Abstract
Early development of the mammalian cerebral cortex proceeds via a sequence of proliferative and differentiative steps from neural stem cells toward neurons and glia. However, how these steps are molecularly orchestrated is still only partially understood. In this issue of The EMBO Journal, Artegiani and colleagues implicate Tox, a HMG-box transcription factor previously known only for its role in lymphocyte development, in early cortical development.
See also: B Artegiani et al (April 2015)
One of the most fascinating problems of developmental neuroscience concerns the building of the cerebral cortex, an organ that is at the same time immensely complex as it is highly organized. A plethora of regulatory molecules including transcription factors, epigenetic modifiers, and regulatory RNAs has been identified that orchestrate its intricate development from a simple sheet of neuroepithelial cells to the six-layered cortex made up of neurons and glia of many different flavors (Lui et al, 2011).
This process involves distinct, albeit nested, phases of proliferation and differentiation. Neural stem cells (first in the shape of neuroepithelial cells, then in the disguise of radial glia) must first expand their pool through symmetric non-differentiative divisions. Then, radial glia cells start to divide asymmetrically giving rise to a new radial glial cell (a process termed self-renewal) and offspring that is destined to differentiate but typically will divide once more before generating neurons and therefore are referred to as progenitors (coined intermediate or basal progenitors). Eventually this period of neurogenesis comes to an end and is supplanted by a period of gliogenesis. It is a fundamental goal of research to unravel the molecular players involved in these distinct steps, but due to the fact that stem and progenitor cells as well as early postmitotic neurons do not neatly segregate but intermingle in time and space, this has been, and continues to be, an experimental challenge. To tackle this challenge, the Calegari laboratory developed a mouse line that allows for separating stem cells from progenitors and neurons due to their differential and combinatorial expression of reporter genes (BtgRFP expressed in progenitors fated to become neurons, and Tubb3GFP expressed in postmitotic neurons) (Aprea et al, 2013). With this at hand, Calegari and colleagues applied next-generation RNA sequencing to determine the transcriptomes of these respective cell populations. This revealed two sets of genes with particularly interesting expression dynamics: genes whose expression went up specifically in the progenitor populations undergoing differentiative divisions and decreased again in neurons (coined ‘on-switch’ genes), and genes behaving just the opposite way, that is, markedly diminishing in the progenitor population but increasing again in the neurons. This latter group of ‘off-switch’ genes has been very little studied so far. One gene in particular attracted the interest of the researchers: Thymocyte selection-associated HMG-box protein, or short Tox.
This name already tells us that it is a high-mobility group-containing transcription factor, and hints at its role in the selection of CD4 T lymphocytes (Aliahmad et al, 2012). During T-cell selection, transient Tox expression is induced by calcineurin signaling downstream of T-cell receptor activation (Aliahmad et al, 2004). Prior to the study by Artegiani et al (2015), it was believed that Tox and the other members of the Tox gene family (named Tox 2, 3, and 4) can interact with distorted or curved DNA, but in contrast to other HMG-box proteins such as Sox, transcription factors cannot induce bending of DNA. Moreover, its HMG-box sequence suggested structure-dependent but sequence-independent binding to DNA (O'Flaherty & Kaye, 2003).
With this light provision of prior knowledge, Artegiani et al (2015) went on a tour de force to obtain insights into the mechanisms that regulate the expression of Tox during mouse cortical development, studied its genomewide binding by elegantly adapting the DNA adenine methyltransferase identification (DamID) method for the first time to a transcription factor, identified and subsequently validated Tox putative target genes revealing a Tox DNA binding motif, and assessed its potential functions following enforced expression in the mouse embryonic cortex in vivo.
First, expression analysis confirmed the off-switch Tox expression profile (Fig1): Tox was expressed in Sox2-positive radial glia in the ventricular zone (VZ), but was turned off in the subventricular zone (SVZ), which in the mouse harbors mostly progenitors undergoing differentiative divisions. Tox remained off in the intermediate zone (IZ) that contains young neurons on their way to the emerging cortical plate, and was finally turned on again in neurons upon settling down in the cortical plate. Following previous work on Tox regulation in T lymphocytes, the authors then showed that Tox expression can be regulated by the calcineurin/Nfat signaling pathway suggesting that calcium signaling in radial glia might trigger the onset of Tox expression. Constitutively active Nfat4 causes ectopic expression of Tox in the SVZ suggesting that Nfat signaling must be quickly turned off in differentiative progenitors.
Figure 1.
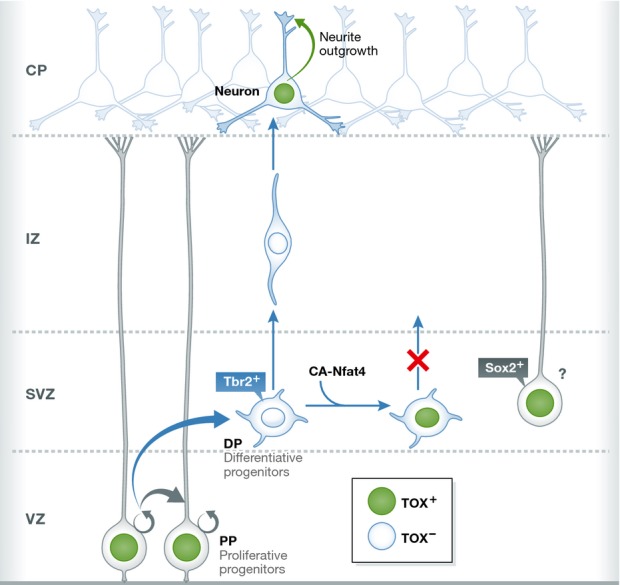
Off-switch type of Tox expression during cortical development
The scheme illustrates the dynamically regulated expression of Tox in radial glia of the ventricular zone (referred to by the authors as proliferative progenitors or pp) and neurons in the developing cortical plate (CP) where Tox expression promotes neurite outgrowth. In Tbr2-positive progenitors (referred to by the authors as differentiative progenitors or dp) located in the subventricular zone (SVZ), Tox expression is absent. Likewise, young neurons migrating through the intermediate zone (IZ) do not express Tox. Forced expression of constitutively active Nfat4 (CA-Nfat4) induces Tox expression in the SVZ and inhibits neurogenesis. Intriguingly, direct Tox overexpression results in the emergence of cells suggestive (as indicated by the question mark) of basal radial glia characterized by Sox2 expression and the absence of Tbr2.
Due to the absence of Tox antibodies suitable for genomewide chromatin immunoprecipitation, the authors turned to DamID (van Steensel & Henikoff, 2000) by fusing the Tox coding sequence to a prokaryotic DNA adenine methyltransferase, an enzyme that methylates adenines specifically at GATC sequences. By this means, methylation is targeted around Tox binding sites and allows for identification of these binding sites by PCR amplification of the methylated genomic DNA fragments and sequencing. This resulted in the identification of more than 9,000 putative Tox target genes in human embryonic kidney (HEK) cells and suggested that, contrary to the theory of structure-dependent binding, Tox may in fact exhibit sequence-specific DNA binding by revealing a 10mer binding motif. Future studies are required to experimentally validate the importance of this DNA binding motif. Intriguingly, despite these experiments being performed in HEK cells, genes expressed within the neural lineage figured very prominently among the putative Tox targets, even more so than genes of the lymphocyte lineage. To further validate the putative targets, Artegiani et al overexpressed Tox in the mouse developing cortex in vivo by in utero electroporation and then performed quantitative RT-PCR of Tox-overexpressing cells. Indeed, many of the genes selected for analysis on the basis of their gene ontology association with signaling, neurogenic commitment, and neurite outgrowth were found regulated upon Tox overexpression. Intriguingly, not all genes were upregulated as one would expect for a transcriptional activator, but several were downregulated suggesting that Tox may act both as a transcriptional activator and repressor.
Finally, the authors addressed the questions regarding the potential function(s) of Tox. They approached this by overexpressing Tox within the E13.5 embryonic cortex in vivo. Consistent with its dynamic expression, Artegiani et al could discern several modes of action: Firstly, Tox expression promoted progenitor expansion resulting in an increase in cells not in the VZ, but surprisingly in the SVZ. The increase in SVZ progenitors was not due to more Tbr2-positive differentiative progenitors—in fact, Tox was found to inhibit neurogenesis—but instead due to the appearance of Sox2-positive cells. Might this emergence of Sox2-positive cells reflect an increase in basal (or outer) radial glia that occur so rarely in the mouse, but are a hallmark of many gyrencephalic mammals and especially the human outer SVZ (Borrell & Götz, 2014)? This reminds of another recently studied off-switch gene called Trnp1, the difference being that it was knockdown rather than overexpression of Trnp1 which caused an increase in the basal radial glia pool (Stahl et al, 2013). The decrease in Tbr2-positive cells is puzzling also given that Tbr2 is a direct target of Tox and is upregulated following forced Tox expression. One explanation for this paradoxical gene regulation favored by the authors is that parallel to Tbr2, which alone would drive neurogenesis, other neurogenesis-inhibiting factors such as Sox2 increase as well and thereby dominate the outcome in the decision between stem cell expansion and differentiation. However, the paradoxical regulation of Tbr2 mRNA may relate to the dynamical role normally played by Tox. In fact, induction of Tbr2 mRNA may not signify automatically also that of Tbr2 protein. Rather, expression of Tbr2 mRNA in Sox2-positive cells might commit these cells to future neuron production without immediately enforcing it.
On the other hand, while inhibiting neuronal differentiation in progenitors, Tox promotes differentiation in neurons already settled in the cortical plate. Consistent with the binding to and regulation of many genes involved in axon guidance and neurite outgrowth, Artegiani found that forced Tox expression in neurons in vivo enhanced neurite formation both in terms of numbers and length. Moreover, Tox might affect also neuronal fate decisions: Most of the Tox-overexpressing neurons were found to be Ctip2 negative and in fact settled just above the layer of Ctip2-positive deep-layer neurons. This is another puzzling result as physiologically expressed Tox is actually mostly co-expressed in Ctip2-positive neurons. Could this discrepancy be due to an overdose of Tox? Clearly, a full understanding of the functional role played by Tox during corticogenesis will require a comparison of the genomewide binding data with transcriptome analyses of cells in which Tox is either overexpressed or knocked down. Also knockdown or genetic deletion of Tox will reveal its physiological roles at the radial glia and the neuronal stage, respectively. Finally, on a system biological level, what is the precise place of Tox and its related family members such as Tox3 within the neural differentiation regulatory networks (Ziller et al, 2014)? Now that we got in-Tox-icated we clearly want to learn more.
References
- Aliahmad P, O'Flaherty E, Han P, Goularte OD, Wilkinson B, Satake M, Molkentin JD, Kaye J. TOX provides a link between calcineurin activation and CD8 lineage commitment. J Exp Med. 2004;199:1089–1099. doi: 10.1084/jem.20040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliahmad P, Seksenyan A, Kaye J. The many roles of TOX in the immune system. Curr Opin Immunol. 2012;24:173–177. doi: 10.1016/j.coi.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprea J, Prenninger S, Dori M, Ghosh T, Monasor LS, Wessendorf E, Zocher S, Massalini S, Alexopoulou D, Lesche M, Dahl A, Groszer M, Hiller M, Calegari F. Transcriptome sequencing during mouse brain development identifies long non-coding RNAs functionally involved in neurogenic commitment. EMBO J. 2013;32:3145–3160. doi: 10.1038/emboj.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B, de Jesus Domingues AM, Bragado Alonso S, Brandl E, Massalini S, Dahl A, Calegari F. Tox: a multifunctional transcription factor and novel regulator of mammalian corticogenesis. EMBO J. 2015;34:896–910. doi: 10.15252/embj.201490061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Götz M. Role of radial glial cells in cerebral cortex folding. Curr Opin Neurobiol. 2014;27:39–46. doi: 10.1016/j.conb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty E, Kaye J. TOX defines a conserved subfamily of HMG-box proteins. BMC Genom. 2003;4:13. doi: 10.1186/1471-2164-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl R, Walcher T, De Juan Romero C, Pilz GA, Cappello S, Irmler M, Sanz-Aquela JM, Beckers J, Blum R, Borrell V, Götz M. Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate. Cell. 2013;153:535–549. doi: 10.1016/j.cell.2013.03.027. [DOI] [PubMed] [Google Scholar]
- van Steensel B, Henikoff S. Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat Biotechnol. 2000;18:424–428. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- Ziller MJ, Edri R, Yaffe Y, Donaghey J, Pop R, Mallard W, Issner R, Gifford CA, Goren A, Xing J, Gu H, Cacchiarelli D, Tsankov AM, Epstein C, Rinn JL, Mikkelsen TS, Kohlbacher O, Gnirke A, Bernstein BE, Elkabetz Y, et al. Dissecting neural differentiation regulatory networks through epigenetic footprinting. Nature. 2014 doi: 10.1038/nature13990. doi: 10.1038/nature13990. [DOI] [PMC free article] [PubMed] [Google Scholar]


