Abstract
The host suitability of five zucchini and three cucumber genotypes to Meloidogyne incognita (MiPM26) and M. javanica (Mj05) was determined in pot experiments in a greenhouse. The number of egg masses (EM) did not differ among the genotypes of zucchini or cucumber, but the eggs/plant and reproduction factor (Rf) did slightly. M. incognita MiPM26 showed lower EM, eggs/plant, and Rf than M. javanica Mj05. Examination of the zucchini galls for nematode postinfection development revealed unsuitable conditions for M. incognita MiPM26 as only 22% of the females produced EM compared to 95% of the M. javanica females. As far as cucumber was concerned, 86% of the M. incognita and 99% of the M. javanica females produced EM, respectively. In a second type of experiments, several populations of M. arenaria, M. incognita, and M. javanica were tested on zucchini cv. Amalthee and cucumber cv. Dasher II to assess the parasitic variation among species and populations of Meloidogyne. A greater parasitic variation was observed in zucchini than cucumber. Zucchini responded as a poor host for M. incognita MiPM26, MiAL09, and MiAL48, but as a good host for MiAL10 and MiAL15. Intraspecific variation was not observed among the M. javanica or M. arenaria populations. Cucumber was a good host for all the tested populations. Overall, both cucurbits were suitable hosts for Meloidogyne but zucchini was a poorer host than the cucumber.
Keywords: Cucumis sativus, Cucurbita pepo, infection, parasitic variation, reproduction, root-knot nematodes
Root-knot nematodes (RKN), Meloidogyne spp., are important nematode pests for vegetable production in temperate, subtropical, and tropical regions (Sikora and Fernández, 2005). Vegetables are grown under protected cultivation in many areas and nematode management in these systems is a major challenge due to crop intensity, short fallowing, and environmental conditions that favor pest and disease development. Cucurbits such as zucchini and cucumber are frequently rotated with tomatoes and peppers in double cropping systems in plastic houses in Spain (Meneses and Castilla, 2009), and thus, management actions taken in a crop may affect the subsequent crop in the rotation (Westphal, 2011). Information on the host suitability of rotational crops is useful because RKN population densities rapidly build up on susceptible crops under the favorable conditions prevailing in plastic houses. Cultivation of less suitable or poor/resistant host crops would benefit the following crop in the rotation as poor hosts are less likely to be damaged than good hosts since invasion, root damage, and nematode reproduction are reduced (Ehwaeti et al., 1999). Lower RKN levels were recorded in cucurbits compared with solanaceous crops (Verdejo-Lucas et al., 2002; Talavera et al., 2012) pointing to different host suitability among these crops. Host range studies have shown that some crop cultivars differ in suitability to RKN (Fourie et al., 2012; Maleita et al., 2012) which also vary with the RKN species or populations. For example, bean and pea cultivars were good hosts for M. hapla and M. chitwoodi but the reproduction factor (Rf, final/initial population density) of M. hapla was greater than that of M. chitwoodi in some of the tested cultivars (Santo and Ponti, 1985). Cultivars of celery and carrot showed varying degrees of host suitability due to the parasitic variation in M. hapla (Melakeberhan and Wang, 2012, 2013). Extensive studies have been conducted on the suitability of watermelon germplasm (Thies and Levi, 2003, 2007) but those on current cultivars of other cucurbits are limited (Edelstein et al., 2010; Mukhtar et al., 2013).
Assessment of RKN reproduction on cucurbits may discriminate tolerant genotypes, even if resistance genes are lacking, as the genetic background of the plants can provide superior vigor, extensive root systems, or tolerance to environmental stresses (i.e., salinity) in the production area. It is generally accepted that cucurbit crops, such as cucumber, melon, zucchini, and watermelon, are susceptible to the most widespread RKN, M. arenaria, M. incognita, and M. javanica (Sikora and Fernández, 2005), although resistance has been found in some Cucumis species (Fassuliotis, 1970; Walters et al., 2006; Faske, 2013). In the context of this study, host suitability refers to the ability of a host plant to reproduce the nematode and it is measured as Rf. Therefore, good hosts show high Rf values whereas poor hosts often show low Rf.
Zucchini and cucumber are cultivated around the world in a variety of environmental conditions and are common ingredients in the daily diet in many countries. In Spain, around 8000 ha zucchini and cucumber are cropped annually of which 70% and 89%, respectively, are grown under protected cultivation (Anonymous, 2011). The economic losses due to RKN on zucchini and cucumber in southeastern Spain were estimated in €640500 and €918300, respectively, in 2010 (Talavera et al., 2012).
This study was conducted to determine i) the host suitability of zucchini and cucumber genotypes to M. incognita and M. javanica, and ii) the parasitic variation of populations of M. arenaria, M. incognita, and M. javanica on these crops.
Materials and Methods
Host plants:
Five genotypes of zucchini (Cucurbita pepo L.) and three of cucumber (Cucumis sativus L.) were selected for the study. The zucchini genotypes represented cultivars that produced fruits of different shapes and colors, and included cv. Amalthee (long light green), cv. Parador (long yellow), cv. Pixar (long green), cv. Floridor (round yellow), and cv. Satelite (round deep green). Cucumber genotypes represented cultivars that produced fruits of different length, cv. Taray (long), cv. Dasher II (medium long), and cv. Urano (short). Zucchini and cucumber seeds were soaked overnight and germinated in seed trays with vermiculite. When the first true leaf was fully expanded, the seedlings were transplanted to Styrofoam pots filled with 500 cm3 of sterilized river sand. Plants were arranged randomly on a greenhouse bench and allowed to grow for 1 wk before nematode inoculation. They were watered daily as needed, and fertilized with a slow-release fertilizer (Osmocote® Scotts Company, Heerlen, Netherlands; 15% N + 10% P2O5 + 12% K2O + 2% MgO2 + microelements) at the beginning of the experiments.
Host suitability:
The suitability of selected genotypes of zucchini and cucumber to M. incognita MiPM26 and M. javanica Mj05 was determined in two experiments conducted in a greenhouse. The RKN isolates, collected from infested tomato roots (Ornat et al., 2001), were established as single egg mass (EM) cultures and maintained on susceptible tomato cv. Roma in spring-summer and on celery cv. D´Elne in autumn-winter in a greenhouse. The nematode was multiplied on tomato cv. Roma to obtain the inoculum for the experiments. Eggs were extracted by blender maceration of infected roots in a 0.5% NaOCl solution for 5 min (Hussey and Barker, 1973). The egg suspension was passed through a 74-µm aperture sieve to remove root debris, and the dispersed eggs were collected on a 25-µm sieve, counted, and used as inoculum. Plants were inoculated with 4,000 eggs by adding aliquots of the respective RKN isolates into two holes made in the soil 3-cm apart from the base of the plant. Non-inoculated plants, included as controls for reference, received the same volume of water. Each treatment (genotype-RKN isolate) was replicated seven times. The hatching rate of the egg inoculum was determined by placing aliquots of egg suspension on three replicated Baermann trays that were incubated at 26 ± 1°C in darkness for 21 d. Emerged second-stage juveniles (J2) were collected once a week, stored at 4°C until counted, and the sum of emerged J2 was used to calculate the hatching rate (%). The egg inoculum was converted to the number of emerged J2 and considered as the Pi for the experiments. Soil temperatures were recorded daily at 30-min intervals with temperature probes (Em50 Data Logger®, Decagon Devices Inc., Pullman, WA, accuracy ± 1°C, resolution 0.1°C) inserted into the pots.
The experiment (Exp. 1) was terminated 65 d after nematode inoculation allowing the nematode to complete one generation. Tops were cut at ground level and their fresh weight determined. Root systems were washed free of soil, weighed, and stained in a 0.1 g/liter erioglaucine solution (Aldrich Chemical Company, St. Louis, MO) for 2 hr (Omwega et al., 1988), then washed in tap water, and the number of EM per plant counted as an indication of nematode infectivity. The infection frequency was calculated by dividing the number of EM by the J2 inoculum × 100. Eggs were extracted from 3-g root subsamples for 10 min as previously described (Hussey and Barker, 1973), to determine the final population densities (Pf) that were expressed as eggs/plant. Host-suitability was based on Rf calculated by dividing the number of eggs/plant (Pf) by the number of emerged J2 (Pi). To determine the fecundity of the females, five EM from each genotype of zucchini and cucumber × RKN combination were hand-picked and placed individually into Eppendorf tubes. The eggs were dispersed in a 0.5% NaOCl solution for 10 min, as described previously, and counted.
Exp. 2 is a repetition of Exp. 1 with similar experimental conditions, plant maintenance and nematode assessments but was run for 74 d.
Postinfection development:
To assess the RKN developmental stages inside the galls, a random sample of about 100 galls per treatment was dissected under a stereoscopic microscope. Root samples had been previously stained with acid fuchsine 0.05% (Bridge and Page, 1982), cleared in acidified glycerol, and stored until the developmental stages were categorized as females with and without EM, distorted females, males, and juvenile stages (J3 and J4). The distorted females were small with an abnormal sausage-like shape with no EM as opposed to the globose pear shape of the females with EM.
Parasitic variation in Meloidogyne:
Thirteen RKN populations were tested on zucchini cv. Amalthee, and five on cucumber cv. Dasher II. These populations had been previously characterized for their Mi-gene virulence status (Ornat et al., 2001, Verdejo-Lucas et al., 2012), and were maintained as described previously. The identity of the populations was verified according to their enzymatic and molecular patterns (Esbenshade and Triantaphyllou, 1990; Zijlstra et al., 2000). They included the three most common species, M. arenaria, M. incognita, and M. javanica, in plastic greenhouses in southern Spain (Verdejo-Lucas et al., 2002; Talavera et al., 2012). Plants were inoculated with 500 freshly hatched J2 (less than 72-hr old) and each treatment was replicated seven times. The experiment was conducted twice. Plants were harvested at 56 and 58 d after nematode inoculation. The root systems were washed free of soil, weighed, and stained in a 0.1-g/liter erioglaucine solution. The number of galls per root system with and without EM was counted. The infection frequency was calculated as described previously.
Statistical analyses:
The SAS system V8 (SAS Institute Inc., Cary, NC) was used for statistical analyses. Analysis of variance was carried out by the general lineal model (Proc GLM). Normality was checked by the Shapiro-Wilk test and homogeneity of variances by the Levene’s test. Data were transformed to (log x+1) when needed to improve homogeneity of error variances prior to the analyses. The experiments on host suitability were analyzed separately due to differences in egg hatching rate of the nematode inoculum. The response of the genotypes to each RKN isolate was compared and Tukey’s honestly significant difference (HSD) test used to separate means (P < 0.05). Comparisons between RKN isolates on individual genotypes and between cucurbit crops for individual populations were done by Student’s t-test (P < 0.05). The experiments on parasitic variation were analyzed together since no differences were found between the repeated experiments. The RKN populations were compared within each cucurbit crop and means separated by Tukey’s HSD test (P < 0.05).
Results
Host suitability:
Inoculated and noninoculated zucchini plants showed similar fresh top weight (data not shown), indicating that RKN infection and reproduction was not associated with plant damage after a single nematode generation. The hatching rate of the egg inoculum in Exp. 1 was 14% and 21% for M. incognita MiPM26 and M. javanica Mj05, respectively, which provided statistically different Pi values of 560 and 840 J2 per plant. The hatching rate in Exp. 2, 26.4% and 27.5% for M. incognita and M. javanica, respectively, provided similar Pi values of 1055 and 1099 J2 per plant, respectively. Nevertheless, the numbers of EM were similar statistically, although numerically different, among zucchini genotypes within RKN isolate and experiment (Table 1). On average, M. incognita showed similar infection frequency (%) in both experiments (8.6 ± 0.5 and 8.7 ± 0.5 in Exp. 1 and 2, respectively) despite differences in J2 inoculum as also did M. javanica (65 ± 3 and 72 ± 4 in Exp. 1 and 2, respectively). Zucchini genotypes differed in eggs/plant and Rf (Table 1), and the general trend in both experiments was that Amalthee supported less M. incognita eggs/plant than Satelite, whereas Parador, Pixar, and Floridor provided intermediate values although statistical differences were only shown in Exp. 2. The Rf of M. incognita on Amalthee and Pixar was lower (P < 0.05) than on Satelite. The M. javanica eggs/plant were lower (P < 0.05) on Amalthee, Parador, and Pixar than on Floridor and Satelite in Exp. 2 (Table 1), and the Rf was lower (P < 0.05) on Amalthee than Parador, Pixar, or Floridor in Exp. 1, but only Amalthee differed from Floridor in Exp. 2 (Table 1). In relation to the RKN isolates, all reproductive traits of M. incognita MiPM26 were lower (P < 0.05) than those of M. javanica Mj05 in both experiments (Table 1). Female fecundity did not differ among the zucchini genotypes but M. incognita (253 ± 6 eggs/EM, mean ± standard error) showed lower (P < 0.05) fecundity than the M. javanica (538 ± 23 eggs/EM).
Table 1.
Root weight, number of egg masses, eggs per plant and reproduction factor (Rf) of Meloidogyne incognita (MiPM26) and Meloidogyne javanica (Mj05) on genotypes of zucchini (Cucurbita pepo) and cucumber (Cucumis sativus) in pot experiments (Exp.) conducted in a greenhouse for 65 and 74 d in Exp. 1 and 2, respectively.
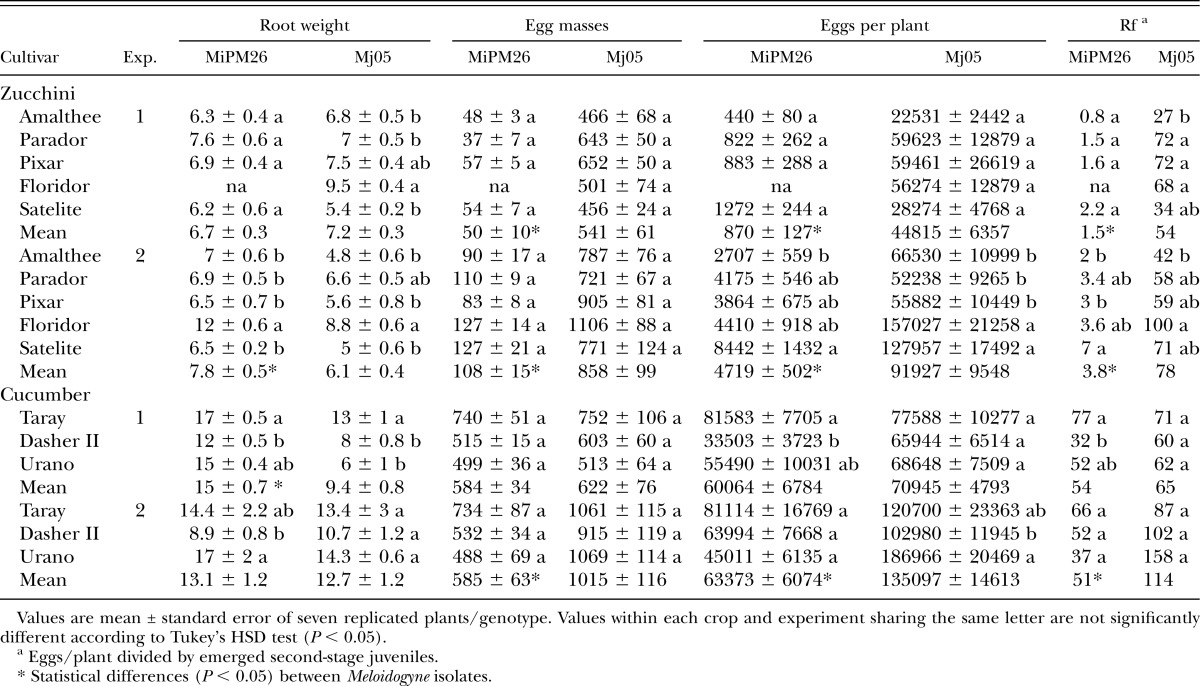
Inoculated and non-inoculated cucumber plants showed similar fresh top weight independently of the genotype. The hatching rate of the egg inoculum was 30.8% and 29.6%, and 26.4% and 27.5%, for M. incognita and M. javanica, in Exp. 1 and 2, respectively, which provided similar Pi values: 1234 and 1184 J2 per plant in Exp. 1, and 1055 and 1099 J2 per plant of M. incognita and M. javanica, respectively, in Exp. 2. The number of EM was similar among cucumber genotypes within RKN isolate in both experiments (Table 1). M. incognita produced less (P < 0.05) eggs/plant on Dasher II than Taray in Exp. 1, whereas M. javanica produced less eggs/plant on Dasher II than Urano in Exp. 2 (Table 1). The Rf of M. incognita on Dasher II was lower (P < 0.05) than that on Taray but only in Exp. 1 (Table 1). Cucumber genotypes did not differ in Rf values when infected by M. javanica. The fecundity of the females in the cucumber plants was similar among genotypes irrespective of the RKN isolate: 568 ± 48 and 553 ± 58 eggs/EM for M. incognita and M. javanica, respectively.
Postinfection development:
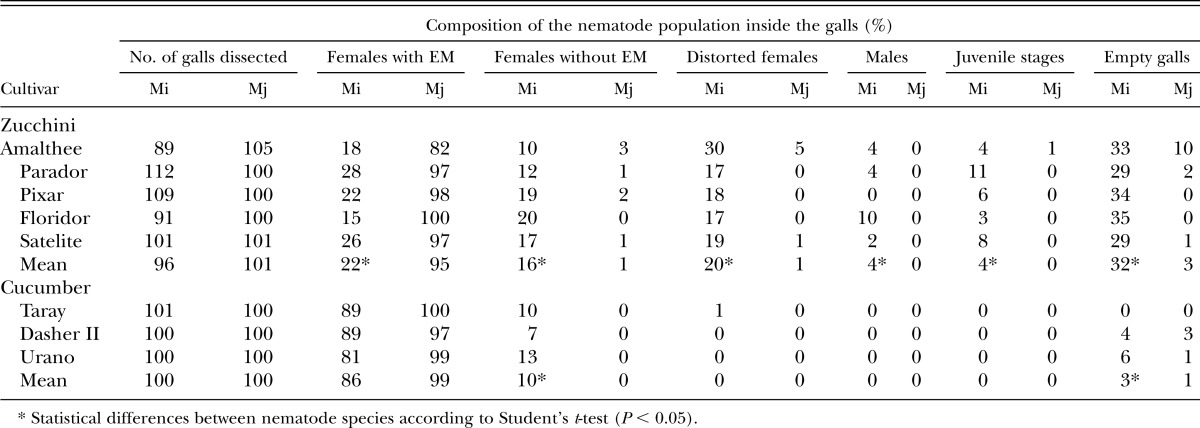
Zucchini galls induced by M. incognita MiPM26 showed both small and large galls. Small galls contained single pear-shaped females with a large EM (22%) exposed on the root surface. Large galls showed hyperplasic and hypertrophic tissue and contained females without EM (16%), distorted females (20%), males (4%), and juvenile stages (4%) or they were empty (32%) (Table 2). M. javanica galls were small, discrete and 82% to 100% contained single pear-shaped females with large EM. Males or juveniles stages were not observed and only 3% of the galls were empty (Table 2). M. incognita females produced fewer (P < 0.05) EM than M. javanica (22% and 95%, respectively) on zucchini (Table 2). Examination of the cucumber galls showed that 86% of the M. incognita females had EM, 10% did not, and 3% of the galls were empty, whereas 99% of the M. javanica galls showed females with EM (Table 2).
Table 2.
Percentage of females with and without egg masses (EM), distorted females, males, juveniles stages (J3 + J4) and empty galls on genotypes of zucchini (Cucurbita pepo) and cucumber (Cucumis sativus) inoculated with 4000 eggs of Meloidogyne incognita MiPM26 (Mi) and M. javanica Mj05 (Mj) in pot experiments conducted in a greenhouse.
Parasitic variation in Meloidogyne:
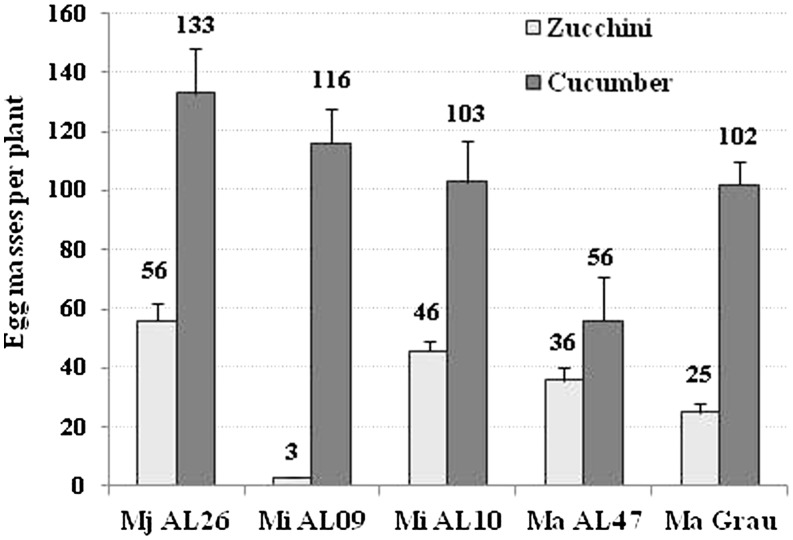
Zucchini showed a large variation in the number of galls (Table 3). As a general trend, the M. javanica populations showed the highest numbers of EM, followed by M. incognita MiAL10 and MiAL15, and those of M. arenaria. However, the exceptions to this trend were M. incognita MiPM26, MiAL09, and MiAL48 that showed high numbers of galls but few EM (Table 3). Cucumber showed a high correspondence between the numbers of galls and EM as more than 75% of the galls had EM. Only M. arenaria MaAL47 showed a lesser (P < 0.05) ability than the remaining populations to form galls and produce EM in the cucumber plants. When populations were grouped by Meloidogyne species (Table 4), M. incognita and M. javanica showed similar gall numbers on zucchini but higher (P < 0.05) than M. arenaria. The number of EM of M. incognita on zucchini was lower (P < 0.05) than that of M. arenaria followed by M. javanica (Table 4). On cucumber, M. incognita and M. javanica showed similar gall and egg mass numbers but M. arenaria produced less (P < 0.05) galls than M. incognita, and fewer (P < 0.05) EM than M. incognita and M. javanica (Table 4). The five individual populations tested on both cucurbit hosts showed lower (P < 0.05) EM numbers on zucchini than cucumber (Fig. 1), which is in support of the differential host status of these cucurbits to Meloidogyne. The average number of EM was 33 ± 3.4 on zucchini, and nearly thrice as many on cucumber (102 ± 12.8 EM).
Table 3.
Total numbers of galls, with and without egg masses (EM) and infection frequency (%) on zucchini cv. Amalthee and cucumber cv. Dasher II inoculated with 500 second-stage juveniles of Meloidogyne arenaria (Ma), M. incognita (Mi), and M. javanica (Mj) in pot experiments conducted in a greenhouse.
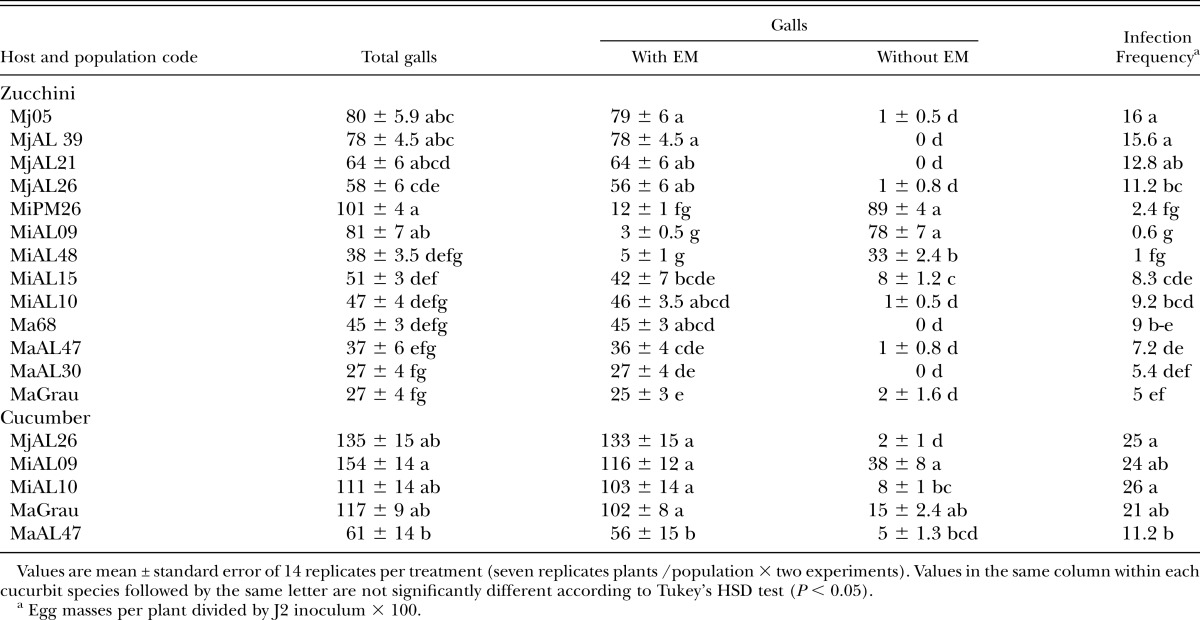
Table 4.
Number of galls and egg masses per plant of Meloidogyne populations grouped by nematode species on zucchini cv. Amalthee and cucumber cv. Dasher II inoculated with 500 second-stage juveniles in pot experiments conducted in a greenhouse.
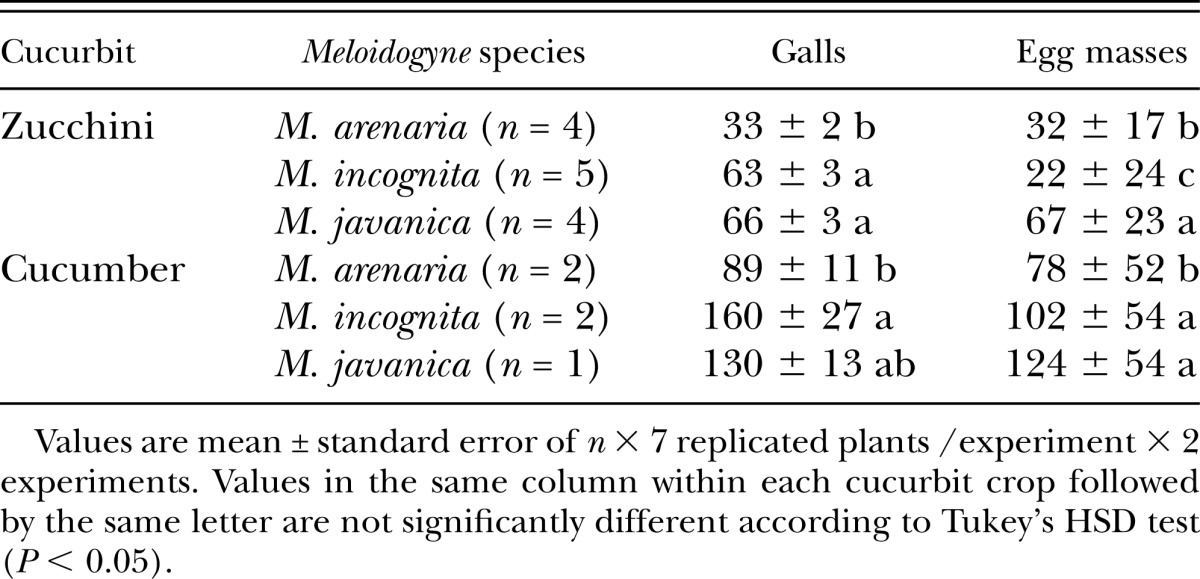
Fig. 1.
Number of egg masses per plant, of Meloidogyne populations tested on both zucchini cv. Amalthee and cucumber cv. Dasher II inoculated with 500 second-stage juveniles in pot experiments conducted in a greenhouse. Bars represent the mean values plus standard error of 14 replicated plants per treatment. Cucurbit hosts differed in number of egg masses according to Student’s t-test (P < 0.05).
Discussion
The interaction between Meloidogyne spp. and two cucurbit crops was investigated in relation to plant genotype and RKN parasitic variation. Differences in host suitability among zucchini genotypes occurred consistently in nematode reproduction (eggs/plant and Rf) but not infection (EM and infection frequency) despite differences in initial J2 inoculum levels. The lack of significant differences in Exp. 1 was probably due to the lower hatching rate of the egg inoculum in this experiment related to Exp. 2 which made any difference among genotypes more difficult to detect. In contrast, the RKN isolate had a striking effect on zucchini revealed by small infection frequency of 8.65% and Rf < 3.8-fold increase for M. incognita MiPM26 compared with a 65% to 72% infection frequency and Rf > 54-fold for M. javanica Mj05. The post-infection exam showed the inability of a high percentage of MiPM26 individuals to reach the egg-laying female stage on zucchini. Because 98% of the inoculated MiPM26 J2 penetrated the zucchini roots and developed into J4 within the first 11 days post-inoculation (López-Gómez and Verdejo-Lucas, 2014), we assumed that no mechanism was preventing root infection but the posterior development of the nematode once the feeding site was established. The slight genetic variability among these genotypes possibly reflects that fruit shape and size, phenotypic characters used for cucurbit domestication, have little effect on the host–nematode interaction.
The emptiness of a third of the galls could be due to crowding of large numbers of invading J2 leading to competition for available feeding sites, which may have stopped nematode development and eventually caused their death or affected their development into males (Stephan and Trudgill, 1982; Faske, 2013). Insufficient nutrient supply by non-fully functional feeding sites may have produced distorted females unable to lay eggs (McClure et al., 1974). Overall, the zucchini genotypes we tested provided unsuitable conditions for MiPM26 development as did Amalthee for MiAL09 and MiAL48 infection. All zucchini genotypes, however, were excellent hosts for Mj05 and all M. javanica populations showed high infectivity on Amalthee. Strawberry genotypes that were susceptible to M. hapla were nonhosts to M. incognita (Edwards et al., 1985). Similarly, soybean genotypes were less suitable hosts to M. incognita than M. arenaria (Kirkpatrick and May, 1989).
The cucumber genotypes we tested showed similar susceptibility levels to both RKN isolates. However, cucumber cultivars grown in the Pothovar region of Pakistan differed in suitability levels to M. incognita with Rf from 0.33- to 10.52-fold increase in response to a 3,000 J2 inoculum (Mukhtar et al., 2013) which point out the genetic variability within this crop.
Parasitic variation was greater on zucchini than cucumber. Thus, the suitability of zucchini varied from being a poor to good host depending on the M. incognita population which suggests that the severity of the disease would change from site to site and site-specific management would be necessary (Melakeberjan et al., 2012; Melakeberjan and Wang, 2012). Intraspecific variation was not observed among the M. javanica or M. arenaria populations on either crop. The reduced parasitic ability of M. arenaria was consistent on both zucchini and cucumber. On cucumber, little parasitic variation was observed, but the high infection and reproduction levels point to the need for nematode management strategies due to the low tolerance limit of cucumber to the nematode (Giné et al., 2014). Cucumber is also a suitable host to M. hapla, M. floridensis, and M. hispanica (Stephan and Trudgill, 1982; Sikora and Fernandez, 2005; Maleita et al., 2012).
Root galling indicates successful establishment of the feeding sites that will allow further nematode development and life cycle completion. However, rating host suitability based on root galling may be misleading (Fassuliotis and Dukes, 1972; Edwards et al., 1985; Fourie et al., 2012; Maleita et al., 2012), as gall formation is not always followed by successful nematode development. This was exemplified by M. incognita on zucchini that produced similar gall numbers but lower EM than M. javanica. Therefore, crop or cultivar recommendation cannot be made based only on the observation of root galling due to the RKN parasitic variation. In general, little host damage has been associated to low nematode reproduction (Ehwaeti et al., 1999), and differences among RKN populations have been observed on poor hosts or resistant genotypes such as pepper, asparagus, tomato, and celery (Khan and Khan, 1991; Dudash and Barker, 1992; Cortada et al., 2009; Melakeberhan and Wang, 2012).
Although the susceptibility of zucchini cv. Amalthee and cucumber Dasher II to RKN had been reported (Coyler et al., 1998; López-Gómez and Verdejo-Lucas, 2014; Giné et al., 2014; Vela et al., 2014), this study showed that zucchini was a poor host for MiPM26 and MiAL09 whereas cucumber was a good one. Also, the infectivity of the RKN populations was less on zucchini (4.8 times) and cucumber (2.5 times) than susceptible tomatoes (Verdejo-Lucas et al., 2012) which support the rotational value of cucurbit crops as contributors to moderate RKN build-up in double cropping systems. Lower remnant populations would remains in the soil after growing cucurbits than solanaceous crops, which concur with field observations (Verdejo-Lucas et al., 2002; Talavera et al., 2012). Additional tests should be done to corroborate the variation in zucchini and cucumber genotypes adapted to protected cultivation.
The size of the M. incognita galls and tissue disturbance on zucchini suggests that damage would be more severe in M. incognita than M. javanica infestations as reported on cucumber and melons (Edelstein et al., 2010) but Pf values will be smaller due to lower EM production. Conversely, higher Pf would be expected in M. javanica- than M. incognita-infested fields. Consequently, it could be argued that it would be more effective to grow zucchini instead of cucumber as a rotation crop in a RKN management program. This choice would be more successful in M. incognita and M. arenaria than M. javanica-infested soils. Populations of M. hapla showed different reproductive potential on celery, carrot, and potato (Melakeberhan and Wang, 2012, 2013; Melakeberhan et al., 2012).
In conclusion, the selected genotypes of both cucurbits were suitable hosts for M. arenaria, M. incognita, and M. javanica but the zucchini was a poorer host than the cucumber regardless of the genotype or RKN population. Postinfection mechanisms involved in the response of zucchini genotypes to M. incognita MiPM26 resulted in reduced egg production, Rf, and female fecundity. The parasitic variation among RKN populations was strongly associated to the host suitability, larger on zucchini than cucumber.
Literature Cited
- Anonymous 2011. MAGRAMA. Estadística agraria. Ministerio de Medio Ambiente, Medio Rural y Marino, Superficies y producciones de cultivos. http://www.magrama.gob.es/es/estadisticas/estadisticas-agrarias/. Accessed March 30, 2014.
- Bridge J, Page LJ. The rice root-knot nematode, Meloidogyne graminicola, on deep water rice (G system Oryza sativa subsp. indica) Revue de Nématologie. 1982;5:225–232. [Google Scholar]
- Cortada L, Sorribas FJ, Ornat C, Andrés MF, Verdejo-Lucas S. Response of tomato rootstocks carrying the Mi-resistance gene to populations of Meloidogyne arenaria, M. incognita and M. javanica. European Journal of Plant Pathology. 2009;124:337–343. [Google Scholar]
- Coyler PD, Kirkpatrick TL, Vernon PR, Barham JD, Baterman RJ. Reducing Meloydogyne incognita injury to cucumber in tomato-cucumber double cropping system. Journal of Nematology. 1998;30:226–231. [PMC free article] [PubMed] [Google Scholar]
- Dudash PJ, Barker KR. Host suitability and response of asparagus cultivars to Meloidogyne species and races. Journal of Nematology. 1992;24:109–116. [PMC free article] [PubMed] [Google Scholar]
- Edelstein M, Oka Y, Burger Y, Eizenberg H, Cohen R. Variation in the response of cucurbits to Meloidogyne incognita and M. javanica. Israel Journal of Plant Sciences. 2010;58:77–84. [Google Scholar]
- Edwards WH, Jones RK, Schmitt DP. Host suitability and parasitism of selected strawberry cultivars by Meloidogyne hapla and M. incognita. Plant Disease. 1985;69:40–42. [Google Scholar]
- Ehwaeti ME, Fargette M, Phillips MS, Trudgill DL. Host status differences and their relevance by Meloidogyne incognita. Nematology. 1999;1:421–432. [Google Scholar]
- Esbenshade PR, Triantaphyllou AC. Isoenzyme phenotypes for identification of Meloidogyne species. Journal of Nematology. 1990;22:10–15. [PMC free article] [PubMed] [Google Scholar]
- Faske TR. Penetration, post-penetration development and reproduction of Meloidogyne incognita on Cucumis melo var. texanus. Journal of Nematology. 2013;45:58–65. [PMC free article] [PubMed] [Google Scholar]
- Fassuliotis G. Resistance of Cucumis spp. to the root-knot nematode, Meloidogyne incognita acrita. Journal of Nematology. 1970;2:174–178. [PMC free article] [PubMed] [Google Scholar]
- Fassuliotis G, Duke PD. Disease reaction of Solanum melongena and S. sisymbriifolium to Meloidogyne incognita and Verticillium albo-atrum. Journal of Nematology. 1972;4:222. [Google Scholar]
- Fourie H, McDonald AH, Mothata TS, Ntidi KN, De Waele D. Indications of variation in host suitability to root-knot nematode populations in commercial tomato varieties. African Journal of Agricultural Research. 2012;7:2344–2352. [Google Scholar]
- Giné A, López-Gómez M, Vela MD, Ornat C, Talavera M, Verdejo-Lucas S, Sorribas FJ. Thermal requirements and population dynamics of root-knot nematodes on cucumber and yield losses under protected cultivation. Plant Pathology. 2014;63:1446–1453. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Khan AA, Khan MW. Suitability of some cultivars of pepper as host for Meloidogyne javanica and races of M. incognita. Nematologia Mediterranea. 1991;19:51–53. [Google Scholar]
- Kirkpatrick TL, May ML. Host suitability of soybean cultivars for Meloidogyne incognita and M. arenaria. Journal of Nematology. 1989;21:666–670. [PMC free article] [PubMed] [Google Scholar]
- López-Gómez M, Verdejo-Lucas S. Penetration and reproduction of root-knot nematodes on cucurbit species. European Journal of Plant Pathology. 2014;138:863–871. [Google Scholar]
- Maleita C, Curtis R, Powers S, Abrantes I. Host status of cultivated plants to Meloidogyne hispanica. European Journal of Plant Pathology. 2012;133:449–460. [Google Scholar]
- McClure MA, Ellis KC, Nigh EL. Resistance of cotton to root-knot nematode, Meloidogyne incognita. Journal of Nematology. 1974;6:17–21. [PMC free article] [PubMed] [Google Scholar]
- Melakeberhan H, Wang W. Suitability of celery cultivars to populations of Meloidogyne hapla. Nematology. 2012;14:623–629. [Google Scholar]
- Melakeberhan H, Wang W. Proof-of-concept for managing Meloidogyne hapla parasitic variability in carrot production soils. Nematology. 2013;15:339–346. [Google Scholar]
- Melakeberhan H, Douches D, Wang W. Interactions of selected potato cultivars and populations of Meloidogyne hapla adapted to the Midwest U.S. soils. Crop Science. 2012;52:1132–1137. [Google Scholar]
- Meneses JF, Castilla N. Protected cultivation in Iberian horticulture. Chronica Horticulturae. 2009;49:37–39. [Google Scholar]
- Mukhtar T, Kayani MZ, Hussain MA. Response of selected cucumber cultivars to Meloidogyne incognita. Crop Protection. 2013;44:13–17. [Google Scholar]
- Omwega C, Thomason IJ, Roberts PA. A non-destructive technique for screening bean germplasm for resistance to Meloidogyne incognita. Plant Disease. 1988;72:970–972. [Google Scholar]
- Ornat C, Verdejo-Lucas S, Sorribas FJ. A population of Meloidogyne javanica in Spain virulent to the resistance gene Mi in tomato. Plant Disease. 2001;85:271–276. doi: 10.1094/PDIS.2001.85.3.271. [DOI] [PubMed] [Google Scholar]
- Santo GS, Ponti RP. Host suitability and reaction of bean and pea cultivars to Meloidogyne chitwoodi and M. hapla. Journal of Nematology. 1985;17:77–79. [PMC free article] [PubMed] [Google Scholar]
- Sikora RA, Fernandez E. 2005. Nematode parasites of vegetables. Pp. 319–392 in M. Luc, R. A. Sikora, and J. Bridge, eds. Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford, UK: CAB International.
- Stephan ZA, Trudgill DL. Development of four populations of Meloidogyne hapla on two cultivars of cucumber at different temperatures. Journal of Nematology. 1982;14:545–549. [PMC free article] [PubMed] [Google Scholar]
- Talavera M, Sayadi S, Chirosa-Ríos M, Salmerón T, Flor Peregrín E, Verdejo-Lucas S. Perception of the impact of root knot nematode induced diseases in horticultural protected crops of south eastern Spain. Nematology. 2012;14:517–527. [Google Scholar]
- Thies JA, Levi A. Resistance of watermelon germplasm to the peanut root knot nematode. HortScience. 2003;38:1417–1421. [Google Scholar]
- Thies JA, Levi A. Characterization of watermelon (Citrullus lanatus var. citroides) germplasm for resistance to root-knot nematodes. HortScience. 2007;42:1530–1533. [Google Scholar]
- Vela MD, Giné A, López-Gómez M, Sorribas FJ, Ornat C, Verdejo-Lucas S, Talavera M. Thermal time requirements of root-knot nematodes on zucchini-squash and population dynamics with associated yield losses on spring and autumn cropping cycles. European Journal Plant Pathology. 2014;140:481–490. [Google Scholar]
- Verdejo-Lucas S, Ornat C, Sorribas FJ, Stchiegel A. Species of root-knot nematodes and fungal egg parasites recovered from vegetables in Almería and Barcelona, Spain. Journal of Nematology. 2002;34:405–408. [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Lucas S, Talavera M, Andrés MF. Virulence response to the Mi.1 gene of Meloidogyne populations from tomato in greenhouses. Crop Protection. 2012;39:97–105. [Google Scholar]
- Walters SA, Wehner TC, Daykin ME, Barker KR. Penetration rates of root-knot nematodes into Cucumis sativus and C. metuliferus roots and subsequent histological changes. Nematropica. 2006;36:231–242. [Google Scholar]
- Westphal A. Sustainable approaches to the management of plant-parasitic nematodes and disease complexes. Journal of Nematology. 2011;43:122–125. [PMC free article] [PubMed] [Google Scholar]
- Zijlstra C, Donkers-Venne DTHM, Fargette M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology. 2000;2:847–853. [Google Scholar]