Abstract
Autophagy plays a key role in the maintenance of cellular homeostasis. In healthy cells, such a homeostatic activity constitutes a robust barrier against malignant transformation. Accordingly, many oncoproteins inhibit, and several oncosuppressor proteins promote, autophagy. Moreover, autophagy is required for optimal anticancer immunosurveillance. In neoplastic cells, however, autophagic responses constitute a means to cope with intracellular and environmental stress, thus favoring tumor progression. This implies that at least in some cases, oncogenesis proceeds along with a temporary inhibition of autophagy or a gain of molecular functions that antagonize its oncosuppressive activity. Here, we discuss the differential impact of autophagy on distinct phases of tumorigenesis and the implications of this concept for the use of autophagy modulators in cancer therapy.
Keywords: adaptive stress responses, Beclin 1, inflammation, KRAS, mitophagy
Introduction
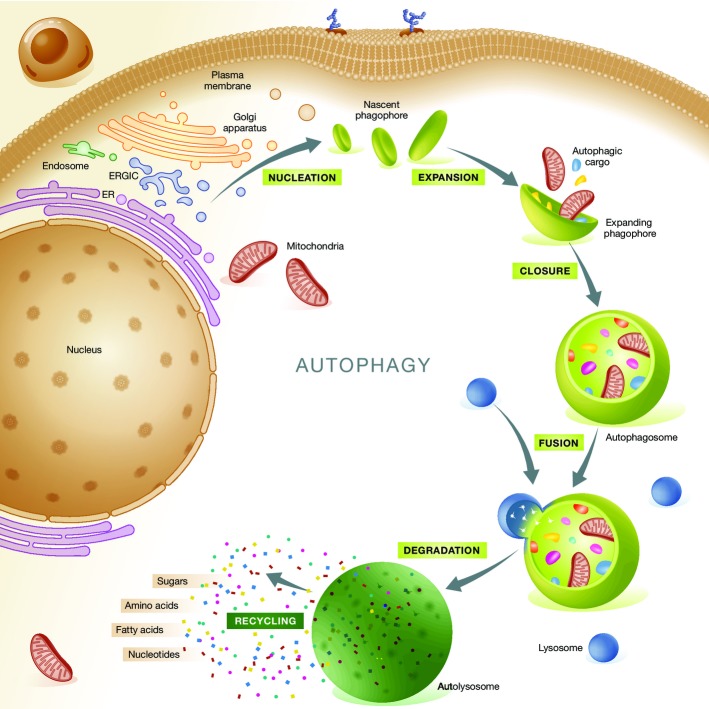
Macroautophagy (herein referred to as autophagy) is a mechanism that mediates the sequestration of intracellular entities within double-membraned vesicles, so-called autophagosomes, and their delivery to lysosomes for bulk degradation (He & Klionsky, 2009). Autophagosomes derive from so-called phagophores, membranous structures also known as ‘isolation membranes’ whose precise origin remains a matter of debate (Lamb et al, 2013). Indeed, the plasma membrane, endoplasmic reticulum (ER), Golgi apparatus, ER-Golgi intermediate compartment (ERGIC), and mitochondria have all been indicated as possible sources for phagophores (Lamb et al, 2013). Upon closure, autophagosomes fuse with lysosomes, forming so-called autolysosomes, and their cargo is exposed to the catalytic activity of lysosomal hydrolases (Mizushima & Komatsu, 2011). The degradation products of the autophagosomal cargo, which includes sugars, nucleosides/nucleotides, amino acids and fatty acids, can be transported back to the cytoplasm and presumably re-enter cellular metabolism (Fig1) (Rabinowitz & White, 2010; Galluzzi et al, 2013). Of note, the molecular machinery that mediates autophagy is evolutionary conserved, and several components thereof have initially been characterized in yeast (He & Klionsky, 2009).
Figure 1.
General organization of autophagic responses
Autophagy initiates with the progressive segregation of cytoplasmic material by double-membraned structures commonly known as phagophores or isolation membranes. Phagophores nucleate from the endoplasmic reticulum (ER), but several other membranous organelles have been shown to contribute to their elongation, including the Golgi apparatus, ER-Golgi intermediate compartment (ERGIC), plasma membrane, mitochondria and recycling endosomes. Completely sealed phagophores, which are known as autophagosomes, fuse with lysosomes to form autolysosomes. This promotes the activation of lysosomal hydrolases and hence causes the breakdown of the autophagosomal cargo. The products of these catabolic reactions reach the cytosol via transporters of the lysosomal membrane and are recycled by anabolic or bioenergetic circuitries.
In physiological scenarios, autophagy proceeds at basal levels, ensuring the continuous removal of superfluous, ectopic or damaged (and hence potentially dangerous) entities, including organelles and/or portions thereof (Green et al, 2011). Baseline autophagy mediates a key homeostatic function, constantly operating as an intracellular quality control system (Mizushima et al, 2008; Green et al, 2011). Moreover, the autophagic flux can be upregulated in response to a wide panel of stimuli, including (but not limited to) nutritional, metabolic, oxidative, pathogenic, genotoxic and proteotoxic cues (Kroemer et al, 2010). Often, stimulus-induced autophagy underlies and sustains an adaptive response to stress with cytoprotective functions (Kroemer et al, 2010; Mizushima & Komatsu, 2011). Indeed, the pharmacological or genetic inhibition of autophagy generally limits the ability of cells to cope with stress and restore homeostasis (Mizushima et al, 2008; Kroemer et al, 2010). This said, regulated instances of cell death that causally depend on the autophagic machinery have been described (Denton et al, 2009; Denton et al, 2012b; Liu et al, 2013b; Galluzzi et al, 2015). The detailed discussion of such forms of autophagic cell death, however, is beyond the scope of this review.
Autophagy is tightly regulated. The best characterized repressor of autophagic responses is mechanistic target of rapamycin (MTOR) complex I (MTORCI) (Laplante & Sabatini, 2012). Thus, several inducers of autophagy operate by triggering signal transduction cascades that result in the inhibition of MTORCI (Inoki et al, 2012). Among other effects, this allows for the activation of several proteins that are crucial for the initiation of autophagic responses, such as unc-51-like autophagy-activating kinase 1 (ULK1, the mammalian ortholog of yeast Atg1) and autophagy-related 13 (ATG13) (Hosokawa et al, 2009; Nazio et al, 2013). A major inhibitor of MTORCI is protein kinase, AMP-activated (PRKA, best known as AMPK), which is sensitive to declining ATP/AMP ratios (Mihaylova & Shaw, 2011). Besides inhibiting the catalytic activity of MTORCI, AMPK directly stimulates autophagy by phosphorylating ULK1 as well as phosphatidylinositol 3-kinase, catalytic subunit type 3 (PIK3C3, best known as VPS34) and Beclin 1 (BECN1, the mammalian ortholog of yeast Atg6), two components of a multiprotein complex that produces a lipid that is essential for the biogenesis of autophagosomes, namely phosphatidylinositol 3-phosphate (Egan et al, 2011; Zhao & Klionsky, 2011; Kim et al, 2013). Autophagy also critically relies on two ubiquitin-like conjugation systems, both of which involve ATG7 (Mizushima, 2007). These systems catalyze the covalent linkage of ATG5 to ATG12 and ATG16-like 1 (ATG16L1), and that of phosphatidylethanolamine to proteins of the microtubule-associated protein 1 light chain 3 (MAP1LC3, best known as LC3) family, including MAP1LC3B (LC3B, the mammalian ortholog of yeast Atg8) (Mizushima, 2007). A detailed discussion of additional factors that are involved in the control and execution of autophagic responses can be found in Boya et al (2013).
Importantly, autophagosomes can either take up intracellular material in a relatively non-selective manner or deliver very specific portions of the cytoplasm to degradation, mainly depending on the initiating stimulus (Weidberg et al, 2011; Stolz et al, 2014). Thus, while non-selective forms of autophagy normally develop in response to cell-wide alterations, most often of a metabolic nature, highly targeted autophagic responses follow specific perturbations of intracellular homeostasis, such as the accumulation of permeabilized mitochondria (mitophagy), the formation of protein aggregates (aggrephagy), and pathogen invasion (xenophagy) (Okamoto, 2014; Randow & Youle, 2014). Several receptors participate in the selective recognition and recruitment of autophagosomal cargoes in the course of targeted autophagic responses (Rogov et al, 2014; Stolz et al, 2014). The autophagy receptor best characterized to date, that is, sequestosome 1 (SQSTM1, best known as p62), recruits ubiquitinated proteins to autophagosomes by virtue of an ubiquitin-associated (UBA) and a LC3-binding domain (Pankiv et al, 2007).
Owing to its key role in the preservation of intracellular homeostasis, autophagy constitutes a barrier against various degenerative processes that may affect healthy cells, including malignant transformation. Thus, autophagy mediates oncosuppressive effects. Accordingly, proteins with bona fide oncogenic potential inhibit autophagy, while many proteins that prevent malignant transformation stimulate autophagic responses (Morselli et al, 2011). Moreover, autophagy is involved in several aspects of anticancer immunosurveillance, that is, the process whereby the immune system constantly eliminates potentially tumorigenic cells before they establish malignant lesions (Ma et al, 2013). However, autophagy also sustains the survival and proliferation of neoplastic cells exposed to intracellular and environmental stress, hence supporting tumor growth, invasion and metastatic dissemination, at least in some settings (Kroemer et al, 2010; Guo et al, 2013b). Here, we discuss the molecular and cellular mechanisms accounting for the differential impact of autophagy on malignant transformation and tumor progression.
Autophagy and malignant transformation
In various murine models, defects in the autophagic machinery caused by the whole-body or tissue-specific, heterozygous or homozygous knockout of essential autophagy genes accelerate oncogenesis. For instance, Becn1+/− mice (Becn1−/− animals are not viable) spontaneously develop various malignancies, including lymphomas as well as lung and liver carcinomas (Liang et al, 1999; Qu et al, 2003; Yue et al, 2003; Mortensen et al, 2011), and are more susceptible to parity-associated and Wnt1-driven mammary carcinogenesis than their wild-type counterparts (Cicchini et al, 2014). Similarly, mice lacking one copy of the gene coding for the BECN1 interactor autophagy/beclin-1 regulator 1 (AMBRA1) also exhibit a higher rate of spontaneous tumorigenesis than their wild-type littermates (Cianfanelli et al, 2015). Mice bearing a systemic mosaic deletion of Atg5 or a liver-specific knockout of Atg7 spontaneously develop benign hepatic neoplasms more frequently than their wild-type counterparts (Takamura et al, 2011). Moreover, carcinogen-induced fibrosarcomas appear at an accelerated pace in autophagy-deficient Atg4c−/− mice (Marino et al, 2007), as do KRASG12D-driven and BRAFV600E-driven lung carcinomas in mice bearing lung-restricted Atg5 or Atg7 deletions, respectively (Strohecker et al, 2013; Rao et al, 2014). The pancreas-specific knockout of Atg5 or Atg7 also precipitates the emergence of KRASG12D-driven pre-malignant pancreatic lesions (Rosenfeldt et al, 2013; Yang et al, 2014).
Several mechanisms can explain, at least in part, the oncosuppressive functions of autophagy. Proficient autophagic responses may suppress the accumulation of genetic and genomic defects that accompanies malignant transformation, through a variety of mechanisms. Reactive oxygen species (ROS) are highly genotoxic, and autophagy prevents their overproduction by removing dysfunctional mitochondria (Green et al, 2011; Takahashi et al, 2013) as well as redox-active aggregates of ubiquitinated proteins (Komatsu et al, 2007; Mathew et al, 2009). In addition, autophagic responses have been involved in the disposal of micronuclei arising upon perturbation of the cell cycle (Rello-Varona et al, 2012), in the degradation of retrotransposing RNAs (Guo et al, 2014), as well as in the control of the levels of ras homolog family member A (RHOA), a small GTPase involved in cytokinesis (Belaid et al, 2013). Finally, various components of the autophagic machinery appear to be required for cells to mount adequate responses to genotoxic stress (Karantza-Wadsworth et al, 2007; Mathew et al, 2007; Park et al, 2014). This said, the precise mechanisms underlying such genome-stabilizing effects remain elusive, implying that the impact of autophagy on DNA-damage responses may be indirect. Further investigation is required to shed light on this possibility.
Autophagy is intimately implicated in the maintenance of physiological metabolic homeostasis (Galluzzi et al, 2014; Kenific & Debnath, 2015). Malignant transformation generally occurs along with a shift from a predominantly catabolic consumption of glycolysis-derived pyruvate by oxidative phosphorylation to a metabolic pattern in which: (1) glucose uptake is significantly augmented to sustain anabolic reactions and antioxidant defenses, (2) mitochondrial respiration remains high to satisfy increased energy demands; and (3) several amino acids, including glutamine and serine, become essential as a means to cope with exacerbated metabolic functions (Hanahan & Weinberg, 2011; Galluzzi et al, 2013). Autophagy preserves optimal bioenergetic functions by ensuring the removal of dysfunctional mitochondria (Green et al, 2011), de facto counteracting the metabolic rewiring that accompanies malignant transformation. Moreover, the autophagic degradation of p62 participates in a feedback circuitry that regulates MTORCI activation in response to nutrient availability (Linares et al, 2013; Valencia et al, 2014).
Autophagy appears to ensure the maintenance of normal stem cells. This is particularly relevant for hematological malignancies, which are normally characterized by changes in proliferation or differentiation potential that alter the delicate equilibrium between toti-, pluri- and unipotent precursors in the bone marrow (Greim et al, 2014). The ablation of Atg7 in murine hematopoietic stem cells (HSCs) has been shown to disrupt tissue architecture, eventually resulting in the expansion of a population of bone marrow progenitor cells with neoplastic features (Mortensen et al, 2011). Along similar lines, the tissue-specific deletion of the gene coding for the ULK1 interactor RB1-inducible coiled-coil 1 (RB1CC1, best known as FIP200) alters the fetal HSC compartment in mice, resulting in severe anemia and perinatal lethality (Liu et al, 2010). Interestingly, murine Rb1cc1−/− HSCs do not exhibit increased rates of apoptosis, but an accrued proliferative capacity (Liu et al, 2010). The deletion of Rb1cc1 in murine neuronal stem cells (NSCs) also causes a functional impairment that compromises postnatal neuronal differentiation (Wang et al, 2013). However, this effect appears to stem from the failure of murine Rb1cc1−/− HNCs to control redox homeostasis, resulting in the activation of a tumor protein p53 (TP53)-dependent apoptotic response (Wang et al, 2013). Finally, Becn1+/− mice display an expansion of progenitor-like mammary epithelial cells (Cicchini et al, 2014). Of note, autophagy also appears to be required for the preservation of normal stem cell compartments in the human system. Indeed, human hematopoietic, dermal, and epidermal stem cells transfected with a short-hairpin RNA (shRNA) specific for ATG5 lose their ability to self-renew while differentiating into neutrophils, fibroblasts, and keratinocytes, respectively (Salemi et al, 2012).
It has been proposed that autophagy contributes to oncogene-induced cell death or oncogene-induced senescence, two fundamental oncosuppressive mechanisms. The activation of various oncogenes imposes indeed a significant stress on healthy cells, a situation that is normally aborted through the execution of a cell death program (Elgendy et al, 2011), or upon the establishment of permanent proliferative arrest (cell senescence) that engages the innate arm of the immune system (Iannello et al, 2013). The partial depletion of ATG5, ATG7 or BECN1 limited the demise of human ovarian cancer cells pharmacologically stimulated to express HRASG12V from an inducible construct (Elgendy et al, 2011). Similarly, shRNAs specific for ATG5 or ATG7 prevented oncogene-induced senescence in primary human melanocytes or human diploid fibroblasts (HDFs) expressing BRAFV600E or HRASG12V (Young et al, 2009; Liu et al, 2013a). Accordingly, the overexpression of the ULK1 homolog ULK3 was sufficient to limit the proliferative potential of HDFs while promoting autophagy (Young et al, 2009). Moreover, both pharmacological inhibitors of autophagy and small-interfering RNAs targeting ATG5, ATG7 or BECN1 prevented spontaneous senescence in HDFs while preventing the degradation of an endogenous, dominant-negative TP53 variant (Horikawa et al, 2014). Finally, ectopic ATG5 expression reduced the colony-forming ability of melanoma cell lines normally characterized by low ATG5 levels, an effect that could be reproduced by the administration of autophagy inducers (Liu et al, 2013a). Apparently at odds with these results, HRASG12V fails to induce senescence in mouse embryonic fibroblasts (MEFs) lacking transformation-related protein 53 binding protein 2 (Trp53bp2), correlating with the stabilization of Atg5/Atg12 complexes and consequent upregulation of the autophagic flux. In line with this notion, ectopic expression of Atg5 prevented Trp53bp2-sufficient MEFs from entering senescence upon overexpression of HRASG12V (Wang et al, 2012b). Thus, while in some cells autophagy appears to inhibit malignant transformation by favoring oncogene-induced senescence, this may not be a general mechanism of autophagy-mediated oncosuppression.
It has been suggested that autophagy is involved in the degradation of oncogenic proteins, including mutant (but not wild-type) TP53 (Rodriguez et al, 2012; Choudhury et al, 2013; Garufi et al, 2014), p62 (Duran et al, 2008; Mathew et al, 2009; Ling et al, 2012), PML-RARA (Isakson et al, 2010; Wang et al, 2011), and BCR-ABL1 (Goussetis et al, 2012). Mutant TP53 often accumulates in neoplastic cells and operates as a dominant-negative factor, thereby interfering with the oncosuppressive function of the wild-type protein (de Vries et al, 2002). Cancer cells depleted of ULK1, BECN1 or ATG5 tend to accumulate increased amounts of mutant TP53, whereas the transgene-driven overexpression of BECN1 or ATG5 results in mutant TP53 depletion (Choudhury et al, 2013). Such an autophagy-dependent degradation of mutant TP53 would therefore restore the ability of wild-type TP53 to inhibit malignant transformation, at least in some settings. It is worth noting that both ATG5 and ATG7 have been involved in the regulation of TP53-dependent adaptive responses to stress (Lee et al, 2012; Salemi et al, 2012). However, this activity appears to be independent of autophagy, at least in the case of ATG7 (Lee et al, 2012). Interestingly, p62 itself has been ascribed with potentially oncogenic functions, including a key role in the transduction of RAS-elicited signals as well as in the activation of a feedforward loop involving the cytoprotective transcription factor NF-κB driven by oncogenic stress (Duran et al, 2008; Mathew et al, 2009; Takamura et al, 2011; Ling et al, 2012). Autophagy may therefore inhibit oncogenesis by limiting p62 availability (Mathew et al, 2009), at least in some settings.
The t(9;22)(q34;q11) translocation is found in about 90% of chronic myeloid leukemia patients, resulting in the synthesis of a fusion protein that involves breakpoint cluster region (BCR) and ABL proto-oncogene 1 (ABL1) (Ben-Neriah et al, 1986). BCR-ABL1 is a constitutively active kinase and is etiologically involved in leukemogenesis, as demonstrated by the outstanding clinical success of imatinib mesylate, a BCR-ABL1-targeting kinase inhibitor (Druker et al, 2001). Arsenic trioxide, a chemotherapeutic agent commonly employed against various forms of leukemia, appears to trigger the p62-dependent and cathepsin B-dependent degradation of BCR-ABL1 in leukemic progenitors (Goussetis et al, 2012). In line with this notion, the pharmacological or genetic inhibition of autophagy or cathepsin B reportedly limits the antileukemic potential of arsenic trioxide (Goussetis et al, 2012). The t(15;17)(q22;q21) translocation can be documented in 95% of promyelocytic leukemia cases, resulting in the expression of a chimera that involves promyelocytic leukemia (PML) and retinoic acid receptor, alpha (RARA) (Goddard et al, 1991). PML-RARA blocks normal retinoic acid-dependent myeloid differentiation, de facto driving leukemogenesis (Rousselot et al, 1994). Patients expressing PML-RARA generally benefit from the administration of all-trans retinoic acid (ATRA), resulting in PML-RARA degradation and restored myeloid differentiation (Wang et al, 2011). Pharmacological and genetic evidence suggests that autophagy is implicated in both ATRA- and arsenic trioxide-driven PML-RARA degradation (Isakson et al, 2010; Wang et al, 2011). Further experimentation is required to understand whether autophagy degrades potentially oncogenic proteins in cells not exposed to chemotherapeutic agents.
Autophagy is implicated in immune responses that prevent the establishment and proliferation of malignant cells (Ma et al, 2013). At least in some circumstances, dying malignant cells are capable of recruiting antigen-presenting cells (APCs) and other cellular components of the immune system, resulting in the elicitation of innate and/or adaptive antitumor immune responses (Deretic et al, 2013; Kroemer et al, 2013). On the one hand, autophagic responses are required for dying neoplastic cells to release ATP in optimal amounts, which not only recruits APCs through purinergic receptor P2Y, G-protein coupled, 2 (P2RY2), but also activates them to release immunostimulatory chemokines through purinergic receptor P2X, ligand-gated ion channel, 7 (P2RX7) (Michaud et al, 2011). On the other hand, autophagy in immune cells is implicated in several steps of both adaptive and innate immune responses (Ma et al, 2013). Thus, both cancer cell-intrinsic and systemic defects in autophagy may prevent the host immune system to properly recognize and eliminate pre-malignant and malignant cells.
Autophagy mediates potent anti-inflammatory effects (Deretic et al, 2013). At least in some cases, malignant transformation is stimulated by an inflammatory microenvironment, which contains high amounts of potentially genotoxic ROS as well as various mitogenic cytokines (Coussens et al, 2013). Proficient autophagic responses limit inflammation as: (1) they efficiently dispose of the so-called inflammasomes (the supramolecular platforms that are responsible for the maturation and secretion of pro-inflammatory interleukin-1β and interleukin-18), as well as damaged mitochondria, which would otherwise release endogenous inflammasome activators (Nakahira et al, 2011; Zitvogel et al, 2012); (2) they are linked to the inhibition of pro-inflammatory signals delivered by some pattern recognition receptors, such as RIG-I-like receptors (Jounai et al, 2007); (3) they limit the abundance of B-cell CLL/lymphoma 10 (BCL10), a protein involved in pro-inflammatory NF-κB signaling (Paul et al, 2012); (4) they are connected to the inhibition of transmembrane protein 173 (TM173, best known as STING), a pattern recognition receptor involved in the delivery of pro-inflammatory cues in response to cytosolic nucleic acids (Saitoh et al, 2009).
Finally, autophagy may suppress carcinogenesis owing to its key role in the first line of defense against viral and bacterial infection (Deretic et al, 2013). Indeed, several potentially carcinogenic pathogens potently activate autophagy upon infection. These pathogens include hepatitis B virus (which promotes hepatocellular carcinoma), human herpesvirus 8 (which causes Kaposi's sarcoma and contributes to the pathogenesis of primary effusion lymphoma and multicentric Castleman's disease), human papillomavirus type 16 and 18 (HPV-16 and HPV-18, which cause cervical carcinoma), Epstein–Barr virus and Helicobacter pylori (both of which are associated with gastric carcinoma), Streptococcus bovis (which causes colorectal carcinoma), Salmonella enterica (which is associated with an increased incidence of Crohn's disease, hence sustaining colorectal carcinogenesis, and gallbladder carcinoma), as well as Chlamydia pneumoniae (an etiological determinant in some forms of lung cancer) (Nakagawa et al, 2004; Travassos et al, 2010; Yasir et al, 2011; Conway et al, 2013; Griffin et al, 2013; Zhang et al, 2014). Such a xenophagic response is required for the rapid clearance of intracellular pathogens as well as for the stimulation of pathogen-specific immune responses (Deretic et al, 2013; Ma et al, 2013). Accordingly, epithelial cells bearing molecular defects in the autophagic machinery, such as those provoked by Crohn's disease-associated point mutations in ATG16L1 and nucleotide-binding oligomerization domain containing 2 (NOD2) (Lassen et al, 2014), are more susceptible to infection by intracellular pathogens than their wild-type counterparts. In line with this notion, reduced levels of autophagic markers including BECN1 have recently been correlated with HPV-16 and HPV-18 infection in a cohort of cervical carcinoma patients (Wang et al, 2014). Thus, autophagy may exert oncosuppressive effects also by virtue of its antiviral and antibacterial activity.
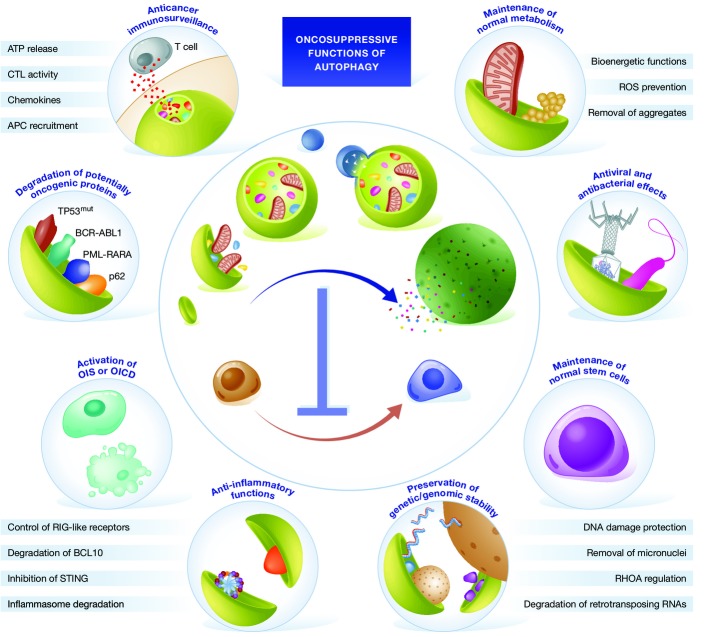
Taken together, these observations suggest that autophagy prevents malignant transformation by preserving both cellular and organismal homeostasis in conditions that pose a risk for oncogenesis (Fig2).
Figure 2.
Oncosuppressive functions of autophagy
Autophagy has been proposed to suppress malignant transformation by several mechanisms, including: (1) the preservation of genetic/genomic stability; (2) the disposal of endogenous sources of potentially mutagenic reactive oxygen species (ROS); (3) the maintenance of normal bioenergetic functions; (4) the degradation of oncogenic proteins; (5) cell-endogenous antiviral and antibacterial effects; (6) the optimal activation of oncogene-induced senescence (OIS) and oncogene-induced cell death (OICD); (7) the maintenance of a normal stem cell compartment; (8) multipronged anti-inflammatory functions; and (9) a key role in the elicitation and execution of anticancer immunosurveillance. ABL1, ABL proto-oncogene 1; APC, antigen-presenting cell; BCL10, B-cell CLL/lymphoma 10; BCR, breakpoint cluster region; CTL, cytotoxic T lymphocyte; TP53mut, mutant tumor protein p53; PML, promyelocytic leukemia; RARA, retinoic acid receptor, alpha; RHOA, ras homolog family member.
Oncoproteins, oncosuppressor proteins and autophagy
In agreement with the oncosuppressive activity of autophagy, several oncoproteins, that is, proteins that drive malignant transformation upon overexpression- or mutation-dependent hyperactivation, inhibit autophagic responses (Maiuri et al, 2009). Along similar lines, many bona fide oncosuppressor proteins, that is, proteins that are inactivated or lost in the course of oncogenesis, stimulate autophagy (Morselli et al, 2011) (Table1).
Table 1.
Oncoproteins, oncosuppressor proteins and autophagy
| Protein | Function(s) | Link(s) to cancer | Link(s) to autophagy | Reference |
|---|---|---|---|---|
| Oncoproteins | ||||
| AKT1 | Serine/threonine kinase | Hyperactivated or overexpressed in various neoplasms | Inhibits BECN1 and activates MTORCI | Carpten et al (2007); Laplante & Sabatini (2012); Wang et al (2012a); Huang et al (2013) |
| Stimulates autophagy via XIAP | ||||
| BCL2 | Anti-apoptotic Bcl-2 family members | Overexpressed in various hematological and solid tumors | Sequester BECN1 in inactive complexes | Pattingre et al (2005); Maiuri et al (2007b); Kang & Reynolds (2009); Anderson et al (2014); Wu et al (2014) |
| BCL-XL | BCL-XL inhibits mitophagy mediated by FUNDC1 | |||
| BRAF | Serine/threonine kinase | Mutated in melanoma and various histiocytoses | Activates MTORCI via ERK\TSC2\RHEB signaling | Davies et al (2002); Sharma et al (2006); Berres et al (2014); Corazzari et al (2014); Hervier et al (2014); Ma et al (2014) |
| BRAF hyperactivation promotes ER stress, in turn triggering autophagy | ||||
| E6 | E3 ubiquitin ligase | Etiological factor inHPV-associated cancers | Inhibits TP53 | Hanning et al (2013); de Freitas et al (2014); Hock & Vousden (2014) |
| E7 | RB1 inhibitor | Etiological factor in HPV-associated cancers | Suppresses autophagy, perhaps as a result of RB1 inhibition | Jiang et al (2010); Hanning et al (2013); de Freitas et al (2014) |
| HIF-1 | Transcription factor | Overexpressed in various tumors | Promotes mitophagy by transactivating BNIP3 and BNIP3L | Tracy et al (2007); Zhang et al (2008); Bellot et al (2009); Luo et al (2009); Wilkinson et al (2009) |
| HRAS | KRAS | Small GTP-binding proteins | Hyperactivated or overexpressed in various neoplasms | Furuta et al (2004); Shaw & Cantley (2006); Wei et al (2008); DeNicola et al (2011); Laplante & Sabatini (2012) |
| NRAS | Activate MTORCI via PI3K signaling | |||
| Derepress BECN1 upon the JNK1-mediated phosphorylation of BCL2 | ||||
| Promote the NRF2-dependent synthesis of p62 and NDP52 | ||||
| MDM2 | E3 ubiquitin ligase | Overexpressed in various neoplasms | Inhibits TP53 | Oliner et al (1992); Hock & Vousden (2014) |
| MYC | Transcription factors | Hyperactivated or overexpressed in various neoplasms | Inhibit autophagy upon 4EBP1 expression | Dalla-Favera et al (1982); Balakumaran et al (2009); Dang (2012); Toh et al (2013); Conacci-Sorrell et al (2014) |
| MYCL | Transactivate MAPK8, coding for the BECN1 derepressor JNK1 | |||
| MYCN | Truncated MYC promotes autophagy independent of transcription | |||
| MYC hyperactivation promotes ER stress, in turn triggering autophagy | ||||
| PDPK1 | Serine/threonine kinase | Hyperactivated in colorectal neoplasms | Activates MTORCI via AKT1\TSC2\RHEB signaling | Laplante & Sabatini (2012); Chinen et al (2014) |
| PI3K | Lipid kinase | Hyperactivated in various neoplasms | Activates MTORCI via AKT1\TSC2\RHEB signaling | Shayesteh et al (1999); Ma et al (2000); Laplante & Sabatini (2012) |
| RHEB | Small GTP-binding protein | Overexpressed in prostate carcinoma | Activates MTORCI | Inoki et al (2003); Nardella et al (2008) |
| RTKs | Tyrosine kinases | Hyperactivated or overexpressed in various neoplasms | Activate MTORCI via PI3K signaling | Slamon et al (1987); Paez et al (2004); Stephens et al (2004); Laplante & Sabatini (2012); Wei et al (2013); Lozy et al (2014) |
| EGFR phosphorylates BECN1, hence inactivating it | ||||
| SRC | Non-receptor tyrosine kinase | Hyperactivated in various cancers | Activates MTORCI via PI3K signaling | Irby et al (1999); Sen & Johnson (2011); Liu et al (2012b) |
| Phosphorylates FUNDC1, hence inactivating it | ||||
| XIAP | E3 ubiquitin ligase | Overexpressed in various tumors | Inhibits the autophagy-blocking activity of cytoplasmic TP53 (?) | Schimmer et al (2006); Huang et al (2013) |
| Oncosuppressor proteins | ||||
| AMBRA1 | Component of class III PI3K complex | Mutated in endometrial, colorectal and urinary tract neoplasms | Key factor for canonical autophagy | Codogno et al (2012); Cianfanelli et al (2015) |
| ATG5 | E3 ubiquitin ligase | Downregulated in melanoma | Key factor for canonical autophagy | Codogno et al (2012); Liu et al (2013a) |
| BECN1 | Component of class III PI3K complex | Monoallelically deleted or downregulated in various solid tumors | Key factor for canonical autophagy | Liang et al (1999); Qu et al (2003); Miracco et al (2007); Codogno et al (2012); Laddha et al (2014) |
| BH3-only proteins | Pro-apoptotic Bcl-2 family members | Downregulated in various hematological and solid tumors | Derepress BECN1 by displacing it from BCL2 and BCL-XL | Ahn et al (2007); Maiuri et al (2007a); Maiuri et al (2007b) |
| BIF1 | Component of class III PI3K complex | Downregulated in colorectal carcinoma | UVRAG interactor | Takahashi et al (2007); Coppola et al (2008); Takahashi et al (2013) |
| DAPK1 | Serine/threonine kinase | Downregulated in various solid tumors | Derepresses BECN1 by displacing it from BCL2 | Raveh et al (2001); Martoriati et al (2005); Christoph et al (2007); Zalckvar et al (2009) |
| Boosts a potentially self-amplifying p19ARF\TP53 response | ||||
| DIRAS3 | GTP-binding protein | Downregulated in breast and ovarian carcinoma | Inhibits MTORCI by antagonizing PI3K signaling | Yu et al (1999); Feng et al (2008); Lu et al (2008) |
| DRAM1 | Lysosomal protein | Downregulated in many tumors as a result of TP53 inactivation | Involved in TP53-dependent autophagy | Crighton et al (2006); Leroy et al (2014) |
| FIP200 | Multifunctional protein | Affected by truncating mutations in breast cancer | Component of the ULK1-ATG13-ATG101 complex | Chano et al (2002); Hara et al (2008) |
| FOXO1 | Transcription factor | Mutated in DLBCL | Interacts with ATG7 in the course of stress-induced autophagy | Huang et al (2005); Zhao et al (2010) |
| LKB1 | Serine/threonine kinase | Germline mutations cause Peutz–Jeghers's syndrome | Activates the TSC complex via AMPK | Hemminki et al (1998); Shaw et al (2004); Ji et al (2007) |
| Mutated in a proportion of SCCs and lung carcinomas | ||||
| NF1 | GTPase | Germline mutations cause type I neurofibromatosis | Activates the TSC complex by antagonizing RAS signaling | Fountain et al (1989); Xu et al (1990); Johannessen et al (2005) |
| RB1 | Transcription factor | Germline mutations cause hereditary retinoblastoma | Stimulates autophagy, perhaps upon BCL2 downregulation and consequent BECN1 derepression | Friend et al (1986); Cryns et al (1994); Gomez-Manzano et al (2001); Jiang et al (2010) |
| Mutated in various solid tumors | ||||
| p19ARF | MDM2 inhibitor | Mutated or deleted in several hematological and solid tumor | Stimulates autophagy via TP53-dependent and -independent mechanisms | Faderl et al (1999); Abida & Gu (2008); Pimkina et al (2009) |
| PTEN | Lipid phosphatase | Germline mutations cause the so-called PHTS | Inhibits MTORCI by antagonizing PI3K signaling | Liaw et al (1997); Marsh et al (1997); Arico et al (2001); Sansal & Sellers (2004) |
| Sporadically mutated in a large fraction of neoplasms | ||||
| smARF | Alternative CDKN2A product | Mutated or deleted in several hematological and solid tumors | Derepresses BECN1 by displacing it from BCL-XL | Faderl et al (1999); Reef et al (2006); Pimkina et al (2009) |
| TP53 | Transcription factor | Germline mutations cause the Li–Fraumeni syndrome | In physiological conditions, inhibits autophagy via non-transcriptional, cytoplasmic mechanisms | Malkin et al (1990); Moll et al (2005); Riley et al (2008); Maiuri et al (2010); Pietrocola et al (2013); Leroy et al (2014); McBride et al (2014) |
| Mutated or inactivated in > 50% of human malignancies | In response to stress, transactivate a panel of genes involved in adaptive responses, including autophagy | |||
| TSC1 | GTPases | Germline mutations cause TSC | The TSC complex inhibits MTORCI via RHEB | van Slegtenhorst et al (1997); Inoki et al (2002) |
| TSC2 | ||||
| UVRAG | Component of class III PI3K complex | Monoallelically deleted in gastric and colorectal cancers | Positive modulator of BECN1 | Liang et al (2006); Kim et al (2008); Zhao et al (2012) |
| VHL | E3 ubiquitin ligase | Germline mutations cause the von Hippel–Lindau syndrome | Inhibits LC3B and TRPM3 via miR-204 | Seizinger et al (1988); Shuin et al (1994); Tracy et al (2007); Zhang et al (2008); Bellot et al (2009); Wilkinson et al (2009); Mikhaylova et al (2012); Hall et al (2014) |
| Sporadically mutated in a large fraction of RCC | Inhibits the HIF-1-dependent synthesis of BNIP3 and BNIP3L | |||
AKT1, v-akt murine thymoma viral oncogene homolog 1; AMBRA1, autophagy/beclin-1 regulator 1; AMPK, 5′-AMP-activated protein kinase; BCL2, B-cell CLL/lymphoma 2; BECN1, Beclin 1; BNIP3, BCL2/adenovirus E1B 19 kDa interacting protein 3; BNIP3L, BNIP3-like; BRAF, B-Raf proto-oncogene, serine/threonine kinase, CDKN2A, cyclin-dependent kinase inhibitor 2A; DAPK1, death-associated protein kinase 1; DIRAS3, DIRAS family, GTP-binding RAS-like 3; DRAM1, DNA-damage-regulated autophagy modulator 1; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; FOXO1, forkhead box O1; FUNDC1, FUN14 domain containing 1; HIF-1, hypoxia-inducible factor 1; HPV, human papillomavirus; HRAS, Harvey rat sarcoma viral oncogene homolog; KRAS, Kirsten rat sarcoma viral oncogene homolog; MTORCI, mechanistic target of rapamycin complex I; MYC, v-myc avian myelocytomatosis viral oncogene homolog; MYCL, v-myc avian myelocytomatosis viral oncogene lung carcinoma-derived homolog; MYCN, v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog; NF1, neurofibromin 1; NRAS, neuroblastoma RAS viral (v-ras) oncogene homolog; PDPK1, phosphoinositide-dependent protein kinase 1; PHTS, PTEN hamartoma tumor syndrome; PI3K, phosphoinositide-3-kinase; PTEN, phosphatase and tensin homolog; RB1, retinoblastoma 1; RCC, renal cell carcinoma; RHEB, Ras homolog enriched in brain; RTK, receptor tyrosine kinase; SCC, squamous cell carcinoma; TP53, tumor protein p53; TRPM3, transient receptor potential nonselective cation channel, subfamily M, member 3; TSC, tuberous sclerosis; ULK1, unc-51-like autophagy-activating kinase 1; UVRAG, UV radiation resistance associated; VHL, von Hippel–Lindau tumor suppressor; XIAP, X-linked inhibitor of apoptosis.
Oncoproteins
BECN1 is inhibited upon sequestration by several members of the Bcl-2 protein family, including B-cell CLL/lymphoma 2 (BCL2) and BCL2-like 1 (BCL2L1, best known as BCL-XL) (Pattingre et al, 2005; Maiuri et al, 2007b). These proteins are overexpressed by a wide variety of hematological and solid tumors, an event that consistently reduces the sensitivity of neoplastic cells to lethal cues of either cell-intrinsic or environmental origin (Kang & Reynolds, 2009; Anderson et al, 2014). Besides inhibiting BECN1, BCL-XL (but not BCL2) also suppresses the ability of phosphoglycerate mutase family member 5 (PGAM5) to dephosphorylate FUN14 domain containing 1 (FUNDC1) on S13, hence suppressing mitophagy (Wu et al, 2014). Interestingly, the robust oncogenic activity of Bcl-2 proteins may also reflect their metabolic and autophagy-inhibitory activity (Pattingre & Levine, 2006; Oh et al, 2011; Wei et al, 2013; Green et al, 2014).
Various oncoproteins stimulate malignant transformation by virtue of their ability to inactivate TP53. These include the human protein MDM2, which is overexpressed by a wide panel of human tumors as a result of gene amplification (Oliner et al, 1992), as well as the early HPV-16 protein E6, which is etiologically involved in the pathogenesis of human cervical carcinoma (de Freitas et al, 2014). Both MDM2 and E6 operate as E3 ligases and target TP53 to proteasomal degradation (Hock & Vousden, 2014), hence suppressing its ability to promote autophagy (and regulated cell death) in response to stress (Vousden & Lane, 2007; Hanning et al, 2013; Pietrocola et al, 2013). Also E7, another early HPV-16 gene product that is required for viral carcinogenesis because it inhibits the oncosuppressor protein retinoblastoma 1 (RB1) (Dyson et al, 1989), has been shown to suppress autophagic responses (Hanning et al, 2013). The ability of E7 to inhibit RB1 may explain its autophagy-suppressing functions, at least in specific settings (see below) (Jiang et al, 2010).
A consistent fraction of human neoplasms is characterized by the hyperactivation of receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR) and v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2, best known as HER2) or downstream signal transducers, including SRC, v-akt murine thymoma viral oncogene homolog 1 (AKT1), class I phosphatidylinositol 3-kinases (PI3Ks) and phosphoinositide-dependent protein kinase 1 (PDPK1) (Slamon et al, 1987; Irby et al, 1999; Shayesteh et al, 1999; Ma et al, 2000; Paez et al, 2004; Stephens et al, 2004; Carpten et al, 2007; Sen & Johnson, 2011; Chinen et al, 2014). According to current views, this promotes neoplastic transformation because it allows cells to proliferate in the absence of growth factors and it increases their resistance to adverse microenvironmental conditions (Manning & Cantley, 2007; Laplante & Sabatini, 2012). In addition, the signal transduction cascades elicited by RTKs often impinge, either directly or upon the engagement of additional signaling modules, on the activation of MTORCI, de facto inhibiting autophagy (Laplante & Sabatini, 2012; Lozy et al, 2014). Of note, AKT1 reportedly suppresses autophagic responses not only by catalyzing the inactivating phosphorylation of the MTOR-repressing tumor sclerosis (TSC) complex, but also by phosphorylating and hence inhibiting BECN1 (Wang et al, 2012a), and perhaps by promoting the ability of TP53 to block autophagy in baseline or near-to-baseline conditions via a X-linked inhibitor of apoptosis (XIAP)- and MDM2-dependent signal transduction cascade (Huang et al, 2013). In line with this notion, although XIAP does not behave as a conventional oncoprotein, several human neoplasms express increased XIAP levels (Schimmer et al, 2006). Moreover, SRC not only suppresses autophagic responses by activating MTORCI via the PI3K\PDPK1\AKT1 signaling module (Laplante & Sabatini, 2012), but also inhibits mitophagy by catalyzing the inactivating phosphorylation of FUNDC1 on Y18 (Liu et al, 2012b). Finally, some RTKs such as EGFR inhibit autophagy in an MTORCI-independent manner, by catalyzing the inactivating phosphorylation of BECN1 on tyrosine residues (Wei et al, 2013).
At least three members of the RAS GTPase family, that is, Harvey rat sarcoma viral oncogene homolog (HRAS), Kirsten rat sarcoma viral oncogene homolog (KRAS) and neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS), are etiologically involved in several paradigms of malignant transformation, either as a result of somatic mutations or upon the hyperactivation of upstream signal transducers (including RTKs) (Shaw & Cantley, 2006). Besides delivering robust mitogenic signals, hyperactivated RAS engages the PI3K\PDPK1\AKT1 signaling cascade, hence potently suppressing autophagic responses (in both mammals and Drosophila) (Furuta et al, 2004; Denton et al, 2012a; Laplante & Sabatini, 2012). However, RAS also transactivates p62 through NF-κB, hence igniting a p62-dependent feedforward signaling loop with pro-autophagic and oncogenic effects accompanied by sustained NF-κB activation (Duran et al, 2008; Ling et al, 2012). Along similar lines, RAS activates mitogen-activated protein kinase 8 (MAPK8, best known as JNK1) and nuclear factor, erythroid 2-like 2 (NFE2L2, best known as NRF2), thereby promoting autophagy via the JNK1-dependent phosphorylation of BCL2 (and the consequent derepression of BECN1), and the NRF2-dependent transactivation of genes encoding several autophagy receptors, including p62 and calcium binding and coiled-coil domain 2 (CALCOCO2, best known as NDP52) (Wei et al, 2008; Jain et al, 2010; DeNicola et al, 2011; Lock et al, 2011; Jo et al, 2014). Interestingly, the oncogene-driven activation of NRF2 may engage a pro-autophagic feedforward loop, stemming from the autophagy-dependent degradation of kelch-like ECH-associated protein 1 (KEAP1, the major negative regulator of NRF2) (Taguchi et al, 2012; Bae et al, 2013), as well as from the NRF2-activating phosphorylation of p62 (Inami et al, 2011; Ichimura et al, 2013). Autophagic responses are indeed recovered along with tumor progression in various models of KRAS-driven oncogenesis (see below) (Guo et al, 2013a; Rosenfeldt et al, 2013; Rao et al, 2014). Another member of the RAS family, that is, Ras homolog enriched in brain (RHEB), is often overexpressed due to genetic amplification in human prostate carcinoma (Nardella et al, 2008). RHEB suppresses autophagy as it operates as a direct activator of MTORCI downstream of AKT1 (Inoki et al, 2003; Nardella et al, 2008).
Hyperactivating mutations in B-Raf proto-oncogene, serine/threonine kinase (BRAF), in particular the C1799T substitution (generating BRAFV600E), are a common finding in biopsies from human melanoma patients (Davies et al, 2002) and have recently been associated with various forms of histiocytosis (a pseudomalignant condition affecting macrophages and dendritic cells) (Berres et al, 2014; Hervier et al, 2014). At odds with its wild-type counterpart, BRAFV600E engages the mitogenic MAPK\ERK signaling pathway even in the absence of RTK-emanated and RAS-transduced signals, de facto rendering cell proliferation independent of extracellular growth factor availability (Sharma et al, 2006). Activated ERK phosphorylates TSC2, hence inhibiting autophagy as a consequence of mTORCI activation (Ma et al, 2005). In addition, constitutive signaling via the BRAF axis results in a chronic state of ER stress that triggers an adaptive autophagic response required for the survival of transformed cells and, at least in some settings, sustaining their resistance to pharmacological BRAF inhibitors (Corazzari et al, 2014; Ma et al, 2014). This may explain, at least in part, the sensitivity of established BRAFV600E-driven lung adenocarcinomas to the deletion of Atg7 (see below) (Strohecker et al, 2013).
v-myc avian myelocytomatosis viral oncogene homolog (MYC), v-myc avian myelocytomatosis viral oncogene lung carcinoma-derived homolog (MYCL) and v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (MYCN) are the major effectors of the mitogenic MAPK\ERK signal transduction cascade (Dang, 2012). MYC, MYCL and MYCN are affected by various mutational events in a wide panel of human malignancies (Dang, 2012). These include point mutations as well as larger genetic rearrangements such as the t(8;14)(q24;q32) translocation, which is etiologically associated with Burkitt's lymphoma (Dalla-Favera et al, 1982). Besides overriding the microenvironmental control on proliferation, the hyperactivation of MYC family members has been linked to suppressed autophagy as a consequence of eukaryotic translation initiation factor 4E binding protein 1 (EIF4EBP1, best known as 4EBP1) overexpression (Balakumaran et al, 2009). Apparently at odds with this notion, MYC depletion reportedly inhibits the formation of autophagosomes, possibly as a consequence of reduced JNK1 expression and consequent BCL2 phosphorylation (Toh et al, 2013). In addition, MYC-driven oncogenesis resembles its BRAF-driven counterparts in that it causes a chronic condition of ER stress that initiates compensatory adaptive responses (Hart et al, 2012). Moreover, it seems that colorectal cancer cells exposed to nutrient deprivation and hypoxia accumulate a transcriptionally inactive, cytoplasmic, calpain-derived cleavage product of MYC that not only supports their survival by triggering autophagy, but also favors other facets of tumor progression, including anchorage-independent growth and genomic instability (Conacci-Sorrell et al, 2014).
In summary, the net effect of RAS hyperactivation and its consequences on autophagy exhibit (at least some degree of) context dependency. This apparent discrepancy may reflect the presence/absence of genetic abnormalities other than the activation of a single oncogene. Irrespective of this hitherto unexplored possibility, autophagy appears to be restored and to be required for optimal tumor progression in rodent models of KRAS-, BRAF- and MYC-driven oncogenesis, perhaps as a compensatory response to oncogenic stress that ensues, rather than accompanies, transformation (Guo et al, 2013a; Rosenfeldt et al, 2013; Strohecker et al, 2013; Conacci-Sorrell et al, 2014; Rao et al, 2014). The molecular mechanisms that are responsible for the reconstitution of autophagic responses in these settings have not yet been elucidated.
Oncosuppressor proteins
The Li–Fraumeni syndrome, a dominantly inherited disorder characterized by the early-onset of various tumors (including bone and soft tissue sarcomas, breast carcinomas, brain cancers and acute leukemias), is caused by germline mutations in TP53 (Malkin et al, 1990; McBride et al, 2014). Moreover, the TP53 system is inactivated as a consequence of somatic mutations or genetic/epigenetic events affecting its regulators, such as MDM2 (see above), in more than 50% of sporadic human malignancies (Leroy et al, 2014). Besides engaging the cell death machinery in cells bearing irreparable molecular defects (mainly DNA damage), via transcriptional as well as transcription-independent mechanisms (Moll et al, 2005; Riley et al, 2008), stress-stabilized TP53 can transactivate various genes involved in autophagic responses (Maiuri et al, 2010; Pietrocola et al, 2013). These include, but are not limited to, the genes coding for the β1 and β2 subunits of AMPK (i.e., PRKAB1, PRKAB2) (Feng et al, 2007), the gene coding for the AMPK activators sestrin 1 (i.e., SESN1) and SESN2 (Budanov et al, 2004; Budanov & Karin, 2008), genes encoding various BH3-only proteins (i.e., BAD, BNIP3) and death-associated protein kinase 1 (i.e., DAPK1), all of which stimulate autophagy by favoring the displacement of BECN1 from inhibitory interactions with BCL2 and BCL-XL (Martoriati et al, 2005; Feng et al, 2007; Maiuri et al, 2007a; Maiuri et al, 2007b; Zalckvar et al, 2009), as well as the gene coding for DNA-damage-regulated autophagy modulator 1 (i.e., DRAM1), a lysosomal protein that sustains TP53-dependent autophagic responses via an unknown mechanism (Crighton et al, 2006). The gene coding for the upstream activator of TP53 p19ARF (i.e., CDKN2A) is also mutated or lost in several solid and hematological malignancies (Faderl et al, 1999). Full-length p19ARF promotes autophagy via TP53- as well as TP53-independent mechanisms (Abida & Gu, 2008). Moreover, a short mitochondrial isoform of p19ARF commonly known as smARF stimulates autophagic responses by displacing BECN1 from BCL-XL (Reef et al, 2006). Interestingly, at least part of the autophagy-promoting functions of DAPK1 may result from its ability to initiate a potentially self-amplifying p19ARF\TP53 signal transduction cascade (Raveh et al, 2001; Martoriati et al, 2005).
The gene encoding BECN1, which is required for canonical autophagy (Liang et al, 1999; Codogno et al, 2012), maps to a tumor susceptibility locus on chromosome 17q21 that is monoallelically deleted in a high proportion of sporadic breast and ovarian carcinomas (Aita et al, 1999). Moreover, the expression levels of BECN1 are reduced in a panel of human neoplasms, including brain tumors as well as gastric and colorectal carcinomas (Rubinsztein et al, 2012). Often, BECN1 is co-deleted with breast cancer 1, early onset (BRCA1), a well-known oncosuppressor gene whose loss is associated with familial forms of breast and ovarian carcinoma (Futreal et al, 1994). This has recently led some investigators to propose that the loss of BECN1 does not etiologically contribute to oncogenesis (Laddha et al, 2014). However, in two large, independent cohorts of breast carcinoma patients, reduced levels of BECN1-coding, but not BRCA1-coding, mRNA have been associated with several adverse prognostic features, including estrogen receptor negativity, HER2 overexpression, basal phenotype, TP53 mutations, and advanced tumor grade, as well as with decreased patient survival (Tang et al, 2015).
Further corroborating the notion that BECN1 constitutes a haploinsufficient oncosuppressor protein (Yue et al, 2003), various physical and functional activators of BECN1 have been ascribed with tumor-suppressive functions. The expression of DAPK1, smARF and several BH3-only proteins is lost or reduced as a result of genetic or epigenetic alterations in human tumors of distinct histological origin (Faderl et al, 1999; Christoph et al, 2007). Along similar lines, AMBRA1 is mutated in a subset of endometrial, colorectal and urinary tract neoplasms (Cianfanelli et al, 2015), the gene coding for the BECN1 activator UV radiation resistance associated (UVRAG) is monoallelically mutated in a high proportion of gastric and colonic tumors (Liang et al, 2006; Kim et al, 2008), and the expression of SH3-domain GRB2-like endophilin B1 (SH3GLB1, a positive modulator of UVRAG best known as BIF1) (Takahashi et al, 2007), is reduced in colorectal carcinomas (Takahashi et al, 2007; Coppola et al, 2008). Thus, the BECN1 system appears to be negatively regulated in the course of several paradigms of malignant transformation. As an exception to this trend, the Becn1+/− genotype delays, rather than accelerates, lymphoid oncogenesis in mice lacking the ATM serine/threonine kinase (Valentin-Vega et al, 2012). Perhaps, such an oncogenic function of BECN1 reflects the accrued dependency of Atm−/− cells on mitochondrial respiration and role of autophagy in the maintenance of a functional mitochondrial network. Of note, BECN1 and several of its interactors, including UVRAG and VPS34, are also involved in the late steps of endocytosis (McKnight et al, 2014). Moreover, UVRAG contributes to genomic stability in an autophagy-unrelated manner (Zhao et al, 2012). Finally, the oncosuppressive functions of AMBRA1 appear to stem, at least in part, from its ability to promote the dephosphorylation-dependent degradation of MYC (Cianfanelli et al, 2015). Thus, the impact of the BECN1 system on oncogenesis and tumor progression may be independent of autophagy, at least in some settings.
Germline mutations in RB1 cause a heritable form of retinoblastoma, a rapidly developing cancer that develops from immature retinal cells (Friend et al, 1986). Sporadic RB1 mutations have been detected in a wide spectrum of neoplasms including osteosarcomas, small cell lung carcinomas and breast carcinomas (Cryns et al, 1994). RB1 is well known for its ability to inhibit various members of the E2F transcription factor family, hence preventing cell cycle progression in the absence of growth factors. The actual role of E2F proteins in autophagic responses, however, remains unclear. On the one hand, the inhibition of E2F by RB1 has also been linked to the activation of autophagy (Jiang et al, 2010), perhaps as a result of declining levels of BCL2 and consequent BECN1 derepression (Gomez-Manzano et al, 2001). On the other hand, E2F1 has been shown to promote the synthesis of various components of the autophagic machinery, including ATG1, ATG5 and LC3 (Polager et al, 2008). Thus, the ability of E2F family members to regulate autophagy may exhibit some degree of context-dependency.
A panel of genetic conditions characterized by an increased risk of breast, thyroid and endometrial tumors cumulatively referred to as PTEN hamartoma tumor syndrome (PHTS) is provoked by germline mutations in phosphatase and tensin homolog (PTEN), an enzyme that promotes autophagy by functionally antagonizing PI3K signaling (Liaw et al, 1997; Marsh et al, 1997; Arico et al, 2001). Moreover, the inactivation of PTEN as a result of sporadic mutations is very common among human cancers (Sansal & Sellers, 2004). DIRAS family, GTP-binding RAS-like 3 (DIRAS3), another functional antagonist of PI3K with pro-autophagic activity (Lu et al, 2008), is frequently underexpressed in breast and ovarian carcinoma upon loss of heterozygosity or promoter hypermethylation (Yu et al, 1999; Feng et al, 2008).
Germline mutations in neurofibromin 1 (NF1), serine threonine kinase 11 (STK11), TSC1 and TSC2 are also associated with syndromes characterized by an increased incidence of both benign and malignant neoplasms, notably, type I neurofibromatosis (NF1), Peutz–Jeghers's syndrome (STK11) and tuberous sclerosis (TSC1 and TSC2) (Fountain et al, 1989; van Slegtenhorst et al, 1997; Hemminki et al, 1998). Moreover, somatic mutations in STK11 have been documented in a considerable percentage of squamous cell carcinomas and lung adenocarcinomas (Ji et al, 2007). As a common denominator, the inactivation of NF1 (an upstream inhibitor of RAS), STK11 (an activator of AMPK best known as LKB1), TSC1 or TSC2 promotes MTORCI signaling, hence stimulating cell proliferation and inhibiting autophagic responses (Xu et al, 1990; Inoki et al, 2002; Shaw et al, 2004; Johannessen et al, 2005). In spite of such an MTORCI-dependent inhibition of autophagy, the monoallelic loss of Becn1 suppresses spontaneous renal tumorigenesis in Tsc2+/− mice (Parkhitko et al, 2011). This may indicate that the Tsc2+/− genotype does not completely block autophagic responses, or that—at least in this model—BECN1 exerts oncosuppressive functions that are mainly autophagy-independent. The deletion of Rb1cc1 has also been shown to suppress, rather than enhance, mammary tumorigenesis driven by the polyoma middle T antigen (PyMT) (Hara et al, 2008; Wei et al, 2011). Although in this setting the absence of FIP200 was indeed associated with autophagic defects in PyMT-expressing malignant cells, such alterations were not etiologically linked to oncogenesis. This is intriguing as FIP200 (which is also involved in the regulation of RB1) is considered as a classical oncosuppressor protein and is lost as a consequence of large deletions in a high fraction of breast carcinomas (Chano et al, 2002).
The von Hippel–Lindau syndrome, an inherited disorder characterized by the formation of tumors and cysts at various anatomical locations, is provoked by germline mutations in von Hippel–Lindau tumor suppressor, E3 ubiquitin protein ligase (VHL) (Seizinger et al, 1988). Moreover, VHL is affected by somatic mutations in a large proportion of renal cell carcinomas (Shuin et al, 1994). In VHL-deficient cells, hypoxia-inducible factor 1 (HIF-1) accumulates irrespective of oxygen concentration (because VHL normally targets it to proteasomal degradation), resulting in the transactivation of genes involved in bioenergetic metabolism and angiogenesis (Maxwell et al, 1999). VHL suppresses autophagy by an epigenetic mechanism involving miR-204 (Mikhaylova et al, 2012; Hall et al, 2014). MiR-204 targets LC3B as well as transient receptor potential cation channel, subfamily M, member 3 (TRPM3), a Ca2+- and Zn2+-conducive channel that potently stimulates autophagy. Moreover, the hyperactivation of HIF-1 supports mitophagy upon the upregulation of BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3) and BNIP3-like (BNIP3L) even in normoxic conditions (Tracy et al, 2007; Zhang et al, 2008; Bellot et al, 2009; Wilkinson et al, 2009). Thus, the loss of VHL may sustain tumor progression not only as it promotes angiogenesis, but also as it stimulates autophagy. Of note, HIF-1 is not a bona fide oncoprotein, yet multiple cancer cells express increased HIF-1 levels and rely on HIF-1 for survival (Luo et al, 2009).
The transcription factor forkhead box O1 (FOXO1) is mutated in a percentage of diffuse large B-cell lymphoma (DLBCL) cases and is etiologically involved in at least one model of murine oncogenesis (Huang et al, 2005). FOXO1 is classically ascribed with oncosuppressive functions by virtue of its multipronged pro-apoptotic activity (Fu & Tindall, 2008). In addition, a cytosolic pool of FOXO1 seems to be required for stress-induced autophagy as a consequence of its physical interaction with ATG7 (Zhao et al, 2010). Along similar lines, ATG5 is expressed at low levels in primary melanomas as compared to benign nevi, resulting in reduced autophagic proficiency (Liu et al, 2013a), and its absence favors oncogenesis in mice (Takamura et al, 2011). Although neither FOXO1 nor ATG5 can be considered as bona fide oncosuppressor proteins, these results support the contention that various factors with oncosuppressive functions promote autophagic responses.
Taken together, the observations presented above indicate that the hyperactivation of oncoproteins, as well the inactivation of oncosuppressor proteins, most often limits autophagic responses and that the partial suppression of autophagy underlies several (though perhaps not all) paradigms of oncogenesis. The actual impact of primary oncogenic events on autophagy, however, may be influenced (at least to some extent) by the presence/absence of additional genetic defects, and hence is likely to evolve along with tumor progression.
Autophagy and tumor progression
Autophagic responses generally support the growth and progression of established tumors by reducing their sensitivity to cell-intrinsic as well as microenvironmental stimuli that would normally promote their demise, in particular upon the so-called epithelial-to-mesenchymal transition (Kroemer et al, 2010; Avivar-Valderas et al, 2013; Cai et al, 2014). This notion is supported by a growing amount of data indicating that defects in the autophagic machinery often restrain the proliferation, dissemination and metastatic potential of malignant cells, as discussed below. Moreover, advanced human tumors generally exhibit an increased autophagic flux, correlating with an invasive/metastatic phenotype, high nuclear grade, and poor disease outcome (Lazova et al, 2012; Mikhaylova et al, 2012).
Although mice with a systemic mosaic deletion of Atg5 or a liver-specific knockout of Atg7 develop spontaneous hepatic neoplasms more frequently than their wild-type counterparts (see above), these malignancies are mostly benign (pointing to defects in tumor progression) and their size can be further decreased by the simultaneous deletion of Sqstm1 (Takamura et al, 2011). In accord with these data, p62 has been shown to support the progression of both endogenous (ERBB2-driven) and xenografted mammary tumors through a variety of mechanisms, including the activation of NRF2 (see above) (Chen et al, 2013; Cai-McRae et al, 2014). As opposed to their autophagy-competent counterparts, highly metastatic hepatocellular carcinoma cell lines infected with lentiviruses that stably downregulate BECN1 or ATG5 are virtually unable to survive within the metastatic niche, although they normally proliferate, invade surrounding tissues and undergo the epithelial-to-mesenchymal transition (Peng et al, 2013). Along similar lines, the shRNA-mediated downregulation of Atg5 or p62 abolishes the ability of Tsc2−/−Trp53−/− MEFs to develop macroscopic tumors upon inoculation into nude mice (Parkhitko et al, 2011). Moreover, the robust antimetastatic effects of N-myc downstream regulated 1 (NDRG1) have recently been ascribed to its ability to suppress stress-induced autophagic responses (Sahni et al, 2014).
The lung-specific deletion of Atg7 during the late stages of BRAFV600E-driven carcinogenesis favors the development of small oncocytomas (which are relatively benign tumors) rather than adenocarcinomas, a shift that is accompanied by the accumulation of dysfunctional mitochondria and an increased dependency on exogenous glutamine (Strohecker et al, 2013). Similar results have been obtained in models of KRASG12D-driven lung and pancreatic carcinogenesis, upon the tissue-specific deletion of Atg5 or Atg7 (Guo et al, 2013a; Rosenfeldt et al, 2013; Rao et al, 2014; Yang et al, 2014), as well as in models of breast carcinoma driven by the mammary-gland specific knockout of partner and localizer of BRCA2 (Palb2), upon the monoallelic deletion of Becn1 (Huo et al, 2013). Intriguingly, the downregulation of Atg1 (the fly ortholog of ULK1) by RNA interference also suppressed a hyperproliferative eye phenotype caused in Drosophila melanogaster by the ectopic expression of Ras112V (a constitutively active variant of the fly ortholog of mammalian RAS proteins) but had an opposite effect on the overgrowth of the eye epithelium provoked by mutations in the oncosuppressor gene scribbled (scrib) (Perez et al, 2014). Similarly, the individual depletion of 12 distinct components of the autophagic machinery (i.e., Atg1, Atg6, Atg12, Atg5, Atg7, Atg4a, Atg4b, Atg8a, Atg8b, Atg3, Atg9 and Atg18) promoted, rather than limited, the overgrowth of adult Drosophila eyes and their larval precursor tissues in so-called eyeful flies (a model of Notch-driven carcino-genesis) (Perez et al, 2014).
In some models of endogenous mammalian carcinogenesis, the Trp53−/− genotype prevents genetic interventions that target the autophagic machinery from provoking metabolic and bioenergetic alterations that limit tumor progression (Huo et al, 2013; Rosenfeldt et al, 2013; Rao et al, 2014). Conversely, the response of pancreatic cancer cells, xenografts and KRASG12D-driven autochthonous adenocarcinomas to genetic or pharmacological autophagy inhibition persists in the context of TP53 loss-of-heterozygosity (Yang et al, 2011; Yang et al, 2014).
KRASG12D-driven pancreatic adenocarcinoma cells entering a state of dormancy (rather than succumbing) in response to oncogene ablation (i.e., the shutdown of oncogenic KRAS signaling) have recently been show to activate autophagy to efficiently counteract metabolic stress (Viale et al, 2014). Of note, whereas primary KRASG12D-expressing cells generally exhibit increased glucose and glutamine uptake, as well as an elevated anabolic flux via the pentose phosphate pathway (Ying et al, 2012; Rosenfeldt et al, 2013), the survival of oncogene-depleted pancreatic adenocarcinoma cells critically relies on oxidative phosphorylation (Viale et al, 2014). Thus, exposing such dormant pancreatic adenocarcinoma cells to the inhibitor of oxidative phosphorylation oligomycin reportedly abolishes their ability to form tumors upon KRASG12D re-expression (Viale et al, 2014).
Interestingly, KRASG12D-expressing pancreatic adenocarcinoma cells driven into dormancy upon oncogene ablation also display functional and phenotypic features of cancer stem cells (CSCs) (Viale et al, 2014). Moreover, mammary CSCs (which propagate in culture as mammospheres) are often characterized by an elevated autophagic flux, and their ability to efficiently form tumors in vivo appears to rely on autophagy, as tumor formation can be abolished by the genetic inhibition of BECN1 or ATG4A (Gong et al, 2013; Wolf et al, 2013). Thus, autophagy may also sustain tumor progression by preserving the viability of the CSC compartment and/or by promoting the persistence of dormant cancer cells (Viale et al, 2014).
Cancer cells isolated from established tumors and subjected to the genetic or pharmacological inhibition of autophagy are less resistant to exogenous stimuli than their wild-type counterparts (Boya et al, 2005; Amaravadi et al, 2007; Kroemer et al, 2010). In line with this notion, autophagy-deficient tumors are often more sensitive to several chemotherapeutic agents as well as to radiation therapy than their autophagy-proficient counterparts (Janku et al, 2011; Ko et al, 2014; Levy et al, 2014). This does not necessarily hold true for immunocompetent mice. Indeed, autophagic responses preceding the demise of cancer cells exposed to a selected panel of agents are required (though not sufficient) for cell death to be perceived as immunogenic and hence to elicit a therapeutically relevant immune response (Kroemer et al, 2013; Ko et al, 2014). Cancer cells exposed to therapeutic interventions can also undergo senescence (Lopez-Otin et al, 2013). Although senescent cells do not proliferate, they may support disease relapse by releasing a wide panel of pro-inflammatory and mitogenic cytokines into the microenvironment (underlying the so-called senescence-associated secretory phenotype, SASP) (Lopez-Otin et al, 2013). Interestingly, these cells are highly dependent on autophagic responses for survival, and pharmacological inhibitors of autophagy have been shown to synergize with various chemotherapeutics in experimental models of lymphoma that are susceptible to acquire the SASP in response to treatment (Young et al, 2009; Dorr et al, 2013).
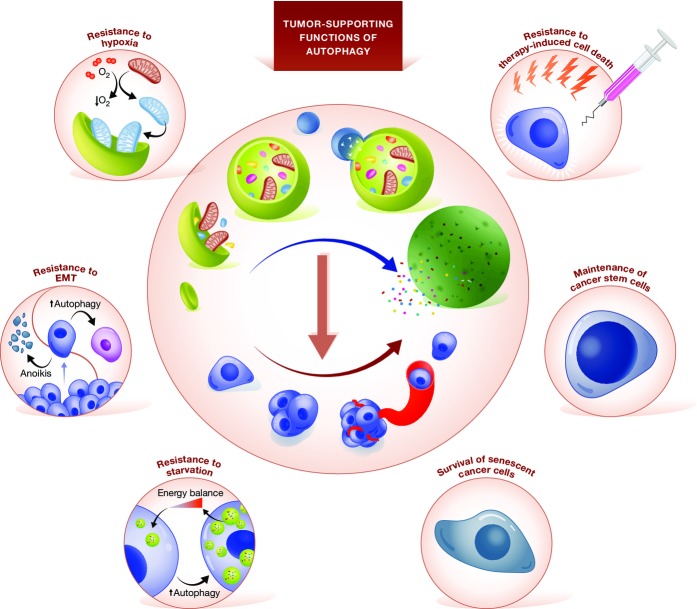
Taken together, these observations suggest that autophagy supports the progression of established neoplasms through several mechanisms (Fig3) and that pharmacological inhibitors of autophagy may exert robust antineoplastic effects, at least in some settings.
Figure 3.
Tumor-supporting functions of autophagy
Once malignant transformation has occurred, autophagy is believed to promote tumor progression and resistance to therapy. Such tumor-supporting functions reflects the ability of autophagy to: (1) improve the resistance of cancer cells to endogenous conditions that normally provoke cell death, such as the detachment from the basal membrane, hypoxia and nutrient deprivation; (2) render transformed cells less sensitive to therapy-induced cell death; (3) sustain the survival of cancer cells that enter a state of dormancy or senescence in response to therapy; and (4) ensure the maintenance of the cancer stem cell compartment. EMT, epithelial-to-mesenchymal transition.
Concluding remarks
Based on the data presented above, it is tempting to speculate that the multistep process leading from a healthy tissue to a metastatic and therapy-resistant, and hence life-threatening, neoplasm involves a temporary loss of autophagic competence (or the gain of molecular functions that antagonize, at least transitorily, autophagy-dependent oncosuppression). Initially, defects in the autophagic process might facilitate the acquisition of malignant features by healthy cells. Later on, once malignancy is established, the restoration of proficient autophagic responses may be essential to support the survival, proliferation and growth of cancer cells in the presence of adverse microenvironmental conditions (Fig4A). How proficient autophagic responses are reconstituted after an initial phase of autophagy inhibition, however, has not yet been established. As a possibility, the genetic or epigenetic instability that characterizes progressing tumors may restore autophagy in specific cells, rendering them able to overcome their neighboring autophagy-incompetent counterparts. Formal experimental evidence in support of this model is lacking. At least in some settings, oncogenesis and tumor progression may indeed rely on a stable loss or gain of autophagic proficiency (Fig4B and C).
Figure 4.
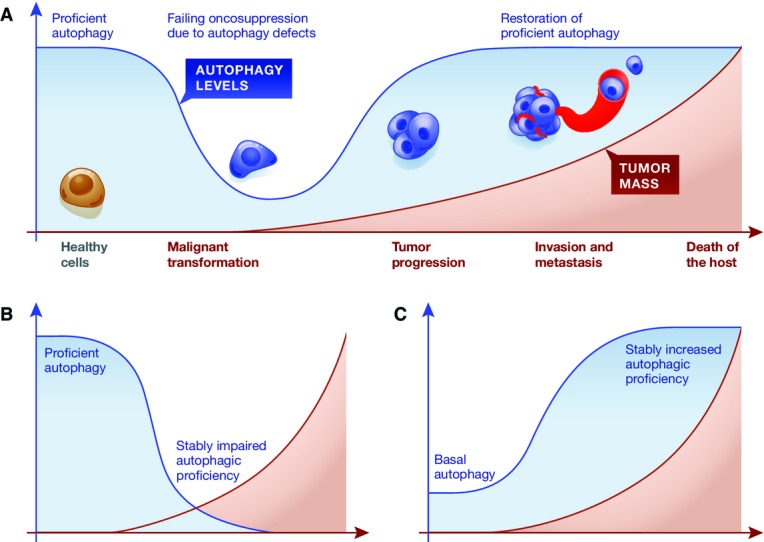
Autophagy in malignant transformation and tumor progression
(A) Healthy cells appear to be protected from malignant transformation by proficient autophagic responses. Conversely, autophagy promotes tumor progression and therapy resistance in a variety of models. Thus, the transition of a healthy cell toward a metastatic and therapy-insensitive neoplasm may involve a temporary (but not a stable) loss in autophagy competence. The mechanisms underlying the restoration of proficient autophagic responses after malignant transformation remain to be elucidated. (B, C) In specific settings, oncogenesis and tumor progression may rely on a permanent loss (B) or gain (C) of autophagic proficiency.
Importantly, genetic data indicating that the inhibition of autophagy exerts bona fide antineoplastic effects against established tumors have been obtained mainly in RAS-driven or RAS-related (BRAFV600E-driven) models of oncogenesis (Guo et al, 2013a; Rosenfeldt et al, 2013; Strohecker et al, 2013; Perez et al, 2014; Rao et al, 2014; Yang et al, 2014). In other scenarios, including the loss of scrib and the eyeful genotype in Drosophila, disabling autophagy by genetic means de facto accelerates tumor progression (Perez et al, 2014). Moreover, the antineoplastic effects of genetic and pharmacological interventions that inhibit autophagy vary in models in which the TP53 system is lost by different modalities (i.e., homozygous knockout versus loss-of-heterozygosity) (Yang et al, 2011; Huo et al, 2013; Rosenfeldt et al, 2013; Rao et al, 2014; Yang et al, 2014). Thus, the impact of autophagy on tumor progression may exhibit a significant degree of context dependency. Accordingly, recent data indicate that only tumors that are addicted to autophagy even in nutrient-rich conditions and in the absence of stressful stimuli respond to autophagy inhibitors in vivo (Maycotte et al, 2014). This suggests that only a fraction of cancer patients may benefit from the administration of autophagy inhibitors. Along similar lines, autophagy has been shown to underlie, at least in part, the therapeutic activity of some anticancer regimens (Salazar et al, 2009; Torres et al, 2011; Vara et al, 2011). Moreover, autophagy is required not only for the emission of immunostimulatory signals by malignant cells succumbing to specific anticancer agents (Kroemer et al, 2013), but also for the activation of tumor-targeting innate and adaptive immune responses (Ma et al, 2013). Efforts should therefore be focused on the identification of precise clinical scenarios in which autophagy supports, rather than counteracts, disease progression and resistance to therapy. This is particularly important not only because autophagy inhibitors may one day become part of the clinical routine, but also because most (if not all) anticancer agents that are currently employed in the clinic modulate autophagy (Kroemer et al, 2010).
Of note, the genetic inhibition of autophagy in models of mammalian carcinogenesis has near-to-invariably been achieved with the whole-body or conditional (heterozygous or homozygous) knockout of Atg5, Atg7 or Becn1 (Guo et al, 2013a; Rosenfeldt et al, 2013; Strohecker et al, 2013; Rao et al, 2014; Yang et al, 2014). As an increasing number of activities is being ascribed to these and other autophagy mediators (Cosse et al, 2010; Lee et al, 2012; Liu et al, 2012a; Moscat & Diaz-Meco, 2012; Zhao et al, 2012; Maskey et al, 2013; Elgendy et al, 2014), it remains possible that other, autophagy-independent functions of ATG5, ATG7 and BECN1 support the progression of KRASG12D- and BRAFV600E-driven tumors.
In experimental settings, the pharmacological inhibition of autophagy is most often realized with the administration of chloroquine (CQ) and hydroxychloroquine (HCQ), two lysosomotropic drugs approved by the US Food and Drug Administration for the prophylactic treatment of malaria and for the management of (chronic, discoid or systemic) lupus erythematosus as well as acute or chronic rheumatoid arthritis (Rubinsztein et al, 2012). Similar to other lysosomotropic agents, both CQ and HCQ block autophagy by inhibiting the fusion between autophagosomes with lysosomes and their degradation (Rote & Rechsteiner, 1983). Results from several Phase I–II clinical data indicate that HCQ can be safely employed at relatively high doses to improve the clinical activity of radiation therapy as well as of distinct anticancer chemotherapeutics, including temozolomide (an alkylating agent), vorinostat (a histone deacetylase inhibitor) and bortezomib (a proteasome inhibitor) (Barnard et al, 2014; Mahalingam et al, 2014; Rangwala et al, 2014a; Rangwala et al, 2014b; Rosenfeld et al, 2014; Vogl et al, 2014; Wolpin et al, 2014). However, the potency and specificity of HCQ and CQ are poor, and both these compounds have been shown to mediate antineoplastic effects via multiple autophagy-independent pathways, including lethal lysosomal destabilization (Boya et al, 2003; Maycotte et al, 2012) and the normalization of the tumor vasculature (Maes et al, 2014). Thus, the abundant scientific literature concluding that pharmacological inhibitors of autophagy constitute a convenient means to arrest tumor progression or sensitize malignant cells to therapy based on results obtained with CQ and HCQ only should be taken with caution. Lys05, a potent dimeric variant of CQ, is currently being characterized in preclinical tumor models (McAfee et al, 2012). Lys05, however, seems to share the limited specificity of CQ and HCQ. Small molecules that block autophagy in a highly specific manner are therefore urgently awaited. Recently, a specific VPS34 inhibitor has been developed and shown to efficiently inhibit autophagy. However, its putative antineoplastic effects may reflect the pleiotropic activity of VPS34, which is also involved in non-autophagic vesicle trafficking (Ronan et al, 2014). Finally, it will be interesting to develop molecules that inhibit autophagy in malignant cells but not in their normal counterparts, perhaps by targeting upstream signal transducers rather than downstream effectors. Proof-of-principle data in support of the therapeutic activity of such an approach in preclinical models have already been generated (Wilkinson et al, 2009). This is particularly relevant given the key contribution of autophagy to the maintenance of homeostasis in healthy cells. Indeed, at least on theoretical grounds, efficiently inhibiting autophagy in non-transformed cells may have deleterious consequences ranging from an accrued propensity to malignant transformation to overt cytotoxicity.
Intriguingly, several experimental maneuvers that increase the lifespan of model organisms as evolutionary distant as nematodes, flies and mice, including caloric restriction as well as the administration of the MTOR inhibitor rapamycin, activate autophagy (Harrison et al, 2009; Morselli et al, 2010; Madeo et al, 2014). Moreover, these interventions generally lose their lifespan-extending activity in autophagy-deficient hosts (Morselli et al, 2010; Madeo et al, 2014). However, to which extent the lifespan-prolonging effects of autophagy directly relate to its major oncosuppressive functions remains to be determined.
Recently, several studies have demonstrated that autophagy is regulated by epigenetic alterations, including histone methylation and acetylation (Artal-Martinez de Narvajas et al, 2013; Lam et al, 2013; Eisenberg et al, 2014). In addition, transcription factors other than TP53 and HIF-1, such as transcription factor EB (TFEB) and cAMP-responsive element binding protein 1 (CREB1), are intimately involved in autophagic responses (Settembre et al, 2011; Seok et al, 2014). The precise mechanisms through which cancer-associated epigenetic alterations (and/or the consequent transcriptional reprogramming) modulate autophagy have not yet been elucidated. Obtaining profound insights into this issue may pave the way to the development of novel, cancer-specific inhibitors of autophagy with therapeutic potential.
Irrespective of these incognita, autophagy stands out as key system for the maintenance of homeostasis, hence exerting a differential impact on malignant transformation and tumor progression (Box 1).
Box 1 Reconciling the effects of autophagy on malignant transformation and tumor progression.
Autophagy is a crucial mechanism for the maintenance of intracellular homeostasis, in baseline conditions as well as in response to stress.
Autophagy has been attributed tumor-suppressive as well as tumor-promoting functions, raising doubts on the actual therapeutic value of autophagy inhibitors for cancer therapy.
According to a growing literature, autophagy inhibits malignant transformation, that is, the conversion of a completely normal cell into a cell that can potentially form a tumor, and is required for efficient anticancer immunosurveillance.
Conversely, autophagy often supports tumor progression, that is, the process whereby a (pre)-neoplastic cell acquires the ability to grow unrestrained locally, promote angiogenesis, undergo the epithelial-to-mesenchymal transition, reach a metastatic niche through circulation and form secondary lesions, as well as resistance to various forms of anticancer therapy.
From a cell-centered perspective, autophagic responses near-to-invariably exert beneficial effects, but these are detrimental for the host when they occur in cells that have already initiated malignant transformation.
In line with this notion, pharmacological inhibitors of autophagy exert antineoplastic effects against established tumors, especially in combination with other forms of therapy.
However, highly targeted inhibitors of autophagy for use in humans are not available, and the molecules employed so far to this aim (i.e., chloroquine and hydroxychloroquine) have several, therapeutically relevant off-target effects.
Moreover, the true impact of autophagy on oncogenesis and tumor progression may exhibit a significant degree of context dependency.
Thus, it is critical to characterize specific neoplasms for their actual dependency on autophagy before implementing the use of autophagy inhibitors in the clinical routine.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). DG is supported by the American Cancer Society (Institutional Research Grant IRG-14-192-40). SK is supported by the National Health and Medical Research Council of Australia Project Grant (1041807) and a Senior Principal Research Fellowship (1002863). MP is supported by grants from the Ministry of Health of Italy (‘Ricerca Corrente’ and ‘Ricerca Finalizzata’) and Associazione Italiana per la Ricerca sul Cancro (AIRC). HUS is supported by the Swiss National Science Foundation, Swiss Cancer League, and the European Commission (MEL-PLEX). GV is supported by grants from the Spanish Ministry of Economy and Competitiveness (MINECO), Fondo Europeo de Desarrollo Regional (FEDER) (PI12/02248, FR2009-0052 and IT2009-0053), Fundación Mutua Madrileña (AP101042012) and Fundació La Marató de TV3 (20134031).
Author contributions
LG, KMR and GK conceived the review. LG wrote the review, centralized and integrated comments from co-authors, conceived the figures, and revised the review upon editorial feedback. JMBSP designed the figures. FP constructed the table. All authors corrected the review and provided valuable input to obtain a consensus view.
Conflict of interest
Vassiliki Karantza is an employee of Merck Research Laboratories. All other authors declare that they have no conflict of interest.
References
- Abida WM, Gu W. p53-Dependent and p53-independent activation of autophagy by ARF. Cancer Res. 2008;68:352–357. doi: 10.1158/0008-5472.CAN-07-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CH, Jeong EG, Lee JW, Kim MS, Kim SH, Kim SS, Yoo NJ, Lee SH. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. APMIS. 2007;115:1344–1349. doi: 10.1111/j.1600-0463.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Huang D, Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol. 2014;51:219–227. doi: 10.1053/j.seminhematol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- Artal-Martinez de Narvajas A, Gomez TS, Zhang JS, Mann AO, Taoda Y, Gorman JA, Herreros-Villanueva M, Gress TM, Ellenrieder V, Bujanda L, Kim DH, Kozikowski AP, Koenig A, Billadeau DD. Epigenetic regulation of autophagy by the methyltransferase G9a. Mol Cell Biol. 2013;33:3983–3993. doi: 10.1128/MCB.00813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivar-Valderas A, Bobrovnikova-Marjon E, Alan DJ, Bardeesy N, Debnath J, Aguirre-Ghiso JA. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene. 2013;32:4932–4940. doi: 10.1038/onc.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, Lee HE, Kang D, Rhee SG. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Balakumaran BS, Porrello A, Hsu DS, Glover W, Foye A, Leung JY, Sullivan BA, Hahn WC, Loda M, Febbo PG. MYC activity mitigates response to rapamycin in prostate cancer through eukaryotic initiation factor 4E-binding protein 1-mediated inhibition of autophagy. Cancer Res. 2009;69:7803–7810. doi: 10.1158/0008-5472.CAN-09-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard RA, Wittenburg LA, Amaravadi RK, Gustafson DL, Thorburn A, Thamm DH. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. 2014;10:1415–1425. doi: 10.4161/auto.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaid A, Cerezo M, Chargui A, Corcelle-Termeau E, Pedeutour F, Giuliano S, Ilie M, Rubera I, Tauc M, Barale S, Bertolotto C, Brest P, Vouret-Craviari V, Klionsky DJ, Carle GF, Hofman P, Mograbi B. Autophagy plays a critical role in the degradation of active RHOA, the control of cell cytokinesis, and genomic stability. Cancer Res. 2013;73:4311–4322. doi: 10.1158/0008-5472.CAN-12-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233:212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Berres ML, Lim KP, Peters T, Price J, Takizawa H, Salmon H, Idoyaga J, Ruzo A, Lupo PJ, Hicks MJ, Shih A, Simko SJ, Abhyankar H, Chakraborty R, Leboeuf M, Beltrao M, Lira SA, Heym KM, Bigley V, Collin M, et al. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. J Exp Med. 2014;211:669–683. doi: 10.1084/jem.20130977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Perfettini JL, Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–3936. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai-McRae X, Zhong H, Karantza V. Sequestosome 1/p62 facilitates HER2-induced mammary tumorigenesis through multiple signaling pathways. Oncogene. 2014 doi: 10.1038/onc.2014.244. doi: 10.1038/onc.2014.244. [DOI] [PubMed] [Google Scholar]
- Cai Q, Yan L, Xu Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene. 2014 doi: 10.1038/onc.2014.264. doi: 10.1038/onc.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- Chano T, Kontani K, Teramoto K, Okabe H, Ikegawa S. Truncating mutations of RB1CC1 in human breast cancer. Nat Genet. 2002;31:285–288. doi: 10.1038/ng911. [DOI] [PubMed] [Google Scholar]
- Chen N, Eritja N, Lock R, Debnath J. Autophagy restricts proliferation driven by oncogenic phosphatidylinositol 3-kinase in three-dimensional culture. Oncogene. 2013;32:2543–2554. doi: 10.1038/onc.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen Y, Kuroda J, Shimura Y, Nagoshi H, Kiyota M, Yamamoto-Sugitani M, Mizutani S, Sakamoto N, Ri M, Kawata E, Kobayashi T, Matsumoto Y, Horiike S, Iida S, Taniwaki M. Phosphoinositide protein kinase PDPK1 is a crucial cell signaling mediator in multiple myeloma. Cancer Res. 2014;74:7418–7429. doi: 10.1158/0008-5472.CAN-14-1420. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Kolukula VK, Preet A, Albanese C, Avantaggiati ML. Dissecting the pathways that destabilize mutant p53: the proteasome or autophagy? Cell Cycle. 2013;12:1022–1029. doi: 10.4161/cc.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph F, Hinz S, Kempkensteffen C, Weikert S, Krause H, Schostak M, Schrader M, Miller K. A gene expression profile of tumor suppressor genes commonly methylated in bladder cancer. J Cancer Res Clin Oncol. 2007;133:343–349. doi: 10.1007/s00432-006-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfanelli V, Fuoco C, Lorente M, Salazar M, Quondamatteo F, Gherardini PF, De Zio D, Nazio F, Antonioli M, D'Orazio M, Skobo T, Bordi M, Rohde M, Dalla VL, Helmer-Citterich M, Gretzmeier C, Dengjel J, Fimia GM, Piacentini M, Di BS, et al. AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c-Myc dephosphorylation and degradation. Nat Cell Biol. 2015;17:20–30. doi: 10.1038/ncb3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini M, Chakrabarti R, Kongara S, Price S, Nahar R, Lozy F, Zhong H, Vazquez A, Kang Y, Karantza V. Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by WNT1 activation and following parity. Autophagy. 2014;10:2036–2052. doi: 10.4161/auto.34398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2012;13:7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Ngouenet C, Anderson S, Brabletz T, Eisenman RN. Stress-induced cleavage of Myc promotes cancer cell survival. Genes Dev. 2014;28:689–707. doi: 10.1101/gad.231894.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KL, Kuballa P, Song JH, Patel KK, Castoreno AB, Yilmaz OH, Jijon HB, Zhang M, Aldrich LN, Villablanca EJ, Peloquin JM, Goel G, Lee IA, Mizoguchi E, Shi HN, Bhan AK, Shaw SY, Schreiber SL, Virgin HW, Shamji AF, et al. Atg16 l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology. 2013;145:1347–1357. doi: 10.1053/j.gastro.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola D, Khalil F, Eschrich SA, Boulware D, Yeatman T, Wang HG. Down-regulation of Bax-interacting factor-1 in colorectal adenocarcinoma. Cancer. 2008;113:2665–2670. doi: 10.1002/cncr.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazzari M, Rapino F, Ciccosanti F, Giglio P, Antonioli M, Conti B, Fimia GM, Lovat PE, Piacentini M. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.183. doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosse JP, Rommelaere G, Ninane N, Arnould T, Michiels C. BNIP3 protects HepG2 cells against etoposide-induced cell death under hypoxia by an autophagy-independent pathway. Biochem Pharmacol. 2010;80:1160–1169. doi: 10.1016/j.bcp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Cryns VL, Thor A, Xu HJ, Hu SX, Wierman ME, Vickery AL, Jr, Benedict WF, Arnold A. Loss of the retinoblastoma tumor-suppressor gene in parathyroid carcinoma. N Engl J Med. 1994;330:757–761. doi: 10.1056/NEJM199403173301105. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- de Freitas AC, Coimbra EC, Leitao Mda C. Molecular targets of HPV oncoproteins: potential biomarkers for cervical carcinogenesis. Biochim Biophys Acta. 2014;1845:91–103. doi: 10.1016/j.bbcan.2013.12.004. [DOI] [PubMed] [Google Scholar]
- de Vries A, Flores ER, Miranda B, Hsieh HM, van Oostrom CT, Sage J, Jacks T. Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. Proc Natl Acad Sci USA. 2002;99:2948–2953. doi: 10.1073/pnas.052713099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH, Kumar S. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol. 2009;19:1741–1746. doi: 10.1016/j.cub.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Chang TK, Nicolson S, Shravage B, Simin R, Baehrecke EH, Kumar S. Relationship between growth arrest and autophagy in midgut programmed cell death in Drosophila. Cell Death Differ. 2012a;19:1299–1307. doi: 10.1038/cdd.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012b;19:87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Dabritz JH, Lisec J, Lenze D, Gerhardt A, Schleicher K, Kratzat S, Purfurst B, Walenta S, Mueller-Klieser W, Graler M, Hummel M, Keller U, Buck AK, Dorken B, Willmitzer L, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Schroeder S, Andryushkova A, Pendl T, Kuttner V, Bhukel A, Marino G, Pietrocola F, Harger A, Zimmermann A, Moustafa T, Sprenger A, Jany E, Buttner S, Carmona-Gutierrez D, Ruckenstuhl C, Ring J, Reichelt W, Schimmel K, Leeb T, et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab. 2014;19:431–444. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42:23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Elgendy M, Ciro M, Abdel-Aziz AK, Belmonte G, Zuffo RD, Mercurio C, Miracco C, Lanfrancone L, Foiani M, Minucci S. Beclin 1 restrains tumorigenesis through Mcl-1 destabilization in an autophagy-independent reciprocal manner. Nat Commun. 2014;5:5637. doi: 10.1038/ncomms6637. [DOI] [PubMed] [Google Scholar]
- Faderl S, Kantarjian HM, Manshouri T, Chan CY, Pierce S, Hays KJ, Cortes J, Thomas D, Estrov Z, Albitar M. The prognostic significance of p16INK4a/p14ARF and p15INK4b deletions in adult acute lymphoblastic leukemia. Clin Cancer Res. 1999;5:1855–1861. [PubMed] [Google Scholar]
- Feng W, Marquez RT, Lu Z, Liu J, Lu KH, Issa JP, Fishman DM, Yu Y, Bast RC., Jr Imprinted tumor suppressor genes ARHI and PEG3 are the most frequently down-regulated in human ovarian cancers by loss of heterozygosity and promoter methylation. Cancer. 2008;112:1489–1502. doi: 10.1002/cncr.23323. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- Fountain JW, Wallace MR, Bruce MA, Seizinger BR, Menon AG, Gusella JF, Michels VV, Schmidt MA, Dewald GW, Collins FS. Physical mapping of a translocation breakpoint in neurofibromatosis. Science. 1989;244:1085–1087. doi: 10.1126/science.2543076. [DOI] [PubMed] [Google Scholar]
- Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta S, Hidaka E, Ogata A, Yokota S, Kamata T. Ras is involved in the negative control of autophagy through the class I PI3-kinase. Oncogene. 2004;23:3898–3904. doi: 10.1038/sj.onc.1207539. [DOI] [PubMed] [Google Scholar]
- Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Bennett LM, Haugen-Strano A, Swensen J, Miki Y, et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994;266:120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;59:1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, Baehrecke EH, Bazan NG, Bertrand MJ, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Campanella M, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garufi A, Pucci D, D'Orazi V, Cirone M, Bossi G, Avantaggiati ML, D'Orazi G. Degradation of mutant p53H175 protein by Zn(II) through autophagy. Cell Death Dis. 2014;5:e1271. doi: 10.1038/cddis.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AD, Borrow J, Freemont PS, Solomon E. Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science. 1991;254:1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- Gomez-Manzano C, Mitlianga P, Fueyo J, Lee HY, Hu M, Spurgers KB, Glass TL, Koul D, Liu TJ, McDonnell TJ, Yung WK. Transfer of E2F-1 to human glioma cells results in transcriptional up-regulation of Bcl-2. Cancer Res. 2001;61:6693–6697. [PubMed] [Google Scholar]
- Gong C, Bauvy C, Tonelli G, Yue W, Delomenie C, Nicolas V, Zhu Y, Domergue V, Marin-Esteban V, Tharinger H, Delbos L, Gary-Gouy H, Morel AP, Ghavami S, Song E, Codogno P, Mehrpour M. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene. 2013;32:2261–2272. doi: 10.1038/onc.2012.252. , 2272e 2261–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goussetis DJ, Gounaris E, Wu EJ, Vakana E, Sharma B, Bogyo M, Altman JK, Platanias LC. Autophagic degradation of the BCR-ABL oncoprotein and generation of antileukemic responses by arsenic trioxide. Blood. 2012;120:3555–3562. doi: 10.1182/blood-2012-01-402578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greim H, Kaden DA, Larson RA, Palermo CM, Rice JM, Ross D, Snyder R. The bone marrow niche, stem cells, and leukemia: impact of drugs, chemicals, and the environment. Ann N Y Acad Sci. 2014;1310:7–31. doi: 10.1111/nyas.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin LM, Cicchini L, Pyeon D. Human papillomavirus infection is inhibited by host autophagy in primary human keratinocytes. Virology. 2013;437:12–19. doi: 10.1016/j.virol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Chitiprolu M, Gagnon D, Meng L, Perez-Iratxeta C, Lagace D, Gibbings D. Autophagy supports genomic stability by degrading retrotransposon RNA. Nat Commun. 2014;5:5276. doi: 10.1038/ncomms6276. [DOI] [PubMed] [Google Scholar]
- Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, Snyder E, Santanam U, Dipaola RS, Jacks T, Rabinowitz JD, White E. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013a;27:1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013b;155:1216–1219. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DP, Cost NG, Hegde S, Kellner E, Mikhaylova O, Stratton Y, Ehmer B, Abplanalp WA, Pandey R, Biesiada J, Harteneck C, Plas DR, Meller J, Czyzyk-Krzeska MF. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell. 2014;26:738–753. doi: 10.1016/j.ccell.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hanning JE, Saini HK, Murray MJ, Caffarel MM, van Dongen S, Ward D, Barker EM, Scarpini CG, Groves IJ, Stanley MA, Enright AJ, Pett MR, Coleman N. Depletion of HPV16 early genes induces autophagy and senescence in a cervical carcinogenesis model, regardless of viral physical state. J Pathol. 2013;231:354–366. doi: 10.1002/path.4244. [DOI] [PubMed] [Google Scholar]
- Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122:4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Hervier B, Haroche J, Arnaud L, Charlotte F, Donadieu J, Neel A, Lifermann F, Villabona C, Graffin B, Hermine O, Rigolet A, Roubille C, Hachulla E, Carmoi T, Bezier M, Meignin V, Conrad M, Marie L, Kostrzewa E, Michot JM, et al. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124:1119–1126. doi: 10.1182/blood-2013-12-543793. [DOI] [PubMed] [Google Scholar]
- Hock AK, Vousden KH. The role of ubiquitin modification in the regulation of p53. Biochim Biophys Acta. 2014;1843:137–149. doi: 10.1016/j.bbamcr.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Horikawa I, Fujita K, Jenkins LM, Hiyoshi Y, Mondal AM, Vojtesek B, Lane DP, Appella E, Harris CC. Autophagic degradation of the inhibitory p53 isoform Delta133p53alpha as a regulatory mechanism for p53-mediated senescence. Nat Commun. 2014;5:4706. doi: 10.1038/ncomms5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wu Z, Mei Y, Wu M. XIAP inhibits autophagy via XIAP-Mdm2-p53 signalling. EMBO J. 2013;32:2204–2216. doi: 10.1038/emboj.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Cai H, Teplova I, Bowman-Colin C, Chen G, Price S, Barnard N, Ganesan S, Karantza V, White E, Xia B. Autophagy opposes p53-mediated tumor barrier to facilitate tumorigenesis in a model of PALB2-associated hereditary breast cancer. Cancer Discov. 2013;3:894–907. doi: 10.1158/2159-8290.CD-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- Isakson P, Bjoras M, Boe SO, Simonsen A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood. 2010;116:2324–2331. doi: 10.1182/blood-2010-01-261040. [DOI] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Jiang H, Martin V, Gomez-Manzano C, Johnson DG, Alonso M, White E, Xu J, McDonnell TJ, Shinojima N, Fueyo J. The RB-E2F1 pathway regulates autophagy. Cancer Res. 2010;70:7882–7893. doi: 10.1158/0008-5472.CAN-10-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, Johnson GV. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, Okuda K. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci USA. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenific CM, Debnath J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015;25:37–45. doi: 10.1016/j.tcb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Hum Pathol. 2008;39:1059–1063. doi: 10.1016/j.humpath.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, Marino G, Kepp O, Michaud M, Perfettini JL, Kroemer G, Deutsch E. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014;21:92–99. doi: 10.1038/cdd.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Laddha SV, Ganesan S, Chan CS, White E. Mutational landscape of the essential autophagy gene BECN1 in human cancers. Mol Cancer Res. 2014;12:485–490. doi: 10.1158/1541-7786.MCR-13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, An CH, Shan B, Franks JM, Haley KJ, Owen CA, Tesfaigzi Y, Washko GR, Quackenbush J, Silverman EK, Rahman I, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen KG, Kuballa P, Conway KL, Patel KK, Becker CE, Peloquin JM, Villablanca EJ, Norman JM, Liu TC, Heath RJ, Becker ML, Fagbami L, Horn H, Mercer J, Yilmaz OH, Jaffe JD, Shamji AF, Bhan AK, Carr SA, Daly MJ, et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci USA. 2014;111:7741–7746. doi: 10.1073/pnas.1407001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18:370–379. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Kawai Y, Fergusson MM, Rovira II, Bishop AJ, Motoyama N, Cao L, Finkel T. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science. 2012;336:225–228. doi: 10.1126/science.1218395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy B, Girard L, Hollestelle A, Minna JD, Gazdar AF, Soussi T. Analysis of TP53 mutation status in human cancer cell lines: a reassessment. Hum Mutat. 2014;35:756–765. doi: 10.1002/humu.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, Morgan MJ, Mirsky DM, Handler MH, Foreman NK, Thorburn A. Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors. Cancer Discov. 2014;4:773–780. doi: 10.1158/2159-8290.CD-14-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, Liu J, Lemischka IR, Hung MC, Chiao PJ. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yan X, Wang HQ, Gao YY, Liu J, Hu Z, Liu D, Gao J, Lin B. Autophagy-independent enhancing effects of Beclin 1 on cytotoxicity of ovarian cancer cells mediated by proteasome inhibitors. BMC Cancer. 2012a;12:622. doi: 10.1186/1471-2407-12-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lee JY, Wei H, Tanabe O, Engel JD, Morrison SJ, Guan JL. FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood. 2010;116:4806–4814. doi: 10.1182/blood-2010-06-288589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, He Z, von Rutte T, Yousefi S, Hunger RE, Simon HU. Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci Transl Med. 2013a;5:202ra123. doi: 10.1126/scitranslmed.3005864. [DOI] [PubMed] [Google Scholar]
- Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012b;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shoji-Kawata S, Sumpter RM, Jr, Wei Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, Shaw SY, Clarke PG, Puyal J, Levine B. Autosis is a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA. 2013b;110:20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, Debnath J. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2011;22:165–178. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozy F, Cai-McRae X, Teplova I, Price S, Reddy A, Bhanot G, Ganesan S, Vazquez A, Karantza V. ERBB2 overexpression suppresses stress-induced autophagy and renders ERBB2-induced mammary tumorigenesis independent of monoallelic Becn1 loss. Autophagy. 2014;10:662–676. doi: 10.4161/auto.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, Kondo S, Kondo Y, Yu Y, Mills GB, Liao WS, Bast RC., Jr The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, Lazova R, Klump V, Pawelek JM, Xu X, Xu W, Schuchter LM, Davies MA, Herlyn M, Winkler J, Koumenis C, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014;124:1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC, Whang-Peng J, Liu JM, Yang DM, Yang WK, Shen CY. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000;19:2739–2744. doi: 10.1038/sj.onc.1203597. [DOI] [PubMed] [Google Scholar]
- Madeo F, Pietrocola F, Eisenberg T, Kroemer G. Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov. 2014;13:727–740. doi: 10.1038/nrd4391. [DOI] [PubMed] [Google Scholar]
- Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion AC, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquiere B, et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26:190–206. doi: 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi RK, Davis LE, Mita AC, Curiel TJ, Espitia CM, Nawrocki ST, Giles FJ, Carew JS. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10:1403–1414. doi: 10.4161/auto.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007a;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007b;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22:181–185. doi: 10.1016/j.ceb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino G, Salvador-Montoliu N, Fueyo A, Knecht E, Mizushima N, Lopez-Otin C. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J Biol Chem. 2007;282:18573–18583. doi: 10.1074/jbc.M701194200. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Dahia PL, Zheng Z, Liaw D, Parsons R, Gorlin RJ, Eng C. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- Martoriati A, Doumont G, Alcalay M, Bellefroid E, Pelicci PG, Marine JC. dapk1, encoding an activator of a p19ARF-p53-mediated apoptotic checkpoint, is a transcription target of p53. Oncogene 24: 2005;146:1–1466. doi: 10.1038/sj.onc.1208256. [DOI] [PubMed] [Google Scholar]
- Maskey D, Yousefi S, Schmid I, Zlobec I, Perren A, Friis R, Simon HU. ATG5 is induced by DNA-damaging agents and promotes mitotic catastrophe independent of autophagy. Nat Commun. 2013;4:2130. doi: 10.1038/ncomms3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 2012;8:200–212. doi: 10.4161/auto.8.2.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P, Gearheart CM, Barnard R, Aryal S, Mulcahy LJ, Fosmire SP, Hansen RJ, Morgan MJ, Porter CC, Gustafson DL, Thorburn A. STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Cancer Res. 2014;74:2579–2590. doi: 10.1158/0008-5472.CAN-13-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, Lynch JP, Uehara T, Sepulveda AR, Davis LE, Winkler JD, Amaravadi RK. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci USA. 2012;109:8253–8258. doi: 10.1073/pnas.1118193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride KA, Ballinger ML, Killick E, Kirk J, Tattersall MH, Eeles RA, Thomas DM, Mitchell G. Li-Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol. 2014;11:260–271. doi: 10.1038/nrclinonc.2014.41. [DOI] [PubMed] [Google Scholar]
- McKnight NC, Zhong Y, Wold MS, Gong S, Phillips GR, Dou Z, Zhao Y, Heintz N, Zong WX, Yue Z. Beclin 1 is required for neuron viability and regulates endosome pathways via the UVRAG-VPS34 complex. PLoS Genet. 2014;10:e1004626. doi: 10.1371/journal.pgen.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di VF, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova O, Stratton Y, Hall D, Kellner E, Ehmer B, Drew AF, Gallo CA, Plas DR, Biesiada J, Meller J, Czyzyk-Krzeska MF. VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer Cell. 2012;21:532–546. doi: 10.1016/j.ccr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, Mannucci S, De Nisi MC, Toscano M, Malagnino V, Falzarano SM, Pirtoli L, Tosi P. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol. 2007;30:429–436. [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Galluzzi L, Kepp O, Marino G, Michaud M, Vitale I, Maiuri MC, Kroemer G. Oncosuppressive functions of autophagy. Antioxid Redox Signal. 2011;14:2251–2269. doi: 10.1089/ars.2010.3478. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, Sadighi-Akha E, Stranks AJ, Glanville J, Knight S, Jacobsen SE, Kranc KR, Simon AK. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208:455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–236. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella C, Chen Z, Salmena L, Carracedo A, Alimonti A, Egia A, Carver B, Gerald W, Cordon-Cardo C, Pandolfi PP. Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes Dev. 2008;22:2172–2177. doi: 10.1101/gad.1699608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, Cecconi F. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- Oh S, Xiaofei E, Ni D, Pirooz SD, Lee JY, Lee D, Zhao Z, Lee S, Lee H, Ku B, Kowalik T, Martin SE, Oh BH, Jung JU, Liang C. Downregulation of autophagy by Bcl-2 promotes MCF7 breast cancer cell growth independent of its inhibition of apoptosis. Cell Death Differ. 2011;18:452–464. doi: 10.1038/cdd.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K. Organellophagy: eliminating cellular building blocks via selective autophagy. J Cell Biol. 2014;205:435–445. doi: 10.1083/jcb.201402054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Park JM, Tougeron D, Huang S, Okamoto K, Sinicrope FA. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS One. 2014;9:e100819. doi: 10.1371/journal.pone.0100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhitko A, Myachina F, Morrison TA, Hindi KM, Auricchio N, Karbowniczek M, Wu JJ, Finkel T, Kwiatkowski DJ, Yu JJ, Henske EP. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc Natl Acad Sci USA. 2011;108:12455–12460. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res. 2006;66:2885–2888. doi: 10.1158/0008-5472.CAN-05-4412. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Paul S, Kashyap AK, Jia W, He YW, Schaefer BC. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-kappaB. Immunity. 2012;36:947–958. doi: 10.1016/j.immuni.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YF, Shi YH, Ding ZB, Ke AW, Gu CY, Hui B, Zhou J, Qiu SJ, Dai Z, Fan J. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy. 2013;9:2056–2068. doi: 10.4161/auto.26398. [DOI] [PubMed] [Google Scholar]
- Perez E, Das G, Bergmann A, Baehrecke EH. Autophagy regulates tissue overgrowth in a context-dependent manner. Oncogene. 2014 doi: 10.1038/onc.2014.285. doi: 10.1038/onc.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, Maiuri MC, Kroemer G. Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol. 2013;23:310–322. doi: 10.1016/j.semcancer.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Pimkina J, Humbey O, Zilfou JT, Jarnik M, Murphy ME. ARF induces autophagy by virtue of interaction with Bcl-xl. J Biol Chem. 2009;284:2803–2810. doi: 10.1074/jbc.M804705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polager S, Ofir M, Ginsberg D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene. 2008;27:4860–4864. doi: 10.1038/onc.2008.117. [DOI] [PubMed] [Google Scholar]
- Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randow F, Youle RJ. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15:403–411. doi: 10.1016/j.chom.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, Tan KS, Heitjan DF, DeMichele AM, Vaughn DJ, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O'Dwyer PJ, et al. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014a;10:1391–1402. doi: 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala R, Leone R, Chang YC, Fecher LA, Schuchter LM, Kramer A, Tan KS, Heitjan DF, Rodgers G, Gallagher M, Piao S, Troxel AB, Evans TL, DeMichele AM, Nathanson KL, O'Dwyer PJ, Kaiser J, Pontiggia L, Davis LE, Amaravadi RK. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 2014b;10:1369–1379. doi: 10.4161/auto.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, Sigl V, Aumayr K, Schmauss G, Fellner N, Handschuh S, Glosmann M, Pasierbek P, Schlederer M, Resch GP, Ma Y, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. doi: 10.1038/ncomms4056. [DOI] [PubMed] [Google Scholar]
- Raveh T, Droguett G, Horwitz MS, DePinho RA, Kimchi A. DAP kinase activates a p19ARF/p53-mediated apoptotic checkpoint to suppress oncogenic transformation. Nat Cell Biol. 2001;3:1–7. doi: 10.1038/35050500. [DOI] [PubMed] [Google Scholar]
- Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M, Kimchi A. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell. 2006;22:463–475. doi: 10.1016/j.molcel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Rello-Varona S, Lissa D, Shen S, Niso-Santano M, Senovilla L, Marino G, Vitale I, Jemaa M, Harper F, Pierron G, Castedo M, Kroemer G. Autophagic removal of micronuclei. Cell Cycle. 2012;11:170–176. doi: 10.4161/cc.11.1.18564. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Rodriguez OC, Choudhury S, Kolukula V, Vietsch EE, Catania J, Preet A, Reynoso K, Bargonetti J, Wellstein A, Albanese C, Avantaggiati ML. Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell Cycle. 2012;11:4436–4446. doi: 10.4161/cc.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogov V, Dotsch V, Johansen T, Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell. 2014;53:167–178. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, Bachelot MF, Lamberton A, Mathieu M, Bertrand T, Marquette JP, El-Ahmad Y, Filoche-Romme B, Schio L, Garcia-Echeverria C, Goulaouic H, Pasquier B. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol. 2014;10:1013–1019. doi: 10.1038/nchembio.1681. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, Mikkelson T, Wang D, Chang YC, Hu J, McAfee Q, Fisher J, Troxel AB, Piao S, Heitjan DF, Tan KS, Pontiggia L, O'Dwyer PJ, Davis LE, Amaravadi RK. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10:1359–1368. doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt MT, O'Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ, Ryan KM. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- Rote KV, Rechsteiner M. Degradation of microinjected proteins: effects of lysosomotropic agents and inhibitors of autophagy. J Cell Physiol. 1983;116:103–110. doi: 10.1002/jcp.1041160116. [DOI] [PubMed] [Google Scholar]
- Rousselot P, Hardas B, Patel A, Guidez F, Gaken J, Castaigne S, Dejean A, de The H, Degos L, Farzaneh F, et al. The PML-RAR alpha gene product of the t(15;17) translocation inhibits retinoic acid-induced granulocytic differentiation and mediated transactivation in human myeloid cells. Oncogene. 1994;9:545–551. [PubMed] [Google Scholar]
- Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni S, Bae DH, Lane DJ, Kovacevic Z, Kalinowski DS, Jansson PJ, Richardson DR. The metastasis suppressor, N-myc downstream-regulated gene 1 (NDRG1), inhibits stress-induced autophagy in cancer cells. J Biol Chem. 2014;289:9692–9709. doi: 10.1074/jbc.M113.529511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T, Yamamoto N, Kawai T, Ishii K, Takeuchi O, Yoshimori T, Akira S. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci USA. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, Gonzalez-Feria L, Iovanna JL, Guzman M, Boya P, Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemi S, Yousefi S, Constantinescu MA, Fey MF, Simon HU. Autophagy is required for self-renewal and differentiation of adult human stem cells. Cell Res. 2012;22:432–435. doi: 10.1038/cr.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, Dalili S, Batey RA, Riedl SJ. Targeting XIAP for the treatment of malignancy. Cell Death Differ. 2006;13:179–188. doi: 10.1038/sj.cdd.4401826. [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Farmer GE, Lamiell JM, Haines J, Yuen JW, Collins D, Majoor-Krakauer D, et al. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988;332:268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Sen B, Johnson FM. Regulation of SRC family kinases in human cancers. J Signal Transduct. 2011;2011:865819. doi: 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, Ma J, Kemper B, Kemper JK. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature. 2014;16:108–111. doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di MC, Polito VA, Garcia AM, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, Dong C, Robertson GP. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–8209. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- Shuin T, Kondo K, Torigoe S, Kishida T, Kubota Y, Hosaka M, Nagashima Y, Kitamura H, Latif F, Zbar B, et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994;54:2852–2855. [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, Stevens C, O'Meara S, Smith R, Parker A, Barthorpe A, Blow M, Brackenbury L, Butler A, Clarke O, Cole J, Dicks E, Dike A, Drozd A, Edwards K, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M, White E. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 2013;3:1272–1285. doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci USA. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mule JJ, Pledger WJ, Wang HG. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Hori T, Cooper TK, Liao J, Desai N, Serfass JM, Young MM, Park S, Izu Y, Wang HG. Bif-1 haploinsufficiency promotes chromosomal instability and accelerates Myc-driven lymphomagenesis via suppression of mitophagy. Blood. 2013;121:1622–1632. doi: 10.1182/blood-2012-10-459826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Sebti S, Titone R, Zhou Y, Isidoro C, Ross TS, Hibshoosh H, Xiao G, Packer M, Xie Y, Levine B. Decreased BECN1 mRNA expression in human breast cancer is associated with estrogen receptor-negative subtypes and poor prognosis. EBioMedicine. 2015 doi: 10.1016/j.ebiom.2015.01.008. doi: 10.1016/j.ebiom.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh PP, Luo S, Menzies FM, Rasko T, Wanker EE, Rubinsztein DC. Myc inhibition impairs autophagosome formation. Hum Mol Genet. 2013;22:5237–5248. doi: 10.1093/hmg/ddt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres S, Lorente M, Rodriguez-Fornes F, Hernandez-Tiedra S, Salazar M, Garcia-Taboada E, Barcia J, Guzman M, Velasco G. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther. 2011;10:90–103. doi: 10.1158/1535-7163.MCT-10-0688. [DOI] [PubMed] [Google Scholar]
- Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, Yuan L, Soares F, Chea E, Le Bourhis L, Boneca IG, Allaoui A, Jones NL, Nunez G, Girardin SE, Philpott DJ. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, Duran A, Castilla EA, Metallo CM, Diaz-Meco MT, Moscat J. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26:121–135. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Vega YA, Maclean KH, Tait-Mulder J, Milasta S, Steeves M, Dorsey FC, Cleveland JL, Green DR, Kastan MB. Mitochondrial dysfunction in ataxia-telangiectasia. Blood. 2012;119:1490–1500. doi: 10.1182/blood-2011-08-373639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–1111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl DT, Stadtmauer EA, Tan KS, Heitjan DF, Davis LE, Pontiggia L, Rangwala R, Piao S, Chang YC, Scott EC, Paul TM, Nichols CW, Porter DL, Kaplan J, Mallon G, Bradner JE, Amaravadi RK. Combined autophagy and proteasome inhibition: a phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy. 2014;10:1380–1390. doi: 10.4161/auto.29264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Wang C, Liang CC, Bian ZC, Zhu Y, Guan JL. FIP200 is required for maintenance and differentiation of postnatal neural stem cells. Nat Neurosci. 2013;16:532–542. doi: 10.1038/nn.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Yang GF, Huang YH, Huang QW, Gao J, Zhao XD, Huang LM, Chen HL. Reduced expression of autophagy markers correlates with high-risk human papillomavirus infection in human cervical squamous cell carcinoma. Oncol Lett. 2014;8:1492–1498. doi: 10.3892/ol.2014.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Reichelt J, Levine B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012a;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang XD, Lapi E, Sullivan A, Jia W, He YW, Ratnayaka I, Zhong S, Goldin RD, Goemans CG, Tolkovsky AM, Lu X. Autophagic activity dictates the cellular response to oncogenic RAS. Proc Natl Acad Sci USA. 2012b;109:13325–13330. doi: 10.1073/pnas.1120193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cao L, Kang R, Yang M, Liu L, Zhao Y, Yu Y, Xie M, Yin X, Livesey KM, Tang D. Autophagy regulates myeloid cell differentiation by p62/SQSTM1-mediated degradation of PML-RARalpha oncoprotein. Autophagy. 2011;7:401–411. doi: 10.4161/auto.7.4.14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Wei S, Gan B, Peng X, Zou W, Guan JL. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25:1510–1527. doi: 10.1101/gad.2051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, Grishin NV, Peyton M, Minna J, Bhagat G, Levine B. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidberg H, Shvets E, Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem. 2011;80:125–156. doi: 10.1146/annurev-biochem-052709-094552. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, O'Prey J, Fricker M, Ryan KM. Hypoxia-selective macroautophagy and cell survival signaled by autocrine PDGFR activity. Genes Dev. 2009;23:1283–1288. doi: 10.1101/gad.521709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Dewi DL, Fredebohm J, Muller-Decker K, Flechtenmacher C, Hoheisel JD, Boettcher M. A mammosphere formation RNAi screen reveals that ATG4A promotes a breast cancer stem-like phenotype. Breast Cancer Res. 2013;15:R109. doi: 10.1186/bcr3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, Fuchs CS, McCleary NJ, Meyerhardt JA, Ng K, Schrag D, Sikora AL, Spicer BA, Killion L, Mamon H, Kimmelman AC. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist. 2014;19:637–638. doi: 10.1634/theoncologist.2014-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xue D, Chen G, Han Z, Huang L, Zhu C, Wang X, Jin H, Wang J, Zhu Y, Liu L, Chen Q. The BCL2L1 and PGAM5 axis defines hypoxia-induced receptor-mediated mitophagy. Autophagy. 2014;10:1712–1725. doi: 10.4161/auto.29568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GF, Lin B, Tanaka K, Dunn D, Wood D, Gesteland R, White R, Weiss R, Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990;63:835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, Von Hoff DD, Maitra A, Kimmelman AC. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905–913. doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell'antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasir M, Pachikara ND, Bao X, Pan Z, Fan H. Regulation of chlamydial infection by host autophagy and vacuolar ATPase-bearing organelles. Infect Immun. 2011;79:4019–4028. doi: 10.1128/IAI.05308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavare S, Arakawa S, Shimizu S, Watt FM, Narita M. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Xu F, Peng H, Fang X, Zhao S, Li Y, Cuevas B, Kuo WL, Gray JW, Siciliano M, Mills GB, Bast RC., Jr NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc Natl Acad Sci USA. 1999;96:214–219. doi: 10.1073/pnas.96.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R, Kimchi A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang L, Sung JJ, Yu J, Ng SC, Wong SH, Cho CH, Ng SS, Chan FK, Wu WK. Xenophagy in Helicobacter pylori- and Epstein-Barr virus-induced gastric cancer. J Pathol. 2014;233:103–112. doi: 10.1002/path.4351. [DOI] [PubMed] [Google Scholar]
- Zhao M, Klionsky DJ. AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metab. 2011;13:119–120. doi: 10.1016/j.cmet.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Oh S, Li D, Ni D, Pirooz SD, Lee JH, Yang S, Lee JY, Ghozalli I, Costanzo V, Stark JM, Liang C. A dual role for UVRAG in maintaining chromosomal stability independent of autophagy. Dev Cell. 2012;22:1001–1016. doi: 10.1016/j.devcel.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343–351. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]