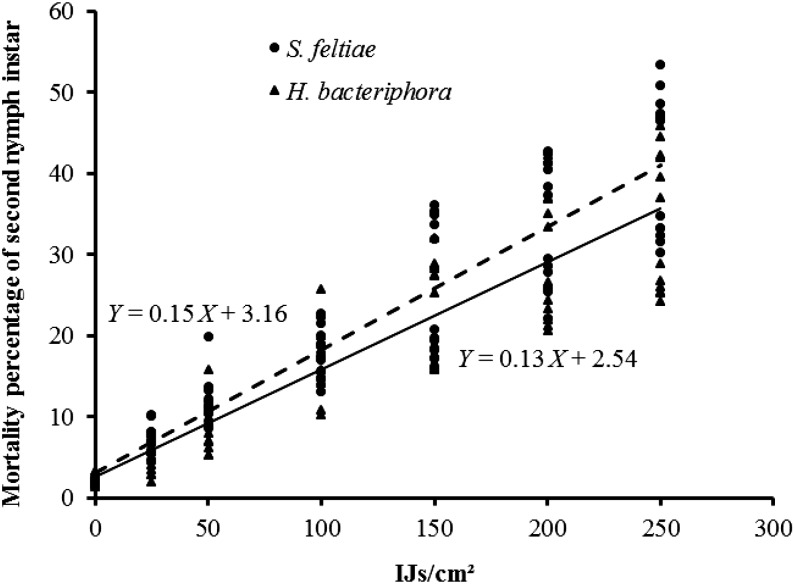
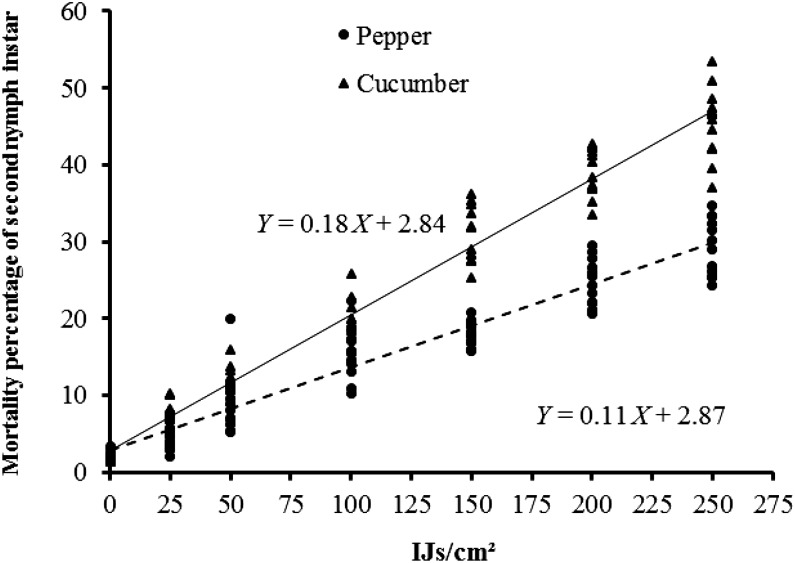
Significant differences in the mortality of second instar nymphs of T. vaporariorum were observed for nematode concentration (F6,139 = 906.26, P < 0.01), EPN species (F1,139 = 68.1, P < 0.01), and host plant (F1,139 = 2.7, P < 0.01). Moreover, the interactions between EPN species × nematode concentration (F6,139 = 5.98, P < 0.01), between nematodes concentration × host plants were significant (F6,139 = 56.22, P < 0.01), and the triple interaction EPN species × concentration × plant (F6,139 = 2.7, P < 0.05) produced statistically significant results (Fig. 1). Therefore, when EPN species and concentrations were considered alone, it was observed that S. feltiae, represented by a regression line with a higher slope and intercept (R2 = 0.85, P < 0.01) than that for H. bacteriophora (R2 = 0.82, P < 0.01) (Fig. 2), was able to kill the same number of second instar nymphs with fewer IJ than those required for H. bacteriophora, and that this mortality significantly increased as concentration increased. Similarly, the application of increasing EPN concentrations produced higher mortality rates on the cucumber plant (R2 = 0.96, P < 0.01) than on the pepper plant (R2 = 0.91) (Fig. 3).
Fig. 1.
Mortality of the second nymphal instar of Trialeurodes vaporariorum after exposition to six concentrations of the EPN species Steinernema feltiae and Heterorhabditis bacteriophora on cucumber and pepper plants. Columns are average of the mortality of second nymphal instar and bars standard error (±SEM) of the mean. Different letters indicate significant difference among combination treatments (nematode species × nematode concentration × host plant type) based on LSD test (P ≤ 0.05). LSD, least significant difference.
Fig. 2.
Linear regression of mean percentage mortality (%) of second nymphal instar of Trialeurodes vaporariorum caused by entomopathogenic nematode (EPN) species Heterorhabditis bacteriophora and Steinernema feltiae at concentrations of 25, 50, 100, 150, 200, and 250 IJ per cm2.
Fig. 3.
Linear regression of mean percentage mortality (%) of second nymphal instar of T. vaporariorum caused by two EPN species at concentrations of 25, 50, 100, 150, 200, and 250 IJ per cm2 applied to two different host plant.
Discussion
Steinernema feltiae and H. bacteriophora are biocontrol agents in commercial use to repress a variety of economically important insect pests (Williams and Walters, 2000; Georgis et al., 2006; Laznik and Trdan, 2014). The results presented herein indicated that EPN may be suitable for use as a foliar application against T. vaporariorum. Although the relatively small size of this insect might be considered as non-optimal for EPN development, and hence a barrier to recycling in the field, as recently evaluated by Bastidas et al. (2014). Some examples have illustrated the potential of EPN as biocontrols for these insects. For example, Hara et al. (1993) reported that the foliar application of some EPN species, including S. feltiae, against the leafminer Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) was viable and can be part of pest control tactics used in greenhouses. There are further examples of satisfactory EPN application against flies in the families of Sciaridae and Phoridae (Jess et al., 2005), leafminers on vegetables (Williams and Walters, 2000), application of high doses of nematodes against F. occidentalis (Thysanoptera: Thripidae) (Ebssa et al., 2001, 2004, 2006), and B. tabaci (Hemiptera: Aleyrodidae) (Cuthbertson et al., 2003a).
This study illustrated how the second instar nymphs and adults of T. vaporariorum were susceptible to EPN attack. Nymphs were more susceptible than adults to EPN activity, S. feltiae being the nematode that provided the lowest LC50 for both instars. Laznik et al. (2011) found similar superior efficacy of S. feltiae against the adult insect. This is more likely due to the immobility of the second instar nymphs attached to the leaves that, consequently, increased the likelihood of EPN contact and penetration. In contrast, adults are capable of flight and could get away from the nematode-treated area; therefore, they were less accessible. These findings are in agreement with those of Cuthbertson et al. (2003a), who reported that the application of S. feltiae (160 IJ/cm2) significantly reduced B. tabaci survival; in their study, the second instar nymphs were most susceptible on both tomato and verbena leaves. Similarly, Qiu et al. (2008) reported that the pathogenicity of S. feltiae differed significantly among B. tabaci instars, and the second instar nymphs suffered the highest mortalities on collard, hibiscus, and cucumber plants. As expected, the mortality of both stages of T. vaporariorum increased with increases in EPN concentrations from 25 to 250 IJ/cm2. Cuthbertson et al. (2007) reported similar evidence about the effects of IJ concentration on the efficiency of S. feltiae in controlling the sweet potato whitefly, B. tabaci. They showed that B. tabaci mortality increased greatly with increasing IJ concentrations, increasing up to 80% after being treated with 10,000 IJ/ml under greenhouse conditions.
The differences between the two EPN species were recorded both in laboratory and greenhouse experiments, with the nematode S. feltiae showing the best results for controlling both instars and in both host plants. Differences in the efficacy of EPN species could be related to the foraging strategy of IJ and their tolerance to environmental fluctuations. In the current study, due to the immobility of the second instar nymph and the mobility of the adult, S. feltiae could eventually use this intermediate behavior as an advantage compared with the typical “cruiser” behavior of the H. bacteriophora. Moreover, the reports showed that nematode species of Steinernematidae tend to be more tolerant of environmental stress than Heterorhabditidae (Grewal, 2000, 2002).
From the plant-host perspective, it was observed in greenhouse trials that the mortality of second nymphal instars was higher on the hairy leaf of the cucumber plant than on the glabrous leaf of the pepper. Previous studies have stated that trichomes over leaf surfaces increase host plant resistance against high evaporation and transpiration (Woodman and Fernandes, 1991). Probably, the high density of hair on the leaf surface of the cucumber increases humidity, critical for enhancing EPN survival. These observations are in agreement with those of Head et al. (2004), who described the higher mortality of B. tabaci caused by S. feltiae on hairy-leaf plants (tomato, cucumber, chrysanthemums, and verbena) than the glabrous leaf plants (poinsettia). Similarly, Head et al. (2004) reported poor efficiency of S. feltiae on poinsettia, probably due to the reduced humidity and nematode movement. However, our findings are in contrast with those of Qiu et al. (2008), who reported that the mortality of B. tabaci was higher on glabrous leaf plants (hibiscus and collard) than on hairy leaf species (cucumber). Perhaps the high density of hair on the leaf surface of the cucumber impedes the movement of nematodes when searching for hosts, this may be a limitation factor for cruiser species including H. bacteriophora. Overall, as Cuthbertson et al. (2007) mentioned suitable conditions when using the EPN are critical factors which allow them to overcome different leaf surface structures.
Cadavers of T. vaporariorum showed discoloration symptoms of infection, the typical phenotype of both physical injury and virulence of symbiotic bacteria. It was noteworthy that the nematodes were dead inside the second instar nymphs, which also confirmed the inability of EPN to reproduce inside. The EPN non-survival and inability to reproduce within the body of the second nymphal instars may be due to insect size (Tomalak, 1994; Shapiro and Lewis, 1999; Ebssa et al., 2001; Bastidas et al., 2014). In general, EPN are considered unable to reproduce in small insects (English-Loeb et al., 1999; Bastidas et al., 2014). However, this inability of reproduction would affect the recycling in the field, but not the direct control of the pest, so our finding still supports their use against this pest.
Foliar application decreases EPN activity due to desiccation, fluctuating temperature, and ultraviolet light (Grewal, 2002). Some adjuvants have been reported to increase the efficiency of EPN; it seems that spraying adjuvants increases adhesion and dispersal of the nematode suspension on the leaf surface (Qui et al., 2008; Hussein et al., 2012; De Waal et al., 2013). In this study, we employed Triton X-100 as the adjuvant to enhance EPN activity, although further investigation might evaluate other products and combinations to enhance the final EPN activity under selected greenhouse conditions.
Our results provide evidence that S. feltiae and H. bacteriophora have the potential to reduce the survival of T. vaporariorum. Ongoing studies indicate that combinations of this EPN with other biological control agents or adjuvants and insecticide may improve them for use in T. vaporariorum management programs (Karimi et al., unpubl. data), as it was suggested by Cuthbertson et al. (2008). Compatability of the both S. feltiae and H. bacteriophora nematodes with other control methods (i.e., azadirachtin and pirimicarp) could provide more chance for successful control of insects in an IPM program. The study reported herein establishes the basis for further applied studies to search for elaborate, new, environmentally compatible strategies that allow the enhancement of pest control and crop health in greenhouse productions.
Literature Cited
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Arthurs S, Heinz KM, Prasifka JR. An analysis of using entomopathogenic nematodes against above-ground pests. Bulletin of Entomological Research. 2004;94:297–306. doi: 10.1079/ber2003309. [DOI] [PubMed] [Google Scholar]
- Bastidas B, Edgar P, San-Blas E. Size does matter: The life cycle of Steinernema spp. in micro-insect hosts. Journal of Invertebrate Pathology. 2014;121:46–55. doi: 10.1016/j.jip.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Bi JL, Toscano NC, Ballmer GR. Greenhouse and field evaluation of six novel insecticides against the greenhouse whitefly Trialeurodes vaporariorum on strawberries. Crop Protection. 2002;21:49–55. [Google Scholar]
- Boemare N. 2002. Biology, taxomony and systematics of Xenorhabdus and Photorhabdus. Pp. 35–56 in R. Gaugler, ed. Entomopathogenic Nematology. Wallinford, UK: CABI Publishing.
- Bonato O, Couton L, Fargues J. Feeding Preference of Macrolophus caliginosus (Heteroptera: Miridae) on Bemisia tabaci and Trialeurodes vaporariorum (Homoptera: Aleyrodidae) Journal of Economic Entomology. 2006;99:1143–1151. doi: 10.1603/0022-0493-99.4.1143. [DOI] [PubMed] [Google Scholar]
- Campbell JF, Gaugler R. Nictation behaviour and its ecological implications in the host search strategies of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) Behaviour. 1993;126:155–169. [Google Scholar]
- Choi WI, Lee EH, Choi BR, Park HM, Ahn YJ. Toxicity of plant essential oils to Trialeurodes vaporariorum (Homoptera: Aleyrodidae) Journal of Economic Entomology. 2003;96:1479–1484. doi: 10.1603/0022-0493-96.5.1479. [DOI] [PubMed] [Google Scholar]
- Cuthbertson A, Walters K, Northing P, Luo W. Efficacy of the entomopathogenic nematode, Steinernema feltiae, against sweetpotato whitefly Bemisia tabaci (Homoptera: Aleyrodidae) under laboratory and glasshouse conditions. Bulletin of Entomological Research. 2007;97:9–14. doi: 10.1017/S0007485307004701. [DOI] [PubMed] [Google Scholar]
- Cuthbertson AG, Head JK, Walters F, Gregory SA. The efficacy of the entomopathogenic nematode, Steinernema feltiae, against the immature stages of Bemisia tabaci. Journal of Invertebrate Pathology. 2003a;83:267–269. doi: 10.1016/s0022-2011(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Cuthbertson AG, Head JK, Walters F, Murray AW. The integrated use of chemical insecticides and the entomopathogenic nematode, Steinernema feltiae, for the control of sweetpotato whitefly, Bemisia tabaci. Nematology. 2003b;5:713–720. [Google Scholar]
- Cuthbertson AG, Mathers JJ, Northing P, Prickett AJ, Walters KF. The integrated use of chemical insecticides and the entomopathogenic nematode, Steinernema carpocapsae (Nematoda: Steinernematidae), for the control of sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) Insect Science. 2008;15:447–453. [Google Scholar]
- Cuthbertson A, North J, Walters K. Effect of temperature and host plant leaf morphology on the efficacy of two entomopathogenic biocontrol agents of Thrips palmi (Thysanoptera: Thripidae) Bulletin of Entomological Research. 2005;95:321–327. doi: 10.1079/ber2005363. [DOI] [PubMed] [Google Scholar]
- Cuthbertson AGS, Walters KFA. Evaluation of exposure time of Steinernema feltiae against second instar Bemisia tabaci. Tests of Agrochemicals and Cultivars. 2005;26:34–35. [Google Scholar]
- De Waal JY, Malan AP, Addison MF. Effect of humidity and a superabsorbent polymer formulation on the efficacy of Heterorhabditis zealandica (Rhabditida: Heterorhabditidae) to control codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae) Biocontrol Science and Technology. 2013;23:62–78. [Google Scholar]
- Ebssa L, Borgemeister C, Berndt O, Poehling HM. Impact of entomopathogenic nematodes on different soil-dwelling stages of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), in the laboratory and under semi-field conditions. Biocontrol Science and Technology. 2001;11:515–525. [Google Scholar]
- Ebssa L, Borgemeister C, Poehling HM. Effectiveness of different species/strains of entomopathogenic nematodes for control of western flower thrips (Frankliniella occidentalis) at various concentrations, host densities, and temperatures. Biological Control. 2004;29:145–154. [Google Scholar]
- Ebssa L, Borgemeister C, Poehling HM. Simultaneous application of entomopathogenic nematodes and predatory mites to control western flower thrips Frankliniella occidentalis. Biological Control. 2006;39:66–74. [Google Scholar]
- English-Loeb G, Villani M, Martinson T, Forsline A, Consolie N. Use of entomopathogenic nematodes for control of grape phylloxera (Homoptera: Phylloxeridae): A laboratory evaluation. Environmental Entomology. 1999;28:890–894. [Google Scholar]
- Georgis R, Koppenhöfer A, Lacey L, Bélair G, Duncan L, Grewal P, Samish M, Tan L, Torr P, Van Tol R. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123. [Google Scholar]
- Glazer I, Lewis EE. 2000. Bioassays of entomopathogenic nematodes. Pp. 229–247 in A. Navon, and K. R. S. Ascher, eds. Bioassays of entomopathogenic microbes and nematodes. New York: CABI Publishing.
- Grewal P. Anhydrobiotic potential and long-term storage of entomopathogenic nematodes (Rhabditida: Steinernematidae) International Journal for Parasitology. 2000;30:995–1000. doi: 10.1016/s0020-7519(00)00080-1. [DOI] [PubMed] [Google Scholar]
- Grewal PS. 2002. Formulation and application technology. Pp. 265–288 in R. Gaugler, ed. Entomopathogenic nematology. Wallinford, UK: CABI Publishing.
- Grewal PS, Ehlers RU, Shapiro DI. 2005. Nematodes as Biocontrol Agents. New York: CABI Publishing.
- Hamdi F, Fargues J, Ridray G, Jeannequin B, Bonato O. Compatibility among entomopathogenic hyphocreales and two beneficial insects used to control Trialeurodes vaporariorum (Hemiptera: Aleurodidae) in Mediterranean greenhouses. Journal of Invertebrate Pathology. 2011;108:22–29. doi: 10.1016/j.jip.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Hara A, Kaya H, Gaugler R, Lebeck L, Mello C. Entomopathogenic nematodes for biological control of the leafminer, Liriomyza trifolii (Dipt.: Agromyzidae) Entomophaga. 1993;38:359–369. [Google Scholar]
- Head J, Lawrence A, Walters K. Efficacy of the entomopathogenic nematode, Steinernema feltiae, against Bemisia tabaci in relation to plant species. Journal of Applied Entomology. 2004;128:543–547. [Google Scholar]
- Hussein HM, Adel MM, Gelbič I. Effectiveness of the entomopathogenic nematode Steinernema feltiae in agar gel formulations against larvae of the Colorado potato beetle, Leptinotarsa decemlineata (Say.)(Coleoptera: Chrysomelidae) Central European Journal of Biology. 2012;7:77–82. [Google Scholar]
- Jess S, Schweizer H, Kilpatrick M. 2005. Mushroom applications. Pp. 191–213 in P. S. Grewal, R. U. Ehlers, and D. I. Shapiro, eds. Nematodes as biocontrol agents. New York: CABI Publishing.
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock S. 1997. Techniques in insect nematology. Pp. 281–284 in L. A. Lacey, ed. Manual of techniques in insect pathology, vol. 1. San Diego: Academic Press.
- Laznik Z, Toth T, Lakatos T, Vidrih M, Trdan S. Control of the Colorado potato beetle (Leptinotarsa decemlineata [Say]) on potato under field conditions: a comparison of the efficacy of foliar application of two strains of Steinernema feltiae (Filipjev) and spraying with thiametoxam. Journal of Plant Diseases and Protection. 2010;117:129–135. [Google Scholar]
- Laznik Z, Trdan S. The influence of insecticides on the viability of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under laboratory conditions. Pest Management Science. 2014;70:784–789. doi: 10.1002/ps.3614. [DOI] [PubMed] [Google Scholar]
- Laznik Ž, Žnidarčič D, Trdan S. Control of Trialeurodes vaporariorum (Westwood) adults on glasshouse-grown cucumbers in four different growth substrates: An efficacy comparision of foliar application of Steinernema feltiae (Filipjev) and spraying with thiamethoxam. Turkish Journal of Agriculture and Forestry. 2011;35:631–640. [Google Scholar]
- Lewis EE, Gaugler R, Harrison R. Response of cruiser and ambusher entomopathogenic nematodes (Steinernematidae) to host volatile cues. Canadian Journal of Zoology. 1993;71:765–769. [Google Scholar]
- Lewis EE. 2002. Behavioural ecology. Pp. 205–224 in R. Gaugler, ed. Entomopathogenic nematology. New York: CABI Publishing.
- Lucas E, Labrecque C, Coderre D. Delphastus catalinae and Coleomegilla maculata lengi (Coleoptera: Coccinellidae) as biological control agents of the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae) Pest Management Science. 2004;60:1073–1078. doi: 10.1002/ps.916. [DOI] [PubMed] [Google Scholar]
- Manzano MR, van Lenteren JC. Life history parameters of Trialeurodes vaporariorum (Westwood)(Hemiptera: Aleyrodidae) at different environmental conditions on two bean cultivars. Neotropical Entomology. 2009;38:452–458. doi: 10.1590/s1519-566x2009000400002. [DOI] [PubMed] [Google Scholar]
- Poprawski T, Greenberg S, Ciomperlik M. Effect of host plant on Beauveria bassiana-and Paecilomyces fumosoroseus-induced mortality of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) Environmental Entomology. 2000;29:1048–1053. [Google Scholar]
- Qiu BL, Mandour NS, Xu CX, Ren SX. Evaluation of the entomopathogenic nematode Steinernema feltiae as a biological control agent of the whitefly, Bemisia tabaci. International Journal of Pest Management. 2008;54:247–253. [Google Scholar]
- Quesada-Moraga E, Maranhao EAA, Valverde-García P, Santiago-Álvarez C. Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements, and toxicogenic activity. Biological Control. 2006;36:274–287. [Google Scholar]
- SAS institute 2002. SAS Software: Version 9.1 for windows, SAS Institute, Cary, NC.
- Schroer S, Ehlers RU. Foliar application of the entomopathogenic nematode Steinernema carpocapsae for biological control of diamondback moth larvae (Plutella xylostella) Biological Control. 2005;33:81–86. [Google Scholar]
- Scorsetti AC, Humber RA, Gregorio C, Lastra CCL. New records of entomopathogenic fungi infecting Bemisia tabaci and Trialeurodes vaporariorum, pests of horticultural crops, in Argentina. BioControl. 2008;53:787–796. [Google Scholar]
- Shapiro D, Lewis EE. Infectivity of entomopathogenic nematodes from cadavers vs. aqueous applications. Environmental Entomology. 1999;28:907–911. [Google Scholar]
- Tomalak M. 1994. Genetic improvement of Steinernema feltiae for integrated control of the western flower thrips Frankliniella occidentalis. Bulletin OILB SROP (France).
- White G. A method for obtaining infective nematode larvae from cultures. Science. 1927;66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]
- Williams E, Walters K. Foliar application of the entomopathogenic nematode Steinernema feltiae against leafminers on vegetables. Biocontrol Science and Technology. 2000;10:61–70. [Google Scholar]
- Wilson MJ, Ehlers R, Glazer I. Entomopathogenic nematode foraging strategies – is Steinernema carpocapsae really an ambush forager? Nematology. 2012;14:389–394. [Google Scholar]
- Woodman RL, Fernandes GW. 1991 Differential mechanical defense: herbivory, evapotranspiration, and leaf-hairs Oikos 11–19. [Google Scholar]