Abstract
The discovery of insulin more than 90 years ago introduced a life-saving treatment for patients with type 1 diabetes, and since then, significant progress has been made in clinical care for all forms of diabetes. However, no method of insulin delivery matches the ability of the human pancreas to reliably and automatically maintain glucose levels within a tight range. Transplantation of human islets or of an intact pancreas can in principle cure diabetes, but this approach is generally reserved for cases with simultaneous transplantation of a kidney, where immunosuppression is already a requirement. Recent advances in cell reprogramming and beta cell differentiation now allow the generation of personalized stem cells, providing an unlimited source of beta cells for research and for developing autologous cell therapies. In this review, we will discuss the utility of stem cell-derived beta cells to investigate the mechanisms of beta cell failure in diabetes, and the challenges to develop beta cell replacement therapies. These challenges include appropriate quality controls of the cells being used, the ability to generate beta cell grafts of stable cellular composition, and in the case of type 1 diabetes, protecting implanted cells from autoimmune destruction without compromising other aspects of the immune system or the functionality of the graft. Such novel treatments will need to match or exceed the relative safety and efficacy of available care for diabetes.
Keywords: beta cells, cell replacement therapy, stem cells, type 1 diabetes
Introduction
The treatment of type 1 diabetes (T1D) is one of the most important medical success stories of the 20th century. Most type 1 diabetics can now expect a life span that is largely equivalent to those of the non-diabetic population. Secondary complications such as retinopathy and nephropathy were initially prevalent, caused by inadequate glycemic control (DCCT-Research-Group, 1993). In the last decades, technical advancements such as the generation of human recombinant insulin, better blood glucose monitoring devices, and insulin pumps have improved the patients' ability to self-regulate their blood glucose levels and reduced the risks of hypoglycemia. A selection of insulin variants now also exist that are either short or long acting and can be taken in combination for tighter glycemic control. But the treatment is still cumbersome and requires careful monitoring and multiple insulin injections each day. Maintaining blood glucose within physiological range is difficult to achieve, especially in children and adolescents. And even with intensive insulin therapy, the accumulative incidence of retinopathy, nephropathy, and cardiovascular disease after 30 years of T1D remains considerable, at 21, 9 and 9%, respectively (Nathan et al, 2013), although these numbers are much lower than decades ago. Therefore, even with ongoing improvements in diabetes care, there is need for a cure.
Because T1D is caused by the loss of beta cells due to autoimmune-mediated destruction, a cure may require the use of replace-ment beta cells. Upon diagnosis, patients show an age-dependent loss of 40–85% of their beta cell mass (Klinke, 2008), and a small amount of beta cells remain for many years in the majority of T1D patients (Oram et al, 2014). In mice, beta cell mass can recover after ablating most cells with a toxin (Nir et al, 2007) or if the autoimmune reaction in NOD mice is halted after beta cell destruction has commenced (Nishio et al, 2006). But human beta cells may not be as competent to regenerate: Immune suppression and maintenance of euglycemia following pancreas transplantation could not restore normal function of the endogenous pancreas (Liu et al, 2009). Even in recent-onset type 1 diabetes, no increase in beta proliferation is seen (Butler et al, 2007). Further, therapies aimed at inhibiting the autoimmune reaction in recent-onset T1D are only able to transiently maintain C-peptide levels in the blood, but not restore normal levels (Herold et al, 2002; Keymeulen et al, 2005). A cure for patients with established T1D is thus likely going to involve the transplantation of functional beta cells. Pancreas transplantation or allogeneic transplantation of donor islets is indeed effective and can often achieve insulin independence in T1D patients. Such therapy is mostly applied for T1D subjects with severe complications such as renal failure, where pancreas transplant is performed in tandem with kidney transplantation (Merani & Shapiro, 2006; Gruessner, 2011). A shortage in donor organs and the potentially adverse side effects of systemic immunosuppression that these grafts require greatly limits the utility of this approach. In addition, beta cells within the graft may still be destroyed by an autoimmune reaction (Tyden et al, 1996; Vendrame et al, 2010). These same limitations to cell therapies for T1D persist even if other sources, such as stem cells, become available. Therefore, other forms of diabetes that are not mediated by autoimmunity may suitable initial targets to determine the efficacy and safety of beta cell transplants. These experimental treatments will need continuous evaluation against existing care options. A source of beta cells very similar or equivalent to the beta cells of the human pancreas is a necessary requirement for the development of such therapies. Such stem cell-derived beta cells are also required for basic research aimed at understanding beta cell development and the mechanisms of beta cell failure in diabetes. Therefore, research and clinical goals can often be aligned, and indeed, progress toward clinical application is currently being made in a basic research context.
Beta cells differentiate from human pluripotent stem cells
Pluripotent stem cells have the ability to differentiate into all cell and tissue types of the body, most notably shown in mice by the generation of chimeric animals. Directed differentiation of human pluripotent stem cells into various cell types in vitro has only recently become possible by applying developmental signals based on lessons learned from developmental biology in animals. Initial attempts to generate beta cells from human embryonic stem cells relied on spontaneous differentiation. By culturing embryonic bodies in stem cell media, Assady and colleagues reported the upregulation of insulin transcripts and occasional insulin-positive cells, but most cells were not of pancreatic origin (Assady et al, 2001). To improve differentiation efficiency into insulin-positive cells, developmental steps were sequentially targeted, progressing from definitive endoderm to primitive gut tube, through posterior foregut, pancreatic endoderm, pancreatic endocrine progenitors and, finally, to beta cells. High levels of activin A induce definitive endoderm formation during development (Thisse et al, 2000; Whitman, 2001; Tam et al, 2003; Kubo et al, 2004) and, together with Wnt3a, can induce definitive endoderm from human embryonic stem cells (D'Amour et al, 2005). Induction of posterior foregut endoderm, characterized by the expression of PDX1, FOXA2, and HNF1beta, can further be achieved by the activation of FGF and retinoic acid signaling pathways and inhibition of the sonic hedgehog signaling pathway (D'Amour et al, 2006; Mfopou et al, 2010). In the embryo, pancreatic endocrine cells develop from Ngn3-positive pancreatic endocrine progenitors. These Ngn3-positive cells are derived from pancreatic progenitors upon downregulation of Notch signaling (Shih et al, 2012). To mimic this stage in vitro, γ-secretase inhibitors have been added to inhibit Notch signaling, resulting in the expression of Ngn3 (D'Amour et al, 2006; Mfopou et al, 2010). Following this step-wise induction, insulin-expressing cells could be derived, although the cells were not responsive to glucose stimulation (D'Amour et al, 2006). This was the first report with clear evidence that cells resembling beta cells could be derived, and a powerful demonstration that developmental knowledge could be employed to direct differentiation of human embryonic stem cells to a specific developmental fate.
Other groups were initially not as successful when replicating these results using a variety of embryonic stem cell lines (Cho et al, 2008; Mfopou et al, 2010). Though PDX1-positive cells were obtained, the efficiency was low, and a high percentage of cells were positive for the liver markers alpha-fetoprotein and albumin, resembling hepatocytes instead of pancreatic cells (Mfopou et al, 2010; Nostro et al, 2011). During mouse development, before the onset of pancreatic lineage commitment, BMP4 favors liver development by inhibiting Pdx1 and by inducing albumin expression (Wandzioch & Zaret, 2009). Inhibition of BMP4 signaling allowed a shift from hepatocyte to the pancreatic lineage, characterized by the generation of 50–80% of PDX1-positive cells from several human embryonic stem cell lines (Mfopou et al, 2010; Nostro et al, 2011). Additional improvements were made to allow for the efficient differentiation of PDX1-positive cells into endocrine cells. Nostro and colleagues found that inhibition of the TGF-β pathway was required for endocrine lineage commitment, increased the expression of the endocrine lineage marker NGN3 and, when combined with BMP4 inhibition, resulted in 25% C-peptide-positive cells (Nostro et al, 2011). These additional manipulations of signaling pathways resulted in a protocol that was effective for many different stem cell lines.
Based on these studies, a number of groups have succeeded in generating insulin-producing cells with an efficiency of about 25% (Kelly et al, 2011; Nostro et al, 2011; Kunisada et al, 2012; Rezania et al, 2012, 2013; Schulz et al, 2012; Hua et al, 2013; Shang et al, 2014). However, none of them reported the derivation of glucose-responsive and monohormonal insulin-positive cells. Most of the cells co-expressed insulin and glucagon, and the secretion of insulin increased only marginally upon a glucose challenge. These studies failed to generate pancreatic progenitors expressing all transcription factors required for pancreatic development and beta cell function. A pancreatic progenitor cell is defined by coexpression of the transcription factors Pdx1, Nkx6.1, Nkx2.2, Ptf1a, and Sox9 in the embryonic pancreas epithelium, and the lack of one of these transcription factors can result in abnormal beta cell development (Gittes, 2009). For instance, pancreatic cells in homozygous Nkx6.1 mutant mice develop into NGN3-positive endocrine progenitors and insulin and glucagon co-expressing cells, but fail to form monohormonal insulin-expressing beta cells at embryonic day 18.5 (Sander et al, 2000). During mouse embryonic development, two waves of Ngn3 expression, initiated before and after Nkx6.1 expression, have a vastly different outcome on the phenotype of endocrine cells. The early wave of NGN3 expression occurs around mouse embryonic day 9.5 in the absence of Nkx6.1 and drives pancreatic cells into polyhormonal and glucagon-positive alpha cells. Only the second wave of NGN3 expression around embryonic day 12.5 in the presence of Nkx6.1 enables differentiation into monohormonal insulin-positive beta cells (Johansson et al, 2007). To generate NKX6.1-positive cells from human pluripotent stem cells, the BMP4 signaling pathway had to be more precisely controlled. In previously published studies, BMP4 was continuously inhibited with the intention to first induce the pancreatic lineage over the hepatocyte lineage and then to induce NGN3 expression (Nostro et al, 2011). However, PDX1-positive cells fail to upregulate NKX6.1 when BMP4 signaling is inhibited (Sui et al, 2013). Therefore, in order to generate NKX6.1-positive cells, BMP4 signaling should be transiently activated after onset of PDX1 expression. In addition, the protein kinase C (PKC) pathway also plays a role in the generation of NKX6.1-positive pancreatic progenitors. Rezania and colleagues demonstrated that activation of the PKC pathway induced NKX6.1 expression before the onset of NGN3 expression (Rezania et al, 2013). NGN3-expressing endocrine progenitors with or without NKX6.1 had markedly different developmental potential after transplantation into mice. Though endocrine cells could be derived from both of these groups, only NKX6.1-positive progenitors gave rise to monohormonal insulin-expressing cells, while the NKX6.1 negative cells gave rise to polyhormonal and glucagon-positive cells. This maturation into monohormonal insulin-producing cells in mice takes several months and is poorly understood.
To generate functional monohormonal beta cells in vitro, Pagliuca and colleagues as well as Rezania and colleagues manipulated additional pathways implicated in the development of the pancreas (Pagliuca et al, 2014; Rezania et al, 2014). TGF-β inhibitor (ALK5 inhibitor) and the BMP4 inhibitor (LDN-193189), thyroid hormone, and a Notch signaling inhibitor were applied for the generation of beta cells from PDX1 and NKX6.1 double-positive pancreatic progenitors. It has previously been shown that three-dimensional cues are important for pancreatic development (Greggio et al, 2013), and in fact, both studies applied 3D culture systems in their differentiation process, starting with either pluripotent stem cells cultured in suspension, or aggregating cells at the pancreatic progenitor stage of differentiation. Approximately 50% insulin-positive cells could be obtained, and most insulin-positive cells coexpressed the beta cell transcription factors NKX6.1 and PDX1, but not glucagon, and increased secretion of C-peptide during incubation in high levels of glucose. The cells derived also responded to multiple sequential high-glucose challenges (Pagliuca et al, 2014). Rezania's study further evaluated the dynamics of insulin secretion to glucose stimulation with a perifusion system. In contrast to adult human islet cells, the cells displayed a slow response to glucose and did not return to the pre-treatment baseline, indicating that these cells are still slightly different from adult human beta cells. Nevertheless, these beta cells were functional and protected mice from diabetes after transplantation. Although differences remain between stem cell-derived beta cells and beta cells of the human pancreas, they are very similar.
The availability of stem cell-derived beta cells now allows the investigation of mechanisms of beta cell failure in a human system. Beta cells differentiated from stem cells of a diabetes patient carry all the genes responsible for the disease, allowing the study of how a particular genotype affects beta cell function.
Disease-specific stem cells and beta cells
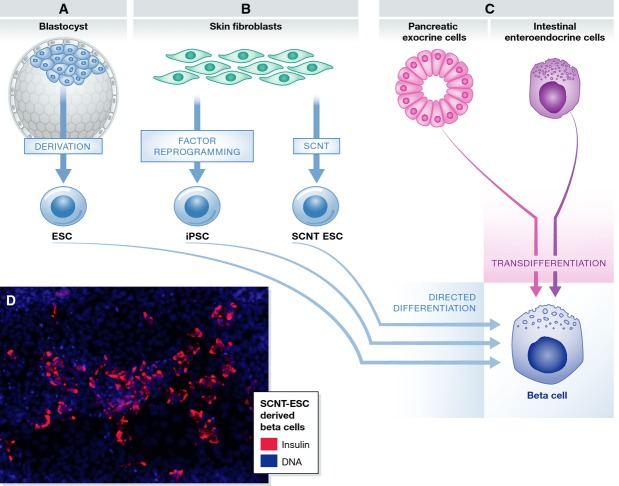
Advances in stem cell technologies have made it possible to generate stem cells from adult-differentiated cells, including from patients with T1D, T2D, and various forms of diabetes caused by single-gene mutations (Fig1) (Takahashi et al, 2007; Maehr et al, 2009; Ohmine et al, 2012; Hua et al, 2013; Teo et al, 2013; Shang et al, 2014; Yamada et al, 2014). Initial protocols to derive these stem cell lines used a retroviral delivery system of four embryonic transcription factors (Takahashi et al, 2007), which resulted in vector integration and low levels of continued expression of the transgenes, and the resulting cells sometimes had reduced differentiation potential (Koyanagi-Aoi et al, 2013). DNA viruses have since been replaced by modified mRNA (Warren et al, 2010) or Sendai virus (Ban et al, 2011), which cannot integrate into the genome. These readily accessible methods have made induced pluripotent stem cells (iPSCs) a popular choice for stem cell biologists. However, several reports have indicated that reprogramming by transcription factors can affect the genetic and epigenetic integrity of iPSCs, including loss of gene imprinting (Nishino et al, 2011), epigenetic memory (Bar-Nur et al, 2011; Kim et al, 2011), alterations in hydroxymethylation (Wang et al, 2013) and appearance of de novo coding mutations (Gore et al, 2011). These defects are not generally seen in embryonic stem cells (ESCs) derived from blastocysts, raising the question regarding their cause and origin.
Figure 1.
Different strategies for making beta cells
(A) Beta cells can be generated by differentiation of pluripotent stem cells derived from IVF blastocysts (ESCs). (B) Autologous stem cells with the genotype of a patient can be derived by reprogramming of somatic cells via somatic cell nuclear transfer (SCNT-ESCs) or factor-mediated reprogramming (iPSCs). (C) Alternatively, pancreatic exocrine cells or intestinal enteroendocrine cells can be trans-differentiated into beta cells by ectopic expression or elimination of specific transcription factors. (D) An example of SCNT-ESC-derived beta cells; insulin is stained in red and DNA in blue.
It has recently become possible to generate patient-specific ESC by somatic cell nuclear transfer (SCNT) (Tachibana et al, 2013; Chung et al, 2014; Yamada et al, 2014). While reprogramming to iPSCs forces transformation by chemical means, reprogramming of a somatic cell in the oocyte cytosol follows a developmental path. Therefore, stem cells derived by nuclear transfer might be more similar to ES cells than iPSCs with regard to the above-described aberrations. But surprisingly, using isogenic NT-ESC and iPSC lines, the two derivation methods resulted in highly similar cell types with regard to gene expression and DNA methylation (Johannesson et al, 2014). And both NT-ESCs and iPSCs differed from ESCs with regard to the number of de novo coding mutations and imprinting aberrations: iPSCs and NT-ESCs showed an average of 10 de novo coding mutations, and 10% of imprinted regions showed changes in DNA methylation consistent with erosion of imprinting. Therefore, the somatic origin of the stem cell lines—as opposed to a germ line origin—and not the specific method of reprogramming is responsible for the elevated mutation rate and aberrations in imprinting. A functional comparison was not performed, and it remains possible that an iPSC-reprogramming more frequently generates outliers with decreased differentiation potential (Koyanagi-Aoi et al, 2013). However, functional studies are challenging to perform in the human system, as they cannot match the stringency of a chimera assay in the mouse system. We do, however, know that both methods of derivation result in stem cell lines that can differentiate into insulin-producing cells with the genotype of a type 1 diabetic (Maehr et al, 2009; Yamada et al, 2014).
Several studies have shown that beta cells can also be generated without the transition though the pluripotent state, by a process termed transdifferentiation (Fig1C). For instance, Zhou and colleagues found that ectopic expression of Pdx1, MafA, and Ngn3 in pancreatic exocrine cells in vivo converted them into beta cells in mice (Zhou et al, 2008). Cells from other developmentally related lineages have also been transdifferentiated into beta cells. The same recipe of Pdx1, MafA, and Ngn3 overexpressed in either mouse or human intestinal crypt cells results in clusters of insulin-positive cells (Chen et al, 2014). Transformation from gut cells to beta cells also occurs when the transcription factor Foxo1 is conditionally deleted in the mouse gut (Talchai et al, 2012). Furthermore, gut organoids derived from human pluripotent stem cells with conditional inhibition of Foxo1 form C-peptide-positive cells, though with low efficiency (Bouchi et al, 2014). The authors speculated that if such cells could be generated in vivo, in the human gut, they might be able to evade the autoimmune response in T1D, as gut-derived cells are intrinsically short-lived and regenerate continuously.
These various methods of generating stem cells and beta cells (Fig1) now allow generation of patient-specific beta cells, providing a tool for studying the molecular and cellular mechanisms of beta cell failure in diabetes, or for experimental testing of cell replacement therapies.
Monogenic diabetes as an entry point for disease modeling and cell replacement
The generation of patient-specific stem cells and their differentiation into specific cell types provides a powerful tool to model disease-specific phenotypes (Robinton & Daley, 2012). As a proof of principle in diabetes, Hua and colleagues generated iPSCs and beta cells from patients with maturity onset diabetes of the young (MODY) 2 (Hua et al, 2013). MODYs are forms of diabetes caused by mutations in genes involved in the function and/or the development of beta cells. In MODY2, this mutation is located in the glucokinase (GCK) gene (for review, see (Vaxillaire & Froguel, 2008). GCK catalyzes a rate-limiting step in glycolysis in beta cells. Reduced GCK levels due to a heterozygous or homozygous mutation result in decreased glycolysis and lower ATP levels, and thereby in reduced insulin secretion. iPSC-derived beta cells indeed showed decreased insulin secretion upon a glucose stimulus. Gene correction of the GCK mutation reversed this phenotype and restored glucose responsiveness to the levels of cells from healthy controls. These phenotypes conformed according to our expectations and showed the utility of patient-specific beta cells for modeling diabetes.
To gain novel insight into the mechanisms of beta cell failure in a less well understood form of diabetes, iPSC-derived beta cells were generated from subjects with Wolfram syndrome. Wolfram syndrome is caused by homozygous mutations in wolframin (Inoue et al, 1998), an endoplasmic reticulum (ER) resident protein that had been implicated in regulating protein folding stress (Fonseca et al, 2005). Beta cells with wolframin mutations showed signs of elevated ER stress under basal conditions and rapidly lost the ability to secrete insulin when additional protein folding stress was induced (Shang et al, 2014). Interestingly, this phenotype could be reversed using chemical chaperones, highlighting the potential of this model for drug screening. Similar strategies should allow investigating whether beta cell intrinsic defects contribute to T2D and perhaps also to T1D. While many of these experiments were performed in vitro, stem cell-derived beta cells can also be transplanted into immunocompromised mice. Kroon and colleagues, and more recently other groups, have shown that mice can be protected from diabetes using stem cell-derived human beta cells (Kroon et al, 2008; Pagliuca et al, 2014; Rezania et al, 2014). This will allow testing of beta cell function under physiologically relevant circumstances, including under conditions of obesity, insulin resistance, aging or other forms of environmental stress. Transplantation studies will also allow continuous optimization and functional testing of grafts for cell replacement therapies.
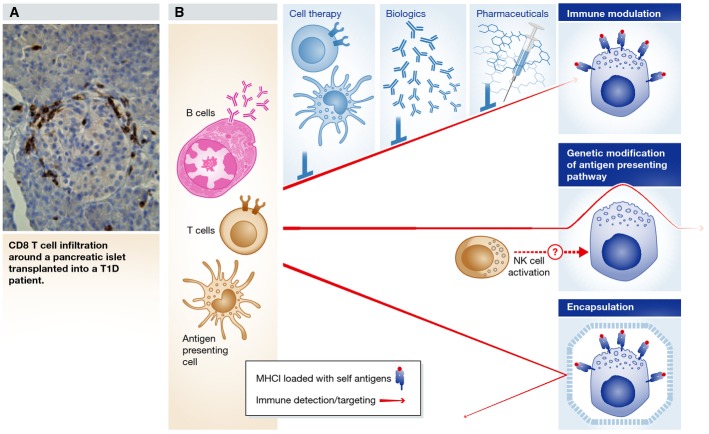
All forms of diabetes are caused by insufficient insulin relative to metabolic need, caused by either decreased ability to produce insulin in the pancreas, or by insulin resistance in the periphery. Therefore, all forms of diabetes might benefit from the transplantation of insulin-producing cells. In the case of T1D, the transplanted beta cells will need to be protected from the immune system as they will likely be subject to the same autoimmune destruction that originally eliminated the native beta cells (Fig2A) (Vendrame et al, 2010). Unlike in T1D, beta cells from individuals with monogenic diabetes do not need to be protected from autoimmune destruction and may therefore be more suitable initial subjects for cell replacement. These monogenetic forms of diabetes could serve as a stepping stone toward cell replacement for T1D. For instance, MODY3 (monogenetic diabetes of the young 3) is one of the most frequent forms, accounting for an estimated 1% of all diabetes cases (Steck & Winter, 2011), and is caused by mutation of the HNF1alpha (hepatocyte nuclear factor 1 alpha) gene. MODY3 subjects can be treated with sulfonylureas, agents that stimulate insulin secretion by modulating the activity of a potassium channel on the beta cell. However, most subjects ultimately become insulin-dependent and are treated like T1D subjects (Thanabalasingham & Owen, 2011). Transplanted beta cells with a corrected allele should be fully functional, and not be subject to an autoimmune response.
Figure 2.
Avoiding autoimmune destruction of grafted beta cells
(A) Reoccurrence of autoimmunity after pancreas transplantation into a T1D patient indicated by infiltration of CD8+ T cells around islets in the graft (Vendrame et al, 2010). (B) There are several potential ways of protecting transplanted beta cells from immune destruction. First, the immune system can be modified either pharmaceutically, with biologics, or by transplantation of regulatory T cells (Tregs) or tolerogenic dendritic cells (DCs). Second, the beta cells can be genetically modified to reduce immunogenicity prior to transplantation, though this could cause the activation of natural killer (NK) cells. Finally, beta cells can be encapsulated in a way that keeps the immune system at bay while allowing for the exchange of nutrients and oxygen. The red arrows indicate detection and targeting of beta cells by the immune system, and the characteristics of the arrows indicate from top to bottom: reduced intensity of the immune response, inability to detect beta cell antigens and inability to penetrate encapsulation devices. Transplanting autologous beta cells into patients with non-autoimmune forms of diabetes would not require such protection.
Engineering functional pancreatic grafts
For successful transplantation of stem cell-derived beta cells, they have to be prepared and transplanted in a way that supports long-term function of the graft and ensures the safety of the host. Proper integration of the transplanted cells into the host tissue has to be promoted while retaining the ability to remove the grafted cells, should they show abnormal function. These aims present a formidable challenge that require innovative tissue engineering and, for T1D, novel ways to modify the immune system or the immunological properties of beta cells. The main goal of tissue engineering is to generate functional tissue. Though we can now generate many cell types from human pluripotent stem cells, these cell types are often immature and do not reach the functionality of tissues in vivo. The lack of appropriate tissue structure is likely part of the under-performance of stem cell-derived products. All cell types and organs in the human body are organized in specific structures composed of multiple cell types. Interactions between different cell types and vascularization, as well as the 3D arrangement, of the cells play an important role in tissue reconstruction (Badylak et al, 2011; Goh et al, 2013). The function of tissues such as the heart or the kidney is highly dependent upon accurate 3D structure, which cannot be achieved by spontaneous or directed differentiation of pluripotent stem cells. Engineering of insulin-producing beta cell constructs is unlikely to require such degree of complexity: human islets can effectively regulate blood glucose levels in isolation from their pancreatic environment, such as after infusion through the portal vein into the liver (Rodriguez Rilo et al, 2003). In addition, clusters of beta cells that differentiate from human stem cells appear to self-organize into spheres that resemble human islets (Pagliuca et al, 2014). Nevertheless, tissue engineering can likely provide an effective method of delivery and improve survival upon transplantation, and the ability to retrieve transplanted cells and immunoprotection through encapsulation.
Encapsulation of transplanted cells
Encapsulation serves a dual function of protecting the graft from immune destruction and protecting the host from the graft (Fig2B). Immune protection is required when non-autologous cells are used for transplantation or if autologous cells are transplanted into an autoimmune environment. This can be achieved by blocking the cellular response via physical isolation of the cells using semi-permeable membranes or scaffolds. This approach reduces the need for immunosuppression and has recently been reviewed in detail elsewhere (Sakata et al, 2012; Qi, 2014). Encapsulation also prevents cells from escaping the location of the graft, and allows for removal if needed. This is particularly relevant, as uncontrolled differentiation and growth, so-called teratomas, have often been observed in mice grafted with stem cell-derived pancreatic precursors (Kroon et al, 2008; Kelly et al, 2011). Teratoma formation may be preventable by generating grafts consisting of a pure population of mature beta cells, either by using cell purification (Kelly et al, 2011) or by improving differentiation methods.
The material used for encapsulation has stringent requirements to maintain long-term function of the graft. The chosen biomaterial needs to be biocompatible and promote the survival of the islet cells. At the same time, the material properties need to permit bi-directional diffusion of glucose, hormones, proteins, and cellular waste produced by the islet cells while allowing the influx of nutrients and oxygen from surrounding tissues. The chosen biomaterial also needs to be inert, in order to avoid triggering a host tissue response and fibrous encapsulation that can adversely affect the long-term functionality of the transplant. Example of biomaterials that have been explored for the encapsulation of islet cells includes alginate (Yang et al, 1997; Lanza et al, 1999b), polysulfone (Lembert et al, 2005), polyvinyl alcohol (Qi et al, 2004), polyacrylonitrile–polyvinyl chloride (Scharp et al, 1994), and poly(ethylene glycol) (Weber et al, 2008). However, this approach requires efficient and reproducible micro-encapsulation protocols, and some cells may die within the encapsulated material without the possibility of being cleared or removed. Some reports have shown micro-encapsulated beta cells remain viable up to 6 months after implantation (Orlando et al, 2014), but it remains unclear how long the survival of these cells can be extended and thus how often the encapsulation device would need to be exchanged. An additional limitation of this approach is that at least one dimension of the graft must be small to allow efficient diffusion as blood vessels cannot permeate the device, restricting the ability to construct 3D tissues.
Vascularization of transplanted cells
One limitation of current islet transplantation approaches is the poor survival of the transplanted cells. Direct transplantation of cells into a tissue cavity mostly relies on diffusion of oxygen and nutrients from the nearest capillary bed for survival of the cells (Qi, 2014). To improve this, a vascularized cavity can be prepared within the recipient prior to delivery of the beta cells. For example, Sernova Corp's approach is to subcutaneously implant a “pouch” with inert rods as place holders into the patient. Once the material has enough surrounding vasculature, the rods are removed and the islet cells are then injected into the pouch where they remain protected but close enough to the vasculature for nutrient exchange to take place. Details of this approach have only been available via public disclosure from the company's Web site (sernova.com). Finally, beta cells can be transplanted in scaffolds that have been engineered to slowly release growth factors that promote neovascularization (Phelps et al, 2013). Although this would not reduce hypoxia immediately post-transplantation, it can speed up vascular integration of the graft.
Decellularized organs as a scaffold for tissue engineering
One of the advantages of using synthetic polymers to encapsulate or deliver islet cells is the reproducibility of the manufacturing process and the precise control over material properties. However, synthetic materials lack the bioactivity and ultrastructural composition of native tissues. Previous reports have shown that synthetic biomaterials, alone or in combination with extracellular matrix (ECM) proteins, can enhance islet survival and function in vitro highlighting the importance of the composition and structure of the chosen material for optimal beta cell function. For example, islet function was enhanced by mimicking the islet extracellular matrix via the combination of ECM proteins such as collagen type I, collagen type IV, fibrinogen, fibronectin, laminin, and vitronectin with a synthetic component such as poly(ethylene glycol) hydrogel (Cheng et al, 2011). However, the recapitulation of the native ultrastructure of the pancreas, which is likely to play an important role during islet cell differentiation and maturation, has not been achieved in vitro.
One exciting new area of research is the use of ECM as a bio-inductive scaffold for functional and phenotypical maintenance of cells. Pancreatic islets cells have been shown to prefer a variety of ECM-derived substrates such as endothelial cell-derived matrix, fibrin, Matrigel, and collagen when compared to synthetic components (Cheng et al, 2011; Daoud et al, 2011; Krishnamurthy et al, 2011). For instance, porcine and rat islet cells seeded on Matrigel displayed improved beta cell function and islet cluster-like morphology when compared to plastic (Hayek et al, 1989; Vukicevic et al, 1992; Lucas-Clerc et al, 1993; Perfetti et al, 1996; Nagata et al, 2001; Oberg-Welsh, 2001). Greggio and colleagues also showed that mouse pancreatic progenitors could be cultured and differentiated in 3D hydrogels containing ECM components such as laminin (Greggio et al, 2013) and by combining 3D hydrogels with Wnt induction. Huch and colleagues showed primary mouse progenitors could be expanded indefinitely (Huch et al, 2013). In other words, the ECM not only serves as a scaffolding material for cells to attach, grow, and populate, but it also provides cues and signals to the cells that help maintain phenotype and function.
Since recreating the exact composition and structure of the pancreas is currently not possible, a logical alternative is to use native pancreatic tissue. The ECM contains multiple proteinaceous and non-proteinaceous components specifically tailored to a given tissue (Badylak et al, 2009). By harnessing the inherent information found in the pancreatic ECM, islet cells can be provided with a more native-like environment (Brizzi et al, 2012). A recent study described a method to isolate porcine pancreatic ECM via perfusion of detergents through an intact pancreas. The resulting scaffold material was a completely decellularized pancreas that can support the attachment and growth of islet and adult stem cells. The pancreatic ECM was shown to help up-regulate markers such as insulin secretion, further supporting their use as potential scaffolding material over synthetics (Mirmalek-Sani et al, 2013). This approach can be further improved by protecting the native vasculature during the decellularization process in order to maintain a patent vascular network that could be used to re-perfuse the pancreatic tissue. Understanding the appropriate ECM ultrastructure needed to maintain beta cell function can also improve emerging techniques such as bioprinting where both cells and ECM can be printed into three-dimensional units (Mendelsohn et al, 2010).
Though we know that bioengineering solutions such as encapsulation devices can protect transplanted cells from an immune response, it is not clear how long these devices will function. Therefore, modifications of the immune system in T1D, or of the immunological properties of the beta cells, would be desirable. Such approach might allow the use of autologous stem cell-derived beta cells and thereby harness the therapeutic potential of reprogramming technologies.
Modification of the immune system to restore tolerance
Although autologous stem cell-derived beta cells would circumvent issues of allogeneic rejection, the underlying autoimmunity still represents a threat to the newly generated and implanted beta cells. Among the many genetic predispositions associated with T1D, a great majority affects the immune system and its ability to remain tolerant to self. In fact, many of these susceptibility genes are shared with other autoimmune and autoinflammatory disorders such as celiac disease, rheumatoid arthritis, vitiligo, and Crohn's disease (www.T1Dbase.org). Long-term restoration of immune tolerance is paramount to the survival of residual endogenous or implanted islets. Two types of approaches have been evaluated or implemented to achieve this goal. The first type aims to eliminate parts of the immune system, such as T or B cells, regardless of whether the targeted immune cells play a role in the autoimmune process or not. This type of approach is quite effective for as long as it is applied, but patients become vulnerable to infections and certain cancers, and autoimmunity usually resurges after treatment interruption. Considering the safety and efficacy of current diabetes care, this is not a viable long-term solution for most T1D subjects. The second type of approach aims to specifically eliminate or tame the immune cells that are responsible for the destruction of the beta cells by exploiting their beta cell antigen specificity. More recently, emphasis has been placed on combination therapies aimed at tackling the disease at multiple levels, by crippling specific immune cells, quenching inflammation and targeting antigen-specific T cells for reprogramming. The evaluation of all these approaches has been made possible thanks to useful animal models, the non-obese diabetic (NOD) mouse, and to a lesser extent the BioBreeding (BB) rat, both of which spontaneously develop T1D and share many of the disease susceptibility genes associated with the human disease. New humanized versions of these animal models, which are reconstituted with a human immune system by transplantation of fetal thymic tissue together with human bone marrow-derived hematopoietic progenitor cells, are being developed and perfected (Greiner et al, 2011; Kalscheuer et al, 2012). Recent advancements in deriving thymic tissue from stem cells could potentially allow the generation of animal models containing immune systems that are fully patient-specific (Parent et al, 2013; Sun et al, 2013), which would greatly facilitate the testing and translation of new therapeutic strategies as well as the assessment of the function of transplanted human islets in vivo. The potential use of autologous stem cell-derived thymic tissue to induce tolerance in T1D patients is, however, less straightforward. First, the loss of central tolerance seen in autoimmune patients may be caused by genetically encoded traits that will also be present in the stem cell-derived thymic tissue. Second, the T-cell pool in adults is largely composed of memory T cells, and it is unlikely that restoration of central tolerance would be sufficient to offset a weak peripheral tolerance characterized by dysfunctional regulatory T cells (Tregs).
Systemic approaches
Immunosuppressive drugs such as cyclosporine are among the most potent in reversing disease in new-onset patients, but they have no durable effect if the treatment is interrupted (Staeva et al, 2013). Long-term treatment with these drugs is not tolerable due to their toxicity and the serious risk of developing opportunistic infections and tumors from weakened immune surveillance. Patients who undergo islet transplantation are subject to even stronger immunosuppression to compensate for the more aggressive allogeneic response against donor islets. Current treatments follow the Edmonton Protocol, which involves rapamycin, FK-506, and anti-CD25, or variants thereof that may include anti-thymocyte globulin (ATG), anti-TNF, anti-CD52 or CTLA4-Ig (Bruni et al, 2014). The use of autologous stem cell-derived beta cells is likely to require treatments that are more in line with those assigned to new-onset T1D patients as described below.
The use of biologics (biomolecules such as antibodies, soluble Ig-fusion receptors, and soluble decoy receptors) offers greater specificity by targeting particular pathways (e.g. blocking costimulation with CTLA4-Ig) or immune cell populations (e.g. non-depleting anti-CD3 or depleting ATG for T cells; anti-CD20 for B cells). Despite showing promising efficacy in animal models, most of these biologics had no or only transient effect on the disease in humans, although prolonged effect after cessation of treatment has been observed in isolated cases, with preservation of C-peptide for up to 2 years (Staeva et al, 2013). These biologics did not induce durable tolerance on their own, but they were generally better tolerated than immunosuppressive small molecule drugs, although complications such as cytokine release syndrome or Epstein–Barr virus reactivation have been observed. Targeting proinflammatory pathways with biologics has proven beneficial in a number of major autoimmune diseases, but anti-TNF and anti-IL1β have shown no or poor efficacy on their own in T1D, suggesting that simply blocking inflammation is insufficient to restore immune regulation in this disease (Staeva et al, 2013). Alpha-1-antitrypsin, another anti-inflammatory candidate with immunoregulatory properties, is under clinical evaluation (Lewis, 2012). Resolving inflammation may still constitute a prerequisite before re-establishing tolerance, and these anti-inflammatory drugs may provide benefit as part of combination therapies.
Cell-based therapies offer an additional path to modifying the immune system, potentially in a safer and more targeted manner. Immune cells used for cell therapy demonstrate unmatched specificity, adaptability, and homing properties and typically consist of natural regulators of the immune system that have been isolated, expanded, boosted, and/or modified (Barcala Tabarrozzi et al, 2013; Fischbach et al, 2013). Autologous polyclonal Tregs that are re-infused after in vitro expansion are currently being tested in the clinics (Thompson et al, 2012), and the suppressive function of endogenous Tregs may be improved with low-dose interleukin-2 (Hartemann et al, 2013). Recombinant human IGF-1 has also recently been shown to stimulate regulatory T cells in vivo and prevent the onset of diabetes in NOD mice (Bilbao et al, 2014). Enforcing expression of particular T-cell receptors in Tregs for recognition of relevant specific antigens is a way to personalize this approach, in a manner analogous to the adoptive cell therapies that are now revolutionizing the treatment of cancer (Brusko et al, 2010). Autologous tolerogenic dendritic cells (DCs) can also promote tolerance by inducing Tregs as well as other protective cell types such as Th2 cells and regulatory B cells (Barcala Tabarrozzi et al, 2013; Creusot et al, 2014). Tolerogenic antigen-presenting cells may be obtained by silencing immunogenic pathways or overexpressing tolerogenic molecules (Giannoukakis et al, 2011; Creusot et al, 2014), and the choice of tolerogenic pathways to be bolstered may be adapted to individual patients.
These approaches may be well tolerated, but their efficacy in treating T1D remains to be firmly demonstrated. Thus far, the above strategies did not involve antigen specificity, which represents the next step toward achieving a more targeted therapy.
Antigen-specific approaches
Overall, antigen-specific therapy involves retraining the immune system to ignore particular self-antigens, in a way similar to desensitization methods used to rectify allergic reactions to particular allergens. Administration of islet antigens (proinsulin, GAD65, HSP60 p277) systematically (intravenous peptides, DNA vaccines) or via the mucosal surface (oral, nasal) has been an extensively studied method of tolerance induction (Coppieters et al, 2013). This has been tested not only to treat overt disease (after onset), but also as an attempt to prevent disease by re-establishing tolerance in high-risk subjects. Successes in animal models have prompted numerous trials in humans that have failed to demonstrate meaningful preservation of beta cell function, despite the therapy being well tolerated and evidence of immune responses reflecting some level of tolerance induction (Coppieters et al, 2013). The relative lack of success of these approaches may be partly explained by epitope spreading, which corresponds to a diversification of the cellular and humoral immune responses to an increasingly large number of epitopes from the target tissue (Di Lorenzo et al, 2007). Once a large number of antigens have been targeted by T cells and antibodies, inducing tolerance to one single antigen may appear preposterous, yet this is what clinical trials have been limited to so far. Thus, targeting multiple antigens after attenuating the local inflammation may represent a sensible strategy that will have to be assessed. Efficient induction of tolerance to these antigens requires presentation by tolerogenic antigen-presenting cells (APCs). When exposed to apoptotic cells, APCs produce TGF-β, which enhances their tolerogenic effect (Hugues et al, 2002; Chatenoud, 2014). Accordingly, spleen or blood cells chemically coupled with specific antigens induce an effective tolerance to these antigens, whereby specific T cells become unresponsive or regulatory (Prasad et al, 2012). The clinical evaluation of this approach, which has already passed phase I safety appraisal for treatment of multiple sclerosis, may soon be extended to T1D patients (Lutterotti et al, 2013). Furthermore, an antigen-specific dimension can easily be added to the cell-based therapies described in the previous section, whereby antigen-specific T-cell receptors can be expressed in Tregs or relevant antigens expressed in tolerogenic antigen-presenting cells in order to exclusively target diabetogenic T cells.
Combinatory approaches
Although many monotherapies have demonstrated adequate efficacy in animal models, it has become clear that long-term inhibition of autoimmune diabetogenic responses in humans will require a combination of different approaches. T1D patients are heterogeneous in their genetic makeup, the environmental conditions in which they live and their age of disease onset. This heterogeneity reflects how a complex combination of genetic and environmental factors differentially modulates several tolerogenic pathways (Todd, 2010). Thus, the same disease outcome can be reached through varying defects that impact the induction and maintenance of tolerance. One type of therapy targeting a particular biological pathway or antigen is therefore unlikely to work to the same extent across all patients. Combination and/or individualized therapies are increasingly considered as necessary to overcome this patient heterogeneity. Combination therapy allows us to tackle multiple issues, not only addressing the inherent defects that led to impaired tolerance induction in the first place, but also extinguishing the persistent inflammation that interferes with homeostasis of the immune system and contributes to improper signals being conveyed to T cells upon recognition of their antigens (Roep et al, 2010; Barthson et al, 2011).
We now have access to a plethora of new therapies, which have shown limited efficacy on their own, but could become effective when different types of approaches are combined. The three main types of therapies include the following: (i) T- and/or B-cell targeting in order to “reset” the immune system, (ii) modulation of the environment from pro- to anti-inflammatory, and (iii) provision of self-antigens to induce antigen-specific deletion (elimination by apoptosis) or anergy (state of unresponsiveness to antigen) in autoreactive T cells or induce/boost antigen-specific Tregs. Particular combinations have already been recommended for prioritization based on preclinical testing, possible synergy, as well as drug safety and availability (Matthews et al, 2010). Some suggested strategies may accomplish all of the above. For instance, as massive cell depletion leads to apoptosis and release of anti-inflammatory TGF-β, the depletion of CD8+ T cells and B cells followed by provision of specific antigens can enhance the differentiation of remaining CD4+ T cells into Tregs that are protective in mouse models of T1D and multiple sclerosis (Kasagi et al, 2014). Cell-based therapies also offer multiple levels of action. For example, tolerogenic APCs may be manipulated to express antigens, anti-inflammatory cytokines, and inhibitory ligands and can be targeted to specific sites of relevance (Creusot et al, 2014). More aggressive approaches such as non-myeloablative bone marrow transplantation have proved efficient in establishing long-term tolerance and preserving beta cell function (Barcala Tabarrozzi et al, 2013); however, the current conditioning regimen, involving immunosuppression and low-dose irradiation, makes it unattractive. Alternative conditioning regimens for bone marrow transplantation are under development, which may benefit both bone marrow and islet transplantation, for tolerance induction and islet replacement, respectively.
Despite limited clinical success of immunological therapies for T1D, much has been learnt in the past decade about their mechanism of action, which will help us formulate the safest and most efficient treatment going forward (Herold et al, 2013). Such treatments will most likely combine multiple approaches to eliminate pre-existing diabetogenic T cells (and possibly antibody-secreting B cells), promote peripheral tolerance with tolerogenic antigen-presenting cells and antigen-specific regulatory T cells, and normalize inflammation durably but locally, so that normal immune responses to pathogens may continue to be elicited as needed. The use of autologous stem cell-derived beta cells, combined with effective tolerance induction, could potentially allow T1D patients who have lost all their islets to live a normal life again. As autologous stem cells can provide an inexhaustible source of beta cells, it may be possible to first administer some of these beta cells in an apoptotic form to boost antigen-specific tolerance in conjunction with other aforementioned regimens before implanting live cells. Such use of apoptotic beta cells for treating new-onset diabetes or people with autoantibodies at risk of developing the disease may be associated with less risk than using living cells for cell transplantation.
Modification of beta cells to escape immune attack
As an alternative to the systemic modification of the immune system, immunological protection may be more readily achieved locally by modifying antigenic properties of the beta cells. The development of specific designer nucleases allows editing of the genome of stem cells without a “genetic footprint” and with high specificity. Zinc finger nucleases and transcription activator-like effector nucleases (TALENs) and more recently clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CAS9) systems mediate site-specific DNA cleavage and induce homologous recombination when provided a repair template (reviewed elsewhere Kim & Kim, 2014). Several reports show that genetic manipulation can be used to reduce the immunological vulnerability of cells. For instance, it has been shown that zinc finger-mediated disruption of human leukocyte antigen A (HLA-A) locus reduces the immunogenicity of various cell types toward cytotoxic lymphocytes (CTLs) (Torikai et al, 2013). As HLA is an essential component of the antigen presentation mechanism, this result is not surprising, but the downside is that the complete ablation of HLA expression usually causes cells to be targeted by natural killer (NK) cells (Ljunggren & Karre, 1990). This can potentially be avoided by reducing, instead of eliminating, HLA expression using short hairpin RNAs (shRNA) (Haga et al, 2006). Viruses commonly target the antigen presentation pathway to reduce their immune detection for instance by inhibiting the transporter associated with antigen-processing (TAP) transporter (Ressing et al, 2013). Wiertz and colleagues utilize this concept by overexpressing cytomegalovirus-encoded TAP inhibitors in human islet cells and showed that this reduced their detection by CTLs in vitro and after transplantation into mice (Zaldumbide et al, 2013). In an alternative approach, Shieh and colleagues overexpressed a chimeric molecule that selectively binds and activates cytotoxic T-lymphocyte-associated protein 4 (CTLA4) in NOD beta cells. CTLA4 is a receptor expressed on T cells that inhibits their function upon activation, and the transgenic expression of this chimeric agonist prevented the onset of diabetes in NOD mice and prolonged graft survival upon transplantation (Shieh et al, 2009). Protection of islets has also been achieved by overexpression of secreted immunoregulatory factors such as IL-4 (Gallichan et al, 1998), TGF-β (Suarez-Pinzon et al, 2002), and IL-35 (Bettini et al, 2012). These are examples of how mouse beta cells can be genetically altered to modulate their own immunological microenvironment. Whether these strategies would also be effective in human system is unknown. A potential caveat for this approach is that the immune system is thought to play an important role in suppressing the growth of tumor cell (reviewed in: Swann & Smyth, 2007), and inhibiting of the antigen presentation pathway has been shown to increase the tumorigenicity of cancer cells (Johnsen et al, 1999). Although the genetic changes discussed above do not induce tumorigenic transformation by themselves, they could plausibly increase the risk of tumor growth.
Discussion
The human body is made of cells, whose function or survival is affected in many incurable and chronic diseases, including diabetes. Drugs, such as insulin, can partially substitute for a cellular deficiency, but generally results in disease management, rather than a cure and a life-long dependence on health care. Therefore, basic research and medicine must learn how to use cells, and preferably the patient's own cells to cure disease (Kind & Colman, 1999; Lanza et al, 1999a). The generation of personalized pluripotent stem cells by either nuclear transfer or factor-mediated reprogramming, and the development of improved protocols for differentiation into beta cells (Pagliuca et al, 2014; Rezania et al, 2014), has moved us closer than ever to this goal. However, not only questions regarding feasibility, but chiefly economic considerations have thus far stalled significant efforts: Production and quality control costs for personalized stem cells would be enormous, as opposed to a single cell line that can be mass-produced. Therefore, companies such as Viacyte are developing a beta cell product based on a single embryonic stem cell line that will be encapsulated for protection from both auto- and allo-immunity. Others proposed the development of haplotype-matched array of stem cell lines to reduce allo-immunity (Turner et al, 2013). However, if patients were able to make a choice, they would likely choose their own cells as a means to restore insulin independence.
Here, we reviewed available cell sources, the methods of delivery, as well as potential avenues of immune protection toward a cell replacement therapy for type 1 and non-immune-mediated forms of diabetes. Several sources of autologous beta cells may be considered. Patient-specific stem cells with the genotype of a type 1 diabetic can be generated either by transcription factors (Maehr et al, 2009), or by nuclear transfer (Yamada et al, 2014), and both methods can give rise to insulin-producing beta cells. Cells generated by either method have gene expression and DNA methylation patterns that are essentially indistinguishable from embryonic stem cell lines derived from IVF embryos, but unlike their IVF counterparts, both methods result in an increased rate of de novo coding mutations and occasional loss of imprinting. Many of these mutations are likely pre-existing in the somatic cell used for reprogramming. This may not disqualify them for clinical use, though appropriate quality controls, such as DNA sequencing to exclude cells with damaging mutations, will likely be required. The group of Nissim Benvinisti recently showed that cells derived by transdifferentiation show fewer chromosomal abnormalities than cells differentiated from stem cells (Weissbein et al, 2014). These findings were made in the nervous system, but they may also apply to other cell types. This unexpected finding indicates that either clonal cell expansion or the transition through the pluripotent state increases the frequency of chromosomal errors. Though pluripotent stem cells often show karyotype abnormalities, they can be grown clonally, allowing the generation of a well-characterized cell population prior to differentiation. The generation of beta cells by trans-differentiation is not as amenable to quality controls: though beta cells can be made by induction from other somatic cells, each cell can represent a separate reprogramming event, cell populations will likely be heterogeneous, and not all cells can be analyzed. Such uncertainties regarding their specific cellular identity will not prevent their use in research, but presents challenges for the development of therapeutic applications.
A prerequisite for developing cell replacement therapies for diabetes is the ability to generate stem cell-derived beta cells that are functionally equivalent to human islet cells. Previous beta cell differentiation protocols resulted in either immature or polyhormonal beta-like cells that were minimally responsive to glucose in vitro. Recent improvements now yield monohormonal and glucose-responsive beta cells that, when transplanted into diabetic mice, restore normoglycemia. This points to their therapeutic potential, but more proximal applications of these cells are in basic research. By generating stem cell-derived beta cells from subjects with various forms of diabetes, genetically encoded defects in beta cell function can be identified and targeted. Monogenetic forms of diabetes caused by defined lesions in genes implicated in beta cell function have thus far been most tractable in this approach (Hua et al, 2013; Shang et al, 2014). Humanized models of T1D (immunodeficient mice hosting a human immune system) are also being developed (Brehm et al, 2012; Kalscheuer et al, 2012). These models may be important to test strategies that can prevent or stop immunity to self on human cells.
One of the main caveats for transplanting stem cell-derived beta cells into T1D patients is recurrence of autoimmunity. Though effective treatments in suppressing autoimmunity are available, the risk that such interventions might compromise the ability of the immune system to fend off infectious diseases and cancer does not compare well to the risks of continued standard diabetes care. Encapsulation of beta cells can in principle avoid the use of immune therapies, but life-long function of such capsules is unlikely. Therefore, a more proximal path to developing a personalized cell replacement therapy for diabetes may be on the forms of diabetes that are not immune mediated, including diabetes caused by single-gene mutations. Though monogenetic forms of diabetes are not as common, they can serve as a stepping stone toward cell therapy for type 1 and perhaps type 2 diabetes. Stem cell-based models of these monogenetic forms of diabetes will allow functional testing of cells with the mutation, and upon gene correction. Such functional testing will not only need to be performed on diabetic mice, but also on an animal model more similar in size and physiology to humans, such as the monkey or the pig. Unfortunately, immune-compromised swine that do not reject transplanted human cells have a life span of only a few months (Basel et al, 2012; Lee et al, 2014). Inducing tolerance to human cells in an immune competent animal or improved methods to allow long-term survival will be critical to advance this goal.
In parallel, immune modulatory approaches targeting the autoimmune reaction may be optimized with the goal to halt and possibly revert new-onset T1D, and to restore tolerance to autologous beta cells in established T1D. Although a number of individual therapies have shown efficacy in animal models, they have not yet been translated into human patients, and few specifically target autoimmunity without affecting other aspects of the immune system. The vital importance of the immune system for fighting infectious diseases and preventing tumor formation requires that immune therapies are specific in suppressing immunity to beta cells only. Another potential approach to protect transplanted beta cells from autoimmune destruction is to genetically modify the beta cells themselves to become less immunogenic and escape immune detection and rejection. Although the use of gene editing technologies requires additional quality controls, there is precedent for genetic modification of somatic cells in gene therapy. However, the in vivo behavior of cells that are invisible to the immune system needs further study.
The use of stem cell-derived beta cells will need to exceed the benefit to risk ratio of current diabetes treatments. As current care, centered around glycemic control through insulin administration, is relatively safe and effective, the bar is set very high for any new approach. Though questions regarding the path of translation and the economic viability of such treatments remain, they may have a transformative effect on medicine, replacing pharmacological care, with the use of a patient's own cells to restore normal physiology.
Acknowledgments
We thank R.L. Leibel for critical reading of the manuscript. D.E. is a NYSCF-Robertson Investigator. B.J. is a NYSCF-Druckenmiller Fellow.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, Nishikawa S. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci USA. 2011;108:14234–14239. doi: 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcala Tabarrozzi AE, Castro CN, Dewey RA, Sogayar MC, Labriola L, Perone MJ. Cell-based interventions to halt autoimmunity in type 1 diabetes mellitus. Clin Exp Immunol. 2013;171:135–146. doi: 10.1111/cei.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Barthson J, Germano CM, Moore F, Maida A, Drucker DJ, Marchetti P, Gysemans C, Mathieu C, Nuñez G, Jurisicova A, Eizirik DL, Gurzov EN. Cytokines tumor necrosis factor-alpha and interferon-gamma induce pancreatic beta-cell apoptosis through STAT1-mediated Bim protein activation. J Biol Chem. 2011;286:39632–39643. doi: 10.1074/jbc.M111.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel MT, Balivada S, Beck AP, Kerrigan MA, Pyle MM, Dekkers JC, Wyatt CR, Rowland RR, Anderson DE, Bossmann SH, Troyer DL. Human xenografts are not rejected in a naturally occurring immunodeficient porcine line: a human tumor model in pigs. BioRes Open Access. 2012;1:63–68. doi: 10.1089/biores.2012.9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao D, Luciani L, Johannesson B, Piszczek A, Rosenthal N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Mol Med. 2014;6:1423–1435. doi: 10.15252/emmm.201303376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, Ratner LE, Egli D, Leibel RL, Accili D. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun. 2014;5:4242. doi: 10.1038/ncomms5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Powers AC, Shultz LD, Greiner DL. Advancing animal models of human type 1 diabetes by engraftment of functional human tissues in immunodeficient mice. Cold Spring Harb Perspect Med. 2012;2:a007757. doi: 10.1101/cshperspect.a007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes. 2014;7:211–223. doi: 10.2147/DMSO.S50789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusko TM, Koya RC, Zhu S, Lee MR, Putnam AL, McClymont SA, Nishimura MI, Han S, Chang LJ, Atkinson MA, Ribas A, Bluestone JA. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS ONE. 2010;5:e11726. doi: 10.1371/journal.pone.0011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50:2323–2331. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- Chatenoud L. Immunology. Teaching the immune system “self” respect and tolerance. Science. 2014;344:1343–1344. doi: 10.1126/science.1256864. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Finkbeiner SR, Weinblatt D, Emmett MJ, Tameire F, Yousefi M, Yang C, Maehr R, Zhou Q, Shemer R, Dor Y, Li C, Spence JR, Stanger BZ. De novo formation of insulin-producing “neo-beta cell islets” from intestinal crypts. Cell Rep. 2014;6:1046–1058. doi: 10.1016/j.celrep.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JYC, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue Eng Part B Rev. 2011;17:235–247. doi: 10.1089/ten.TEB.2011.0004. [DOI] [PubMed] [Google Scholar]
- Cho YM, Lim JM, Yoo DH, Kim JH, Chung SS, Park SG, Kim TH, Oh SK, Choi YM, Moon SY, Park KS, Lee HK. Betacellulin and nicotinamide sustain PDX1 expression and induce pancreatic beta-cell differentiation in human embryonic stem cells. Biochem Biophys Res Commun. 2008;366:129–134. doi: 10.1016/j.bbrc.2007.11.112. [DOI] [PubMed] [Google Scholar]
- Chung YG, Eum JH, Lee JE, Shim SH, Sepilian V, Hong SW, Lee Y, Treff NR, Choi YH, Kimbrel EA, Dittman RE, Lanza R, Lee DR. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 2014;14:777–780. doi: 10.1016/j.stem.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Coppieters KT, Harrison LC, von Herrath MG. Trials in type 1 diabetes: antigen-specific therapies. Clin Immunol. 2013;149:345–355. doi: 10.1016/j.clim.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creusot RJ, Giannoukakis N, Trucco M, Clare-Salzler MJ, Fathman CG. It's time to bring dendritic cell therapy to type 1 diabetes. Diabetes. 2014;63:20–30. doi: 10.2337/db13-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Daoud JT, Petropavlovskaia MS, Patapas JM, Degrandpré CE, Diraddo RW, Rosenberg L, Tabrizian M. Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds. Biomaterials. 2011;32:1536–1542. doi: 10.1016/j.biomaterials.2010.10.036. [DOI] [PubMed] [Google Scholar]
- DCCT-Research-Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: the next pillar of medicine. Sci Transl Med. 2013;5:179ps177. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280:39609–39615. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- Gallichan WS, Kafri T, Krahl T, Verma IM, Sarvetnick N. Lentivirus-mediated transduction of islet grafts with interleukin 4 results in sustained gene expression and protection from insulitis. Hum Gene Ther. 1998;9:2717–2726. doi: 10.1089/hum.1998.9.18-2717. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34:6760–6772. doi: 10.1016/j.biomaterials.2013.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua BelmonteJC, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M, Grapin-Botton A. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–4462. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner DL, Brehm MA, Hosur V, Harlan DM, Powers AC, Shultz LD. Humanized mice for the study of type 1 and type 2 diabetes. Ann N Y Acad Sci. 2011;1245:55–58. doi: 10.1111/j.1749-6632.2011.06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR) Rev Diabet Stud. 2011;8:6–16. doi: 10.1900/RDS.2011.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K, Lemp NA, Logg CR, Nagashima J, Faure-Kumar E, Gomez GG, Kruse CA, Mendez R, Stripecke R, Kasahara N, Cicciarelli JC. Permanent, lowered HLA class I expression using lentivirus vectors with shRNA constructs: averting cytotoxicity by alloreactive T lymphocytes. Transpl Proc. 2006;38:3184–3188. doi: 10.1016/j.transproceed.2006.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- Hayek A, Lopez AD, Beattie GM. Enhancement of pancreatic islet cell monolayer growth by endothelial cell matrix and insulin. In vitro Cell Dev Biol. 1989;25:146–150. doi: 10.1007/BF02626171. [DOI] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- Herold KC, Vignali DA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol. 2013;13:243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H, Shang L, Martinez H, Freeby M, Gallagher MP, Ludwig T, Deng L, Greenberg E, Leduc C, Chung WK, Goland R, Leibel RL, Egli D. iPSC-derived beta cells model diabetes due to glucokinase deficiency. J Clin Invest. 2013;123:3146–3153. doi: 10.1172/JCI67638. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, Ringnalda F, Begthel H, Hamer K, Mulder J, van Es JH, de Koning E, Vries RG, Heimberg H, Clevers H. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Mougneau E, Ferlin W, Jeske D, Hofman P, Homann D, Beaudoin L, Schrike C, Von Herrath M, Lehuen A, Glaichenhaus N. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity. 2002;16:169–181. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Crock P, Rogers D, Mikuni M, Kumashiro H, Higashi K, Sobue G, Oka Y, Permutt MA. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nat Genet. 1998;20:143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- Johannesson B, Sagi I, Gore A, Paull D, Yamada M, Golan-Lev T, Li Z, LeDuc C, Shen Y, Stern S, Xu N, Ma H, Kang E, Mitalipov S, Sauer MV, Zhang K, Benvenisty N, Egli D. Comparable frequencies of coding mutations and loss of imprinting in human pluripotent cells derived by nuclear transfer and defined factors. Cell Stem Cell. 2014;15:634–642. doi: 10.1016/j.stem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Johnsen AK, Templeton DJ, Sy M, Harding CV. Deficiency of transporter for antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J Immunol. 1999;163:4224–4231. [PubMed] [Google Scholar]
- Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, Greenberg E, Spitzer TR, Savage DG, Tahara H, Choi G, Yang YG, Sykes M. A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med. 2012;4:125ra130. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasagi S, Zhang P, Che L, Abbatiello B, Maruyama T, Nakatsukasa H, Zanvit P, Jin W, Konkel JE, Chen W. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci Transl Med. 2014;6:241ra278. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D'Amour KA, Kroon E, Moorman M, Baetge EE, Bang AG. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, Li H, Collins JJ, Feinberg AP, Daley GQ. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- Kind A, Colman A. Therapeutic cloning: needs and prospects. Semin Cell Dev Biol. 1999;10:279–286. doi: 10.1006/scdb.1999.0277. [DOI] [PubMed] [Google Scholar]
- Klinke DJ., II Extent of beta cell destruction is important but insufficient to predict the onset of type 1 diabetes mellitus. PLoS ONE. 2008;3:e1374. doi: 10.1371/journal.pone.0001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi-Aoi M, Ohnuki M, Takahashi K, Okita K, Noma H, Sawamura Y, Teramoto I, Narita M, Sato Y, Ichisaka T, Amano N, Watanabe A, Morizane A, Yamada Y, Sato T, Takahashi J, Yamanaka S. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:20569–20574. doi: 10.1073/pnas.1319061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy M, Li J, Fellows GF, Rosenberg L, Goodyer CG, Wang R. Integrin {alpha}3, but not {beta}1, regulates islet cell survival and function via PI3K/Akt signaling pathways. Endocrinology. 2011;152:424–435. doi: 10.1210/en.2010-0877. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8:274–284. doi: 10.1016/j.scr.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Cibelli JB, West MD. Human therapeutic cloning. Nat Med. 1999a;5:975–977. doi: 10.1038/12404. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Jackson R, Sullivan A, Ringeling J, McGrath C, Kuhtreiber W, Chick WL. Xenotransplantation of cells using biodegradable microcapsules. Transplantation. 1999b;67:1105–1111. doi: 10.1097/00007890-199904270-00004. [DOI] [PubMed] [Google Scholar]
- Lee K, Kwon DN, Ezashi T, Choi YJ, Park C, Ericsson AC, Brown AN, Samuel MS, Park KW, Walters EM, Kim DY, Kim JH, Franklin CL, Murphy CN, Roberts RM, Prather RS, Kim JH. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci USA. 2014;111:7260–7265. doi: 10.1073/pnas.1406376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembert N, Wesche J, Petersen P, Doser M, Zschocke P, Becker HD, Ammon HP. Encapsulation of islets in rough surface, hydroxymethylated polysulfone capillaries stimulates VEGF release and promotes vascularization after transplantation. Cell Transplant. 2005;14:97–108. [PubMed] [Google Scholar]
- Lewis EC. Expanding the clinical indications for alpha(1)-antitrypsin therapy. Mol Med. 2012;18:957–970. doi: 10.2119/molmed.2011.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu EH, Digon BJ, 3rd, Hirshberg B, Chang R, Wood BJ, Neeman Z, Kam A, Wesley RA, Polly SM, Hofmann RM, Rother KI, Harlan DM. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia. 2009;52:1369–1380. doi: 10.1007/s00125-009-1342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Lucas-Clerc C, Massart C, Campion JP, Launois B, Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9–20. doi: 10.1016/0303-7207(93)90046-m. [DOI] [PubMed] [Google Scholar]
- Lutterotti A, Yousef S, Sputtek A, Stürner KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, Schippling S, Miller SD, Sospedra M, Martin R. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5:188ra175. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M Group I.-J.T.D.C.T.A. Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol. 2010;160:176–184. doi: 10.1111/j.1365-2249.2010.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AD, Bernards DA, Lowe RD, Desai TA. Patterning of mono- and multilayered pancreatic beta-cell clusters. Langmuir. 2010;26:9943–9949. doi: 10.1021/la1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merani S, Shapiro AM. Current status of pancreatic islet transplantation. Clin Sci. 2006;110:611–625. doi: 10.1042/CS20050342. [DOI] [PubMed] [Google Scholar]
- Mfopou JK, Chen B, Mateizel I, Sermon K, Bouwens L. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010;138:2233–2245. doi: 10.1053/j.gastro.2010.02.056. 2245 e2231-2214. [DOI] [PubMed] [Google Scholar]
- Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Farney AC, Stratta RJ, Atala A, Opara EC, Soker S. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34:5488–5495. doi: 10.1016/j.biomaterials.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N, Gu Y, Hori H, Balamurugan AN, Touma M, Kawakami Y, Wang W, Baba TT, Satake A, Nozawa M, Tabata Y, Inoue K. Evaluation of insulin secretion of isolated rat islets cultured in extracellular matrix. Cell Transplant. 2001;10:447–451. [PubMed] [Google Scholar]
- Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, Lorenzi G, Zinman B Group D.E.R. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62:3976–3986. doi: 10.2337/db13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino K, Toyoda M, Yamazaki-Inoue M, Fukawatase Y, Chikazawa E, Sakaguchi H, Akutsu H, Umezawa A. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet. 2011;7:e1002085–e1002085. doi: 10.1371/journal.pgen.1002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J, Gaglia JL, Turvey SE, Campbell C, Benoist C, Mathis D. Islet recovery and reversal of murine type 1 diabetes in the absence of any infused spleen cell contribution. Science. 2006;311:1775–1778. doi: 10.1126/science.1124004. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, Daley GQ, Elefanty AG, Stanley EG, Keller G. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg-Welsh C. Long-term culture in matrigel enhances the insulin secretion of fetal porcine islet-like cell clusters in vitro. Pancreas. 2001;22:157–163. doi: 10.1097/00006676-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Ohmine S, Squillace KA, Hartjes KA, Deeds MC, Armstrong AS, Thatava T, Sakuma T, Terzic A, Kudva Y, Ikeda Y. Reprogrammed keratinocytes from elderly type 2 diabetes patients suppress senescence genes to acquire induced pluripotency. Aging. 2012;4:60–73. doi: 10.18632/aging.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando G, Gianello P, Salvatori M, Stratta RJ, Soker S, Ricordi C, Dominguez-Bendala J. Cell replacement strategies aimed at reconstitution of the beta-cell compartment in type 1 diabetes. Diabetes. 2014;63:1433–1444. doi: 10.2337/db13-1742. [DOI] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AV, Russ HA, Khan IS, LaFlam TN, Metzger TC, Anderson MS, Hebrok M. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell. 2013;13:219–229. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti R, Henderson TE, Wang Y, Montrose-Rafizadeh C, Egan JM. Insulin release and insulin mRNA levels in rat islets of Langerhans cultured on extracellular matrix. Pancreas. 1996;13:47–54. doi: 10.1097/00006676-199607000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Headen DM, Taylor WR, Thule PM, Garcia AJ. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34:4602–4611. doi: 10.1016/j.biomaterials.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Xu D, Miller SD. Tolerance strategies employing antigen-coupled apoptotic cells and carboxylated PLG nanoparticles for the treatment of type 1 diabetes. Rev Diabet Stud. 2012;9:319–327. doi: 10.1900/RDS.2012.9.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Gu Y, Sakata N, Kim D, Shirouzu Y, Yamamoto C, Hiura A, Sumi S, Inoue K. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials. 2004;25:5885–5892. doi: 10.1016/j.biomaterials.2004.01.050. [DOI] [PubMed] [Google Scholar]
- Qi M. Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 diabetes mellitus. Adv Med. 2014;2014:429710. doi: 10.1155/2014/429710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressing ME, Luteijn RD, Horst D, Wiertz EJ. Viral interference with antigen presentation: trapping TAP. Mol Immunol. 2013;55:139–142. doi: 10.1016/j.molimm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, Ao Z, Warnock GL, Kieffer TJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O'Neil JJ, Kieffer TJ. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31:2432–2442. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Rilo HL, Ahmad SA, D'Alessio D, Iwanaga Y, Kim J, Choe KA, Moulton JS, Martin J, Pennington LJ, Soldano DA, Biliter J, Martin SP, Ulrich CD, Somogyi L, Welge J, Matthews JB, Lowy AM. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978–989. doi: 10.1016/j.gassur.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F, Marchetti P, Dotta F. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol. 2010;159:338–343. doi: 10.1111/j.1365-2249.2009.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata N, Sumi S, Yoshimatsu G, Goto M, Egawa S, Unno M. Encapsulated islets transplantation: past, present and future. World J Gastrointest Pathophysiol. 2012;3:19–26. doi: 10.4291/wjgp.v3.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- Scharp DW, Swanson CJ, Olack BJ, Latta PP, Hegre OD, Doherty EJ, Gentile FT, Flavin KS, Ansara MF, Lacy PE. Protection of encapsulated human islets implanted without immunosuppression in patients with type I or type II diabetes and in nondiabetic control subjects. Diabetes. 1994;43:1167–1170. doi: 10.2337/diab.43.9.1167. [DOI] [PubMed] [Google Scholar]
- Schulz TC, Young HY, Agulnick AD, Babin MJ, Baetge EE, Bang AG, Bhoumik A, Cepa I, Cesario RM, Haakmeester C, Kadoya K, Kelly JR, Kerr J, Martinson LA, McLean AB, Moorman MA, Payne JK, Richardson M, Ross KG, Sherrer ES, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS ONE. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. sernova.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Hua H, Foo K, Martinez H, Watanabe K, Zimmer M, Kahler DJ, Freeby M, Chung W, LeDuc C, Goland R, Leibel RL, Egli D. beta-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63:923–933. doi: 10.2337/db13-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh SJ, Chou FC, Yu PN, Lin WC, Chang DM, Roffler SR, Sytwu HK. Transgenic expression of single-chain anti-CTLA-4 Fv on beta cells protects nonobese diabetic mice from autoimmune diabetes. J Immunol. 2009;183:2277–2285. doi: 10.4049/jimmunol.0900679. [DOI] [PubMed] [Google Scholar]
- Shih HP, Kopp JL, Sandhu M, Dubois CL, Seymour PA, Grapin-Botton A, Sander M. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. 2012;139:2488–2499. doi: 10.1242/dev.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes. 2013;62:9–17. doi: 10.2337/db12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck AK, Winter WE. Review on monogenic diabetes. Curr Opin Endocrinol Diabetes Obes. 2011;18:252–258. doi: 10.1097/MED.0b013e3283488275. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Marcoux Y, Ghahary A, Rabinovitch A. Gene transfection and expression of transforming growth factor-beta1 in nonobese diabetic mouse islets protects beta-cells in syngeneic islet grafts from autoimmune destruction. Cell Transplant. 2002;11:519–528. [PubMed] [Google Scholar]
- Sui L, Geens M, Sermon K, Bouwens L, Mfopou JK. Role of BMP signaling in pancreatic progenitor differentiation from human embryonic stem cells. Stem Cell Rev. 2013;9:569–577. doi: 10.1007/s12015-013-9435-6. [DOI] [PubMed] [Google Scholar]
- Sun X, Xu J, Lu H, Liu W, Miao Z, Sui X, Liu H, Su L, Du W, He Q, Chen F, Shi Y, Deng H. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell. 2013;13:230–236. doi: 10.1016/j.stem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012;44:406–412. doi: 10.1038/ng.2215. S401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Kanai-Azuma M, Kanai Y. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev. 2003;13:393–400. doi: 10.1016/s0959-437x(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Teo AK, Windmueller R, Johansson BB, Dirice E, Njolstad PR, Tjora E, Raeder H, Kulkarni RN. Derivation of human induced pluripotent stem cells from patients with maturity onset diabetes of the young. J Biol Chem. 2013;288:5353–5356. doi: 10.1074/jbc.C112.428979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY) BMJ. 2011;343:d6044. doi: 10.1136/bmj.d6044. [DOI] [PubMed] [Google Scholar]
- Thisse B, Wright CV, Thisse C. Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature. 2000;403:425–428. doi: 10.1038/35000200. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Perry D, Brusko TM. Autologous regulatory T cells for the treatment of type 1 diabetes. Curr Diab Rep. 2012;12:623–632. doi: 10.1007/s11892-012-0304-5. [DOI] [PubMed] [Google Scholar]
- Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Torikai H, Reik A, Soldner F, Warren EH, Yuen C, Zhou Y, Crossland DL, Huls H, Littman N, Zhang Z, Tykodi SS, Kebriaei P, Lee DA, Miller JC, Rebar EJ, Holmes MC, Jaenisch R, Champlin RE, Gregory PD, Cooper LJ. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122:1341–1349. doi: 10.1182/blood-2013-03-478255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Leslie S, Martin NG, Peschanski M, Rao M, Taylor CJ, Trounson A, Turner D, Yamanaka S, Wilmut I. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Tyden G, Reinholt FP, Sundkvist G, Bolinder J. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. N Engl J Med. 1996;335:860–863. doi: 10.1056/NEJM199609193351205. [DOI] [PubMed] [Google Scholar]
- Vaxillaire M, Froguel P. Monogenic diabetes in the young, pharmacogenetics and relevance to multifactorial forms of type 2 diabetes. Endocr Rev. 2008;29:254–264. doi: 10.1210/er.2007-0024. [DOI] [PubMed] [Google Scholar]
- Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, Diamantopoulos S, Standifer N, Geubtner K, Falk BA, Ichii H, Takahashi H, Snowhite I, Chen Z, Mendez A, Chen L, Sageshima J, Ruiz P, Ciancio G, Ricordi C, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59:947–957. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wu H, Li Y, Szulwach KE, Lin L, Li X, Chen IP, Goldlust IS, Chamberlain SJ, Dodd A, Gong H, Ananiev G, Han JW, Yoon YS, Rudd MK, Yu M, Song CX, He C, Chang Q, Warren ST, Jin P. Subtelomeric hotspots of aberrant 5-hydroxymethylcytosine-mediated epigenetic modifications during reprogramming to pluripotency. Nat Cell Biol. 2013;15:700–711. doi: 10.1038/ncb2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 2008;16:1049–1057. [PubMed] [Google Scholar]
- Weissbein U, Ben-David U, Benvenisty N. Virtual karyotyping reveals greater chromosomal stability in neural cells derived by transdifferentiation than those from stem cells. Cell Stem Cell. 2014;15:687–691. doi: 10.1016/j.stem.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Whitman M. Nodal signaling in early vertebrate embryos: themes and variations. Dev Cell. 2001;1:605–617. doi: 10.1016/s1534-5807(01)00076-4. [DOI] [PubMed] [Google Scholar]
- Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E, Goland RS, Leibel RL, Solomon SL, Benvenisty N, Sauer MV, Egli D. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533–536. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- Yang H, O'Hali W, Kearns H, Wright JR., Jr Long-term function of fish islet xenografts in mice by alginate encapsulation. Transplantation. 1997;64:28–32. doi: 10.1097/00007890-199707150-00006. [DOI] [PubMed] [Google Scholar]
- Zaldumbide A, Alkemade G, Carlotti F, Nikolic T, Abreu JR, Engelse MA, Skowera A, de Koning EJ, Peakman M, Roep BO, Hoeben RC, Wiertz EJ. Genetically engineered human islets protected from CD8-mediated autoimmune destruction in vivo. Mol Ther. 2013;21:1592–1601. doi: 10.1038/mt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]