Abstract
Inflammation entails a complex set of defense mechanisms acting in concert to restore the homeostatic balance in organisms after damage or pathogen invasion. This immune response consists of the activity of various immune cells in a highly complex manner. Inflammation is a double-edged sword as it is reported to have both detrimental and beneficial consequences. In this review, we discuss the effects of inflammation on stem cell activity, focusing primarily on neural stem/progenitor cells in mammals and zebrafish. We also give a brief overview of the effects of inflammation on other stem cell compartments, exemplifying the positive and negative role of inflammation on stemness. The majority of the chronic diseases involve an unremitting phase of inflammation due to improper resolution of the initial pro-inflammatory response that impinges on the stem cell behavior. Thus, understanding the mechanisms of crosstalk between the inflammatory milieu and tissue-resident stem cells is an important basis for clinical efforts. Not only is it important to understand the effect of inflammation on stem cell activity for further defining the etiology of the diseases, but also better mechanistic understanding is essential to design regenerative therapies that aim at micromanipulating the inflammatory milieu to offset the negative effects and maximize the beneficial outcomes.
Keywords: disease, inflammation, neural stem cell, proliferation, regeneration
Introduction
Etymologically, inflammation (in + flammō) denotes a metaphoric blaze caricaturized as sumptuous mythological creatures. A tale about Phoenix for instance narrates the fire devouring its body into ashes, from which a new bird arises. Thus, the state of torrid heat in the myths is both devastating and revitalizing. In biological systems, the situation may be quite similar. Inflammation in organisms points to a non-homeostatic response of the vascular tissues to various stimuli such as pathogens, injury or xenobiotics 1. During evolution, inflammation was developed as a mechanism of self-defense and survival pertaining to both beneficial and detrimental outcomes depending on the timing, cell types involved and the severity of the insult to the tissue. Generically, the effect of inflammation on tissues is therefore closely linked to how inflammation is initiated, maintained and resolved (Fig1).
Figure 1.
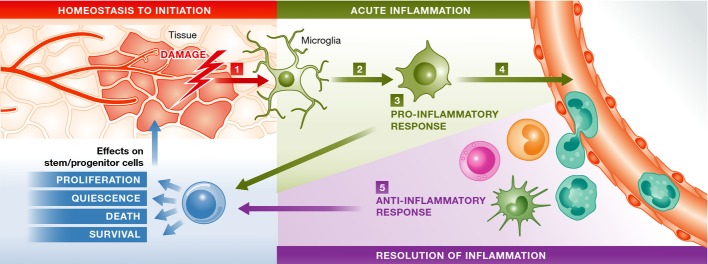
A simplified generic scheme of initiation and resolution of inflammation in six steps
1) A tissue when compromised during its homeostatic state initiates inflammation programs, through damage cues (such as intracellular content, apoptosis and cytokines). 2) Breach of homeostasis triggers the morphological and functional transformation of the resident macrophages (green). 3) Acute inflammation is initiated upon secretion of several pro-inflammatory cytokines and chemokines such as TNF-α, IL-1β, IL-6 and MCP-1. Complement system is also activated. 4) This process calls for peripheral immune cells, and recruited immune cells potentiate the inflammation by secreting more pro-inflammatory factors. 5) The immune cells also partake in active resolution of inflammation through secretion of anti-inflammatory factors such as IL-4, IL-10, C5a, IFN-γ, TGF-β and NO. A stem/progenitor cell undergoes the influence of the dynamic inflammatory milieu. The final outcome on the tissue manifests depending on the context.
Different phases of the inflammatory reaction are well defined 1. Acute period is the initial phase where the earliest reactions to the insult set on with the help of resident immune cells such as macrophages and dendritic cells 2. Secretion of pro-inflammatory cytokines such as IL-8 and TNF-α initiates a cascade of molecular events in neutrophils, fibroblasts and endothelial cells: Macrophages are recruited to the site, and the complement system is concomitantly activated 3. Acute inflammation contains an ensuing phase of active resolution by anti-inflammatory agents such as steroids, IL-10, nitric oxide, TGF-β or regulatory T cells 3. During the acute inflammation phase, the exudative component from the blood plasma-containing immunoglobulins or fibrins flushes into the inflamed site causing edema. This tissue swelling is relinquished by the lymphatic system where antigens are recognized by the T and B cells of the adaptive immune system, which is a recent evolutionary invention in vertebrates 4. Acuity of the reaction can be succeeded by chronicity based on the resolution dynamics of the inflammatory response. Chronic inflammation is hazardous to tissues, as exemplified by its involvement in the onset or progression of neurodegenerative disorders, cardiovascular diseases, autoimmunity, cancer and various metabolic diseases 5. Therefore, the regulation of inflammatory response is of utmost importance for restoration of tissue integrity and homeostasis.
In this review, we will focus specifically on the crosstalk between inflammation and stem cells. Various tissue stem cells react differently to inflammation, and the interplay between inflammation and stem cell activity is an immense research field. Here, we will mainly concentrate on the relationship between neural stem cells and inflammation, molecules known to be mediating this reciprocity and the effect of the inflammatory milieu on the ability to regenerate tissue.
Tissue microenvironment and stem cells during inflammation
Inflammation in a tissue is profoundly affecting the homeostatic measures thereof and thus has a strong impact on nearby cells. In adult organisms, tissues contain resident niches of stem cells that are specialized to form new cells contingent with their surrounding 6. Stem cell activity is regulated by intrinsic mechanisms and extrinsic cues that emanate from the niche environment, and inflammation is one of them. Inflammation was documented to be negatively affecting tissue restoration in various systems 7, 8, 9, 10. Therefore, understanding the crosstalk between inflammation and stem cells is important to elucidate the mechanisms of how stem cells respond to tissue damage. Additionally, tweaking the effects of inflammation on stem cell behavior will constitute a possible intervention point for regenerative therapies.
Inflammation was shown to regulate several stem cell niches non-exhaustively including mesenchymal stem cells (MSCs), intestinal stem cells (ISSs), satellite cells or myogenic precursors of the muscle (MPCs), liver progenitor cells (LPCs), epidermal stem cells and neural stem/progenitor cells (NSPCs). In this review, we will mainly focus on NSPCs and their regulation by inflammation.
Neural stem progenitor cells
Neural stem/progenitor cells (NSPC) are multipotent cells that generate the cell types of the nervous system: neurons, glia and oligodendrocytes 11. In vertebrate development, multipotent neuroepithelial cells progressively differentiate into cell types of the nervous system, while sparing undifferentiated cells that maintain glial identity and act as resident stem/progenitor cells of the adult nervous system 12, 13, 14, 15. Stem cell niches in vertebrates show diverse localizations. In adult mammalian brain, neurogenic stem cell niches are restricted to the telencephalon 13, where neural stem cells are found in the subventricular zone (SVZ) of the lateral ventricles and in the subgranular zone of the dentate gyrus in the hippocampus (SGZ) 12, 16. Non-mammalian vertebrates contain a more widespread activity and distribution of stem cells in their brains. Zebrafish, for instance, possesses sixteen different stem cell niches that are distributed along the entire rostro-caudal brain axis 17. As a result, the regulation of the neural stem cell activity in vertebrates is a highly complex interplay between intrinsic and extrinsic cues 18, 19, 20.
Nervous system contains cells of immune system origin, called microglia—the resident macrophages 21, 22. These cells have various functions such as regulating developmental synaptogenesis 23, homeostatic surveillance of the nervous tissue throughout life 24, managing neuronal cell death 25 and eliciting an innate immunity reaction upon various forms of pathogenesis 26. Microglia are main modulators of the inflammatory milieu in the nervous system 25, 27, 28, 29. Homeostatically, microglia are ramified in shape to fulfill its surveillance function. Upon pathology, pathogen invasion or insults, they retract the protrusions, become amoeboid, increase their migratory behavior and secrete molecules to retract peripheral immune cells 30. In mammals, the early phase of the inflammation entails subsequent infiltration of neutrophils, microglia/macrophages and T cells, while the later phase is an extended prevalence of various cell types and consequent resolution of inflammation over long periods of time (reviewed in 31). In regenerating organisms such as zebrafish or newt, initial events of acute inflammation manifest similar to mammals, while prolonged inflammation response is not observed 32, 33. In regenerating organisms, neural stem/progenitor cells are also activated even in inflammation conditions and fulfill regenerative neurogenesis—as a striking difference to mammalian tissues 32, 34, 35, 36. Therefore, the mutual interaction between inflammation and neural stem cells has emerged as an important research area not only because inflammation might exert different effects on stem cells in different species, but also because a majority of neurological disorders or neurodegenerative diseases in humans involve varying levels of inflammation in the neural microenvironment 27, 37. Hypothetically, what we learn from regenerating organisms in terms of the interplay between inflammation and stem cells could help design regenerative therapies.
First studies elaborating on the instructive crosstalk of inflammation on neural stem cells showed that in a mouse model of multiple sclerosis and experimental autoimmune encephalomyelitis (EAE), transplantation of non-inflamed neurospheres ameliorated the demyelination phenotype in various regions of the brain, suggesting that inflammation impairs the neurophysiological properties of neural stem cells and their descendants, such as oligodendrocytes 38, 39, 40. The exact role of inflammation on neural stem/progenitor cells (NSPCs) has been controversial, because both detrimental and beneficial effects have been assigned to inflammation 31, 41, suggesting that the interactions between immune cells and NSPCs are context dependent. Interestingly, a recent study in adult mouse brain showed that neural stem cells can improve the neuronal survival at the host by transforming microglia from a harmful to a neuroprotective phenotype 42.
Negative role of inflammation on neural stem cells
Several studies indicate that inflammation exerts a negative regulation of NSPC proliferation 43, 44, 45 (Fig2). In fetal mouse, maternal inflammation reduces ventricular cell proliferation in developing brain 46. In a medial cerebral arterial occlusion model of mice, chronic immunosuppression was shown to reduce the activation of macrophages, which increased the number of newborn neurons in mouse hippocampus 47. TNFR1 null mice showed significantly elevated levels of cell proliferation in the dentate gyrus and increased number of newborn neurons in the granular zone 44, suggesting that the pro-inflammatory cytokine TNF-α hampers NPSC activity. NSPCs were shown to express the receptors for proinflammatory cytokine IFN-γ, which is secreted predominantly by cytotoxic T cells and reduces the proliferative ability of NSPCs through STAT1 signaling 48, 49. NSPCs of the hippocampus also express the complement receptor 2 (Cr2) that binds to C3d and INF-α. Cr2 null mouse showed increased basal neurogenesis in adult hippocampus 50, suggesting that complement system might suppress neural stem cell activity. In the adult subventricular zone (SVZ) of the mouse brain, anti-inflammatory cytokine IL-10 keeps NSPCs in undifferentiated proliferative state at the expense of neurogenic differentiation 51, an observation that could partially explain why regenerative neurogenesis does not take place after injuries in rodent brains while SVZ cells increase their proliferation as a reaction to damage 48. Leukotrienes were implicated in suppression of NPSC activity in rodents, because blockage of cysteinyl leukotriene receptor 1 (CYSTLR1) using the antagonist montelukast enhanced NSPC proliferation in cultured rat neurospheres 52, suggesting that lipid modifiers of inflammation negatively regulate NSPCs, possibly due to post-inflammatory brain damage through upregulation of TNF-α and IL-1β 53. Consistent with this finding, in Parkinson's disease (PD) patients and MPTP-induced PD model in mice, CD8+ and CD4+ T cells were shown to contribute to dopaminergic toxicity through expression of FasL and exacerbate the pathology 54. Inflammation was also suggested to cause NSPC dysfunction and lead to neurodegenerative disorders 55. In an experimental autoimmune encephalomyelitis model, inflammation results in reduced neuroblast generation and alleviated olfactory bulb neurogenesis, a phenotype reminiscent of multiple sclerosis (MS) patients 56. One reason of impaired neurogenesis in such an inflammatory condition was suggested to be the hampered cell cycle progression of NSPC and reduced migratory behavior of neuroblasts 57.
Figure 2.
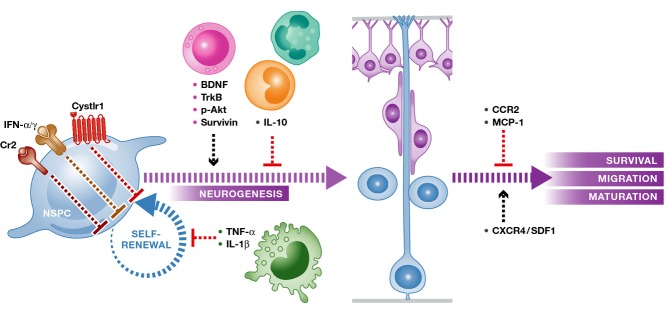
Mammalian neural stem/progenitor cells are generally negatively affected by inflammation
Signaling cascades through Cystlr1, IFN-α and IFN-γ receptors and Cr2, which are expressed by neural stem/progenitor cells (orange, NSPC), as well as macrophage-derived TNF-α and IL-1β, suppress self-renewal. IL-10 secreted by monocytes blocks neuronal differentiation, while CCR2 and MCP-1 hamper neuronal survival, migration and maturation. T cells secrete BDNF to positively regulate neurogenesis from stem cells through its receptor TrkB and intracellular cascades of phosphorylated Akt and survivin. CXCR4/SDF1 chemokine signaling is required for directed migration of neural stem/progenitor cells and neurons.
Positive role of inflammation on neural stem cells
Neural stem/progenitor cells were also shown to be positively affected by inflammatory conditions. In several disease models of mice, grafting efficiency of transplanted NSPCs was shown to be promoted by the inflammation milieu 58, 59. In hippocampal slice cultures, grafted NSPCs migrate to the site of injury upon presentation of cytokines in the tissue and activation of the CCR2 signal cascade 60. In addition, monocyte chemoattractant protein-1 (MCP-1) knockout mice display deficient NSPC migration in vivo and in neurosphere cultures 60, 61. NSPCs require stromal-cell-derived inflammatory chemoattractant SDF1/CXCR4 signaling to migrate to the infracted area of the brain upon lesions or neuro-degenerative conditions 62, 63. NSPCs also increase proliferation upon inflammation. After an immune response upon bacterial enterotoxins, adult mice increase progenitor cell proliferation in the hippocampus 64. In postnatal rats, intrauterine infection using E. coli increases NSPC proliferation at developmental stage P7 by increasing the expression levels of BDNF, TrkB, p-Akt and survivin 65. In vitro studies also suggest that inflammatory signals such as TNF-α or IL-1β could trigger proliferation of NSPCs through NFκB and JNK signaling pathways, respectively 61, 62. Interestingly, NPSCs were also shown to exert immunomodulatory effects in a way to promote NSPC activity. In a chemically induced demyelination assay in rats, transplanted NSPCs inhibited the proliferation and activation of T lymphocytes through peripheral immuno-suppression, which resulted in attenuated experimental autoimmune encephalomyelitis 66. In a mouse model of chronic CNS inflammation, systemically injected NSPCs start expressing antigens of immune cells such as α4 subunit of integrin and various chemokine receptors. These proteins were shown to be required for proliferation and long-term persistence of those stem cells in vivo through induction of selective apoptosis of CNS-infiltrating pro-inflammatory Th1 but not anti-inflammatory Th2 cells 65. This effect is mediated through inhibiting IL-2-mediated phosphorylation of JAK3 in Th1 lymphocytes 44, suggesting that NSPCs might hijack molecular programs of immune cells to positively favor their own proliferation and survival. In a mouse model of EAE, chronic inflammation was suggested to impose a fate switch in spinal cord-derived neural progenitor cells as they transit from being gliogenic to neurogenic 67. Several studies also showed that inflammatory cells exert a protective effect on the neural stem cell function through helping the resolution of the acute inflammation in an orchestrated manner 68, 69. Thus, taken together, documented detrimental and beneficial effects of inflammation clearly demonstrate a context- and time-dependent contribution of inflammatory response to stem cell activity (Table1). The effect of inflammation on NSPCs is binary as it can either support or inhibit proliferation, survival or differentiation depending on the onset of the inflammation, the cell types involved in the process and the chronicity of the response 58, 70. Therefore, studies aiming to determine the correct time of intervention to inflammatory environment will provide an important insight for designing therapeutic clinical strategies which could be customized to individual stem cell niches.
Table 1.
An overview of the effects of inflammatory cues on various stem cell niches
| Stem cell niche | Model | Cytokine/chemokine signaling involved | Effect on stem cell proliferation, migration or engraftment | References |
|---|---|---|---|---|
| Neural stem/progenitor cell | Rodent | TNF-α | − | 44, 114, 115 |
| IFN-γ | − | 48, 49 | ||
| C3d, IFN-α | − | 50 | ||
| LTC4 | − | 52 | ||
| IL-1β | − | 53 | ||
| IL-2 | − | 44, 65 | ||
| CCR2 | + | 60, 61 | ||
| SDF1 | + | 63, 138 | ||
| IL-10 | + | 51 | ||
| Zebrafish | LTC4 | + | 32 | |
| SDF1 | + | 71 | ||
| Cxcr5 | + | 72 | ||
| Satellite cells | Rodent | CX3CR1, IL-10, CCL2 | + | 86 |
| TNF-α | − | 112, 113 | ||
| Pancreatic β-cell | Rodent | IL-1β, IL-6, TNF-α, CXCL8 | − | 88, 139 |
| TGF-β | + | 93 | ||
| Intestinal stem cells | Rodent | IL-6, epimorphin | + | 94 |
| IL-17 | + | 95, 98 | ||
| Liver progenitor cell | Rodent | IL-22 | + | 102 |
| C3, TNF-α, IL-6 | + | 103, 104, 120, 121 | ||
| IFN-γ | + | 105 | ||
| Hair stem cell | Rodent | TNF-α, MCP-1 | − | 106, 107 |
Inflammation in zebrafish nervous system
In zebrafish, several studies showed that chemokine signaling is required for activity of NSPCs at different locations of the nervous system 71, 72, 73, 74, suggesting that an immune-neural crosstalk similar to that of mammals might exist in non-mammalian vertebrates (Fig3). In adult zebrafish brain, acute inflammation through leukotriene C4 (LTC4) binding to its receptor Cystlr1 is sufficient and necessary for activating NSPCs and priming them for regenerative neurogenesis 32. LTC4 seems not to be required for homeostatic NSPC function, but it is necessary for injury-activated proliferation response of the radial glial cells 32 that are the neurogenic progenitors in the adult zebrafish brain 75, 76. Upon cerebroventricular microinjection into the brain fluid 77, 78, LTC4 is also sufficient to induce a regeneration-specific molecular program of zebrafish telencephalon that includes the injury-induced activity of the zinc-finger transcription factor gata3 35. gata3 is not expressed in the homeostatic NSPCs of the adult zebrafish telencephalon but is induced after injury and is required for regeneration of lost neurons as gata3 knockdown inhibits the reactive proliferation response of the NSPCs 35. In case of sterile inflammation using pathogenic cell wall extract or LTC4 itself, gata3 expression can be induced 32. Furthermore, partial immunosuppression using dexamethasone significantly reduces the reactivity of NSPCs to injury and suppresses the induction of gata3 expression in NSPCs 32, demonstrating that acute inflammation is positively affecting the NSPCs in zebrafish brain and is involved in activating molecular programs that enable efficient tissue regeneration, which is poorly manifested in mammals.
Figure 3.
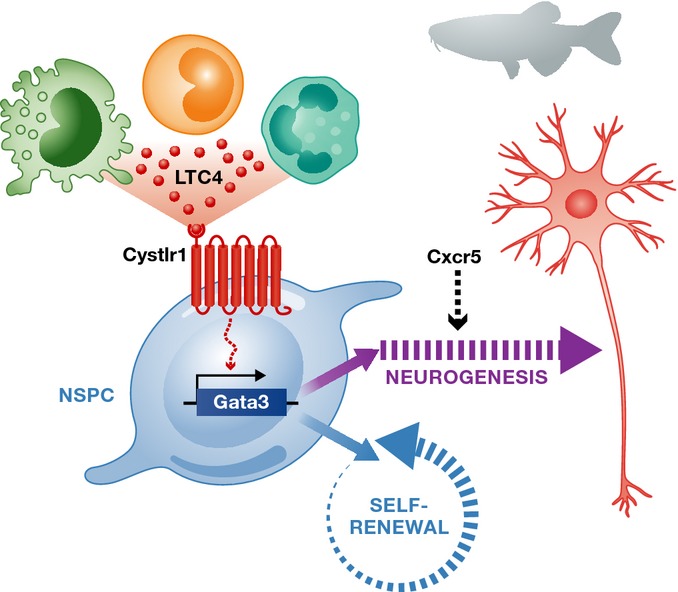
Inflammation promotes neurogenesis and regeneration in zebrafish
In zebrafish, inflammation elicited by immune cells leads to secretion of leukotriene C4 (LTC4, green circles), which bind to its receptor Cystlr1 on neural stem/progenitor cell (NSPC). LTC4/Cystlr1 signaling leads to transcriptional activation of zinc-finger transcription factor Gata3, which is a key molecule that promotes proliferation and regenerative neurogenesis. Chemokine signaling through Cxcr5 is also required for differentiation of proliferating NSPCs into neurons.
Effects of inflammation on stem cells outside the nervous system
The binary role of inflammation in neural stem/progenitor cells also holds true for other stem cell niches. In this section, we will give brief information on the documented effects of inflammation in other stem cell niches outside the nervous system.
Mesenchymal stem cells are multipotent stromal cells that are found in a variety of tissues such as bone marrow, adipose, umbilical cord and muscle. They can differentiate into bone, cartilage and fat cells. In vitro studies showed that MSCs react to the inflammatory milieu. IL-1β-conditioned macrophage medium converts adipose-derived MSCs to smooth muscle cells through a prostaglandin F(2a)-mediated mechanism 79. In an experimental allergic encephalomyelitis model of CD70-transgenic mice, endogenous MSCs were shown to be mobilized dependent on IFN-γ 8. In another study using a mouse model of liver fibrosis, the regenerative role of MSCs was revoked upon immunosuppression with the steroid dexamethasone 80. MSCs also prevent allorecognition and impede macrophage function to modulate different immune phenotypes and cell fate decisions 81, indicating that inflammation orchestrates patterning of the local tissue and its restoration. One example to this phenomenon is seen in satellite cells, which are the resident stem cells of the adult skeletal muscle and give rise to myofibers. Monocytes/macrophages play an intricate role in regulating proliferation and differentiation capacities of satellite cells in muscle tissue 9, 82. Macrophages that are co-injected with myoblasts into injured skeletal muscle led to a significantly improved survival as well as expansion and migration to the dystrophic muscle 83. Myogenic precursor cells crosstalk with monocytes/macrophages to recruit them to the site of injury in order to elicit a chemotactic response, which in turn favors muscle growth and fiber reconstitution 84, 85. This communication is established through chemokine and growth factor signaling such as CX3CR1, IL-10, CCL2/MCP-1 and IGF-1, suggesting that satellite cell behavior is licensed by inflammatory cues secreted by monocytes/macrophages 86.
The role of the inflammatory milieu in generating a micro-patterning environment can also be observed in other organs, such as the pancreas. Elevated release of several pro-inflammatory chemokines and cytokines including IL-1β, IL-6, TNF-α and CXCL8 from the macrophages accumulated around the adipocytes results in activation of stress-induced kinases IKKβ and JNK 87, 88 and initiates a signaling cascade involving NFκB and NLRP3 in β-cells 5, 89. This signaling, in turn, activates FAS ligand on the surface of β-cells, which consequently undergo apoptosis 90, suggesting a negative effect of inflammation in pancreas. Pathogen-related inflammation through TLR4/TLR9 signaling has also been shown to induce β-cell death and insulin resistance through induction of pro-inflammatory cytokines by M1 macrophages 91. Several clinical studies aiming to block pro-inflammatory signaling have been designed to treat T2D 92, suggesting that inflammation has a profound effect on the progression of metabolic diseases. Interestingly, a recent study showed that TGF-β signaling is required for baseline β-cell proliferation by promoting the overall mass of β-cells 93, suggesting that homeostatic and pathological inflammatory state in pancreas might have different effects on progenitor cell proliferation and tissue homeostasis.
Inflammation has been described to play a crucial role in proliferation of intestinal progenitors. Upon damage, IL-6 is secreted by the intestinal myofibroblasts and dendritic cells leading to inflammation and increased proliferation of the crypt cells through epimorphin signaling 94. IL-6 mediates STAT3 activation through SOCS3, which is required for bringing back the injury-induced crypt hyperproliferation to constitutive levels 10. The proliferation of crypt epithelium is also regulated by the cytokine IL-17 secreted by T-helper lymphocytes in the intestine 95. Similar to what leukocytes and other monocytes do, paneth cells, the niche-organizing secretory epithelial cells located at the apex of the crypts 96, secrete proteolytic enzymes and cytokines such as alpha-defensin, NOD2, and TNF-α into the lumen 7, suggesting that modulation of local inflammatory milieu in the intestines is important for stem cell function. In a recent study, it was shown that after intestinal tissue damage, the soluble IL-22 receptor (IL-22BP) was downregulated and thereafter its ligand IL-22 remained in excess in the surrounding environment. For the repair of the intestine, this is a crucial step, but in uncontrolled conditions, during the recovery phase, excess of IL-22 promoted tumor development 97. In another study, by using a mouse model of colorectal tumorigenesis, it was shown that IL-23 signaling promotes tumor growth and initiates an IL-17-dependent neoplastic response 98. A recent study identified STAT5 as a factor mitigating the effects of inflammation, namely reduced LGR5-positive intestinal epithelial stem cell proliferation and their regenerative capacity 99, suggesting context-dependent effects of inflammation on intestinal stem cells.
A well-known example of the supportive effect of inflammation in stem cell proliferation is seen in liver, which contains resident stem cells that generate a transient niche of precursor cells called liver progenitor cells (LPC) 100. Macrophages and inflammatory response are potent determinants of liver progenitor cell (LPC) expansion 101. Recent findings suggest that IL-22 produced by inflammatory cells promotes LPC proliferation via STAT3 102. TNF-α secreted from resident macrophages upon liver damage or pathology is required for the proliferation of liver progenitor cells through binding to TNFR1 103. Pro-inflammatory complement system signaling is also required for LPC proliferation. C3-null mice show impaired liver regeneration due to attenuated production of TNF-α and IL-6, which induces NFκB/STAT3-dependent priming of the hepatocytes 104. A second wave of inflammatory signaling is initiated by cytotoxic T cells that secrete IFN-γ, which induces LPC proliferation 105. Thus, the inflammatory milieu in liver is necessary for stem cell activity during growth and regeneration.
Finally, a clear negative effect of inflammation is seen in hair follicle stem cells. Inflammation has been shown to negatively regulate epidermal stem cell activity in keratin-15/CD34-positive hair follicle cells 106. In a mouse model of permanent hair loss, immunoprivileged hair follicle sac shows elevated levels of inflammation with increased TNF-α and MCP-1 upon macrophage and dendritic cell invasion, whereas immunosuppression rescues the normal physiology of the stem cells 107. Several chronic inflammatory states compromise the function of the hair stem cell niche through modulation of interferon-inducible cytokine expression 108, suggesting that inflammation negatively affects epidermal stem cell activity.
Collectively, inflammation entails a plethora of factors acting in concert in a highly context-dependent manner to regulate the behavior of various stem cell compartments. One practical conclusion emanating from these studies is that besides alleviating negative effects in a given tissue, systemic modulation of inflammation will certainly have other—possibly unwanted—collateral effects in other tissues and stem cells. Given that most age-related diseases manifest concomitantly, clinical therapies aiming at certain diseases should consider either local modulation of immune response or should take into account the effects in other stem cell niches. A more thorough understanding of spatiotemporal dynamics of individual inflammatory cells and stem cell compartments is likely to improve clinical practice as well as efforts toward regenerative therapies.
Inflammation as a regulator of stem cell activity underlying the regenerative capacity
Regeneration is a mechanism that restores lost cells or tissues analogous to the original forms of the precedent structures 109. The capacity of regeneration differs widely among animals. In phylogeny, regeneration capacity tends to decrease or become more restricted 110. Inflammation is generally believed to be a by-product of damage or pathology, and it has been considered to impair tissue regeneration in vertebrates. For instance, TNF-α was shown to impair repair in liver 111, muscle 112, 113 and nervous system 114, 115. Presence of inflammation was suggested to counteract the regenerative capacity in vertebrates 116, 117, 118. Thus, regenerative therapies generally aimed to block inflammation for tissue restoration to take place. However, there is a growing body of evidence for positive regulation of regenerative capacity by inflammation in different tissues due to beneficial effects of the early inflammatory response. Hydrogen peroxide as a stable reactive oxygen species (ROS) is necessary for mounting a regeneration program via recruitment of leukocytes to the injured site in zebrafish 119. TNF-α, IL-6 and complement system are required for regeneration of murine liver by the activation of liver progenitor cells 103, 120, 121. Anti-inflammatory macrophages and their cytokine secretome promote muscle precursor cell proliferation and myogenesis 113 in part by CCR2 and IGF-1 signaling 122, 123. During zebrafish fin regeneration, ablation of macrophages impairs regenerative outgrowth through reduced resolution of inflammation 36, 124. After optic nerve crush, sterile inflammation enhances axonal regeneration in mice 125, 126. T-cell activity can protect nervous system from secondary damage after axotomy 127. Acute inflammation in adult zebrafish brain through LTC4/Cystlr1 signaling initiates reactive proliferation of NSPCs and activates injury-induced molecular programs including gata3, which enables regenerative neurogenesis 32, 35.
Inflammation has also been associated with various disease conditions, such as metabolic disorders, progressive neurodegeneration and cancer 128. In all these conditions, rampant or elevated inflammation plays a role in either exacerbating the pathophysiology or eliciting the onset of the phenotypes. Regenerative therapies to circumvent the disease state largely aim to either supply cells externally or activate the endogenous stem cells of the patient to replenish the lost cell types. In both cases, the inflammatory milieu and its crosstalk to stem cells are important parameters to consider. Several studies addressed the interaction between inflammation and disease states in different contexts.
Specifically in the nervous system, non-resolving inflammation is associated with nervous system pathologies 129, 130. Macrophages—the resident immune cells of the CNS—switch to production of pro-inflammatory cytokines and chemokines (e.g. TNF-α, IL-1β and TGF-β) following pathogen invasion or damage 55. This acute response impinges on neuronal viability by elevating the reactive oxygen species and subsequently causing apoptosis in neurons. When the acute inflammation is resolved, anti-apoptotic factors start to be expressed leading to increased neuronal survival 55. In neurodegenerative disorders such as Alzheimer's disease (AD), Parkinson's disease (PD) or amyotrophic lateral sclerosis (ALS), formation of non-physiological protein aggregates exacerbates the inflammation, which in turn leads to reduced NSPC activity despite neuronal death 55. In AD, amyloid plaques recruit macrophages 131 and lead to elevated levels of pro-inflammatory cytokines and astrogliogenesis 132, which may lead to neuronal death 133. In PD, microglia engulf extracellular α-synuclein aggregates and induce production of neurotoxic reactive oxygen species (ROS) 134, which potentiates the recruitment of CD4+ T cells and expression of pro-apoptotic FasL in neurons 54. Motor neuron degeneration in ALS also involves a feed-forward loop of inflammation through pro-inflammatory cytokines TNF-α and IL-1β, recruitment of M1 macrophages and CD4+ and CD8+ T-cell accumulation, nitric oxide (NO) synthesis, expression of FasL and cell death 135, 136. Several therapeutic applications for counteracting neurodegeneration are devised to reduce inflammation 130.
Conclusion and outlook
The role of inflammation on stem cells and tissue regeneration is multi-faceted. The general belief that early pro-inflammatory signaling is detrimental while anti-inflammatory signaling is beneficial for stem cell activity has been challenged by findings of positive consequences of pro-inflammatory cytokines and negative effects of anti-inflammatory signaling for tissue recovery. In zebrafish brain, upon deployment of immune cells into the tissue, leukotriene signaling—a part of the acute inflammatory response—mounts a special crosstalk to the stem cells urging them to generate more neurons even in the absence of damage 31, 32 by initiating a special regeneration program that does not prevail during homeostasis 35, 74, 76. Leukotriene signaling is an evolutionarily conserved mechanism that is also present in mammals 137. This raises the question whether the crosstalk between immune system and stem cells in the organisms that have regenerative ability could be used to learn how mammalian immune system and inflammation should be tweaked to provide the stem cells a permissive environment and coax them into re-forming the lost cells. Such an approach would have profound ramifications in treatment of chronic diseases that involve progressive degenerative conditions. By performing an in silico comparison of the epistatic targets of inflammatory pathways (e.g. leukotriene signaling and gata3) and their interaction partners in high-throughput expression datasets in mammalian and zebrafish brain, several candidate genes and pathways that could constitute the difference between the regenerative capacities of mammals and zebrafish might be identified 74. In conclusion, as we dwell more on the effects of inflammation on stem cells in various model organisms and disease models, possible spatiotemporal micromanipulation of the inflammatory milieu may emerge as a means of reactivating or unlocking the regeneration potential of mammalian tissues and herald new possibilities for regenerative therapies.
Sidebar A: In need of answers.
What are the inflammatory factors affecting the stem cell activity?
What is the underlying reason why inflammation affects stem cell behavior differently in different tissues?
What are the molecular signaling cascades inflammation initiates in stem cells?
What is the relationship of inflammation with regeneration?
Can regeneration be activated in mammals via immunomodulation?
What are the points of intervention to tweak the inflammation and increase the beneficial outcomes?
Can we learn from regenerating organisms how inflammation enables tissue restoration?
Can inflammation be harnessed for regenerative therapies?
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (SFB-655-A3), The European Union (Zf-Health), Technische Universität Dresden (CK, NK, MB) and German Centre for Neurodegenerative Diseases (DZNE) within Helmholtz Association through Young Investigators Group (CK). We would like to thank Eylem Delikanli for proofreading an earlier version of this manuscript.
Glossary
- CCR2
C-C chemokine receptor type-2
- CNS
central nervous system
- Cr
complement receptor
- Cystlr1
cysteinyl leukotriene receptor 1
- EAE
experimental autoimmune encephalomyelitis
- FasL
Fas ligand; tumor necrosis factor superfamily member 6
- Gata3
GATA family zinc-finger transcription factor 3
- IFN-γ
interferon gamma
- IGF-1
insulin growth factor-1
- IKKβ
nuclear factor NFκB inhibitor kinase beta
- IL
interleukin
- JAK
janus kinase
- JNK
c-Jun N-terminal kinase
- LTC4
leukotriene C4
- MCP-1
monocyte chemoattractant protein-1
- MSC
mesenchymal stem cell
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NSPC
neural stem/progenitor cell
- SOCS3
suppressor of cytokine signaling 3
- STAT3
signal transducer and activator of transcription 3
- TGF-β
transforming growth factor beta
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor alpha
Author contributions
CK, NK and MB wrote the manuscript. CK and MB revised the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abbas AK, Lichtmann AH, Pillai S. Cellular and Molecular Immuno-logy. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Liddiard K, Rosas M, Davies LC, Jones SA, Taylor PR. Macrophage heterogeneity and acute inflammation. Eur J Immunol. 2011;41:2503–2508. doi: 10.1002/eji.201141743. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- Keshav S. Paneth cells: leukocyte-like mediators of innate immunity in the intestine. J Leukoc Biol. 2006;80:500–508. doi: 10.1189/jlb.1005556. [DOI] [PubMed] [Google Scholar]
- Koning JJ, Kooij G, de Vries HE, Nolte MA, Mebius RE. Mesenchymal stem cells are mobilized from the bone marrow during inflammation. Front Immunol. 2013;4:49. doi: 10.3389/fimmu.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- Grandel H, Brand M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev Genes Evol. 2013;223:131–147. doi: 10.1007/s00427-012-0425-5. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto A, Lu H, Miranda AS, Ransohoff RM. Ontogeny and functions of central nervous system macrophages. J Immunol. 2014;193:2615–2621. doi: 10.4049/jimmunol.1400716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Cuadros MA, Martin-Oliva D, Navascues J. Microglia and neuronal cell death. Neuron Glia Biol. 2011;7:25–40. doi: 10.1017/S1740925X12000014. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P, Zhang J, Zhao F, Aschner M, Chen J, Luo W. The interaction between microglia and neural stem/precursor cells. Brain Res Bull. 2014;109:32–38. doi: 10.1016/j.brainresbull.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Kettenmann H. Neuroscience: the brain's garbage men. Nature. 2007;446:987–989. doi: 10.1038/nature05713. [DOI] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Brand M. Neuroinflammation and central nervous system regeneration in vertebrates. Trends Cell Biol. 2014;24:128–135. doi: 10.1016/j.tcb.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- Kirkham M, Hameed LS, Berg DA, Wang H, Simon A. Progenitor cell dynamics in the Newt Telencephalon during homeostasis and neuronal regeneration. Stem Cell Rep. 2014;2:507–519. doi: 10.1016/j.stemcr.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Kizil C, Kyritsis N, Dudczig S, Kroehne V, Freudenreich D, Kaslin J, Brand M. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev Cell. 2012;23:1230–1237. doi: 10.1016/j.devcel.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Petrie TA, Strand NS, Tsung-Yang C, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. 2014;141:2581–2591. doi: 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41:73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry VH, Popovich PG, Weaver LC. Debate: “is increasing neuroinflammation beneficial for neural repair?”. J Neuroimmune Pharmacol. 2006;1:195–211. doi: 10.1007/s11481-006-9021-7. [DOI] [PubMed] [Google Scholar]
- Wu HM, Zhang LF, Ding PS, Liu YJ, Wu X, Zhou JN. Microglial activation mediates host neuronal survival induced by neural stem cells. J Cell Mol Med. 2014;18:1300–1312. doi: 10.1111/jcmm.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Stolp HB, Turnquist C, Dziegielewska KM, Saunders NR, Anthony DC, Molnar Z. Reduced ventricular proliferation in the foetal cortex following maternal inflammation in the mouse. Brain. 2011;134:3236–3248. doi: 10.1093/brain/awr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24:623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Makela J, Koivuniemi R, Korhonen L, Lindholm D. Interferon-gamma produced by microglia and the neuropeptide PACAP have opposite effects on the viability of neural progenitor cells. PLoS ONE. 2010;5:e11091. doi: 10.1371/journal.pone.0011091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Fukuhara T, Britschgi M, He Y, Narasimhan R, Villeda S, Molina H, Huber BT, Holers M, Wyss-Coray T. Complement receptor 2 is expressed in neural progenitor cells and regulates adult hippocampal neurogenesis. J Neurosci. 2011;31:3981–3989. doi: 10.1523/JNEUROSCI.3617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Asensio FJ, Perpina U, Planas AM, Pozas E. Interleukin-10 regulates progenitor differentiation and modulates neurogenesis on adult brain. J Cell Sci. 2013;126:4208–4219. doi: 10.1242/jcs.127803. [DOI] [PubMed] [Google Scholar]
- Huber C, Marschallinger J, Tempfer H, Furtner T, Couillard-Despres S, Bauer HC, Rivera FJ, Aigner L. Inhibition of leukotriene receptors boosts neural progenitor proliferation. Cell Physiol Biochem. 2011;28:793–804. doi: 10.1159/000335793. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepavcevic V, Lazarini F, Alfaro-Cervello C, Kerninon C, Yoshikawa K, Garcia-Verdugo JM, Lledo PM, Nait-Oumesmar B, Baron-Van Evercooren A. Inflammation-induced subventricular zone dysfunction leads to olfactory deficits in a targeted mouse model of multiple sclerosis. J Clin Invest. 2011;121:4722–4734. doi: 10.1172/JCI59145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, Porcheri C, Brambilla E, Cavasinni F, Bergamaschi A, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131:2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu P, Battista D, Depino A, Roca V, Graciarena M, Pitossi F. The more you have, the less you get: the functional role of inflammation on neuronal differentiation of endogenous and transplanted neural stem cells in the adult brain. J Neurochem. 2009;112:1368–1385. doi: 10.1111/j.1471-4159.2009.06548.x. [DOI] [PubMed] [Google Scholar]
- Park DH, Eve DJ, Musso J, III, Klasko SK, Cruz E, Borlongan CV, Sanberg PR. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009;18:693–702. doi: 10.1089/scd.2009.0008. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fu S, Wang Y, Yu P, Hu J, Gu W, Xu XM, Lu P. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Wengner A, Lipp M, Kammertoens T, Kempermann G. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. FASEB J. 2009;23:3121–3128. doi: 10.1096/fj.08-113944. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Covacu R, Perez Estrada C, Arvidsson L, Svensson M, Brundin L. Change of fate commitment in adult neural progenitor cells subjected to chronic inflammation. J Neurosci. 2014;34:11571–11582. doi: 10.1523/JNEUROSCI.0231-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diotel N, Vaillant C, Gueguen MM, Mironov S, Anglade I, Servili A, Pellegrini E, Kah O. Cxcr4 and Cxcl12 expression in radial glial cells of the brain of adult zebrafish. J Comp Neurol. 2010;518:4855–4876. doi: 10.1002/cne.22492. [DOI] [PubMed] [Google Scholar]
- Kizil C, Dudczig S, Kyritsis N, Machate A, Blaesche J, Kroehne V, Brand M. The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 2012;7:27. doi: 10.1186/1749-8104-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka N, Knaut H, Yoshihara Y. Cxcl12/Cxcr4 chemokine signaling is required for placode assembly and sensory axon pathfinding in the zebrafish olfactory system. Development. 2007;134:2459–2468. doi: 10.1242/dev.001958. [DOI] [PubMed] [Google Scholar]
- Cosacak MI, Papadimitriou C, Kizil C. Regeneration, plasticity and induced molecular programs in adult zebrafish brain. BioMed Res Int. 2014 doi: 10.1155/2015/769763. Article ID 769763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72:429–461. doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- Kizil C, Brand M. Cerebroventricular microinjection (CVMI) into adult zebrafish brain is an efficient misexpression method for forebrain ventricular cells. PLoS ONE. 2011;6:e27395. doi: 10.1371/journal.pone.0027395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C, Iltzsche A, Kaslin J, Brand M. Micromanipulation of gene expression in the adult zebrafish brain using cerebroventricular microinjection of morpholino oligonucleotides. J Vis Exp. 2013;75:e50415. doi: 10.3791/50415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Kim MY, Heo SC, Kwon YW, Kim YM, Do EK, Park JH, Lee JS, Han J, Kim JH. Macrophages regulate smooth muscle differentiation of mesenchymal stem cells via a prostaglandin F(2)alpha-mediated paracrine mechanism. Arterioscler Thromb Vasc Biol. 2012;32:2733–2740. doi: 10.1161/ATVBAHA.112.300230. [DOI] [PubMed] [Google Scholar]
- Chen X, Gan Y, Li W, Su J, Zhang Y, Huang Y, Roberts AI, Han Y, Li J, Wang Y, et al. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis. 2014;5:e1009. doi: 10.1038/cddis.2013.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518–524. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharraz Y, Guerra J, Mann CJ, Serrano AL, Munoz-Canoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013;2013:491497. doi: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesault PF, Theret M, Magnan M, Cuvellier S, Niu Y, Gherardi RK, Tremblay JP, Hittinger L, Chazaud B. Macrophages improve survival, proliferation and migration of engrafted myogenic precursor cells into MDX skeletal muscle. PLoS ONE. 2012;7:e46698. doi: 10.1371/journal.pone.0046698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Martinez CO, Ochoa O, Ruiz-Willhite L, Bonilla JR, Centonze VE, Waite LL, Michalek JE, McManus LM, Shireman PK. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 2009;23:382–395. doi: 10.1096/fj.07-095901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi L, Manfredi AA, Rovere-Querini P. Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Front Immunol. 2011;2:62. doi: 10.3389/fimmu.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Schumann DM, Maedler K, Franklin I, Konrad D, Størling J, Böni-Schnetzler M, Gjinovci A, Kurrer MO, Gauthier BR, Bosco D, et al. The Fas pathway is involved in pancreatic beta cell secretory function. Proc Natl Acad Sci USA. 2007;104:2861–2866. doi: 10.1073/pnas.0611487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell Metab. 2013;17:883–894. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Xiao X, Wiersch J, El-Gohary Y, Guo P, Prasadan K, Paredes J, Welsh C, Shiota C, Gittes GK. TGFbeta receptor signaling is essential for inflammation-induced but not beta-cell workload-induced beta-cell proliferation. Diabetes. 2013;62:1217–1226. doi: 10.2337/db12-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Invest. 2010;120:2081–2093. doi: 10.1172/JCI40676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther. 2007;114:94–106. doi: 10.1016/j.pharmthera.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W, Jr, Murphy AJ, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S, Nivarthi H, Mayhew CN, Lo YH, Noah TK, Vallance J, Rülicke T, Müller M, Jegga AG, Tang W, et al. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Rep. 2015;4:209–225. doi: 10.1016/j.stemcr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Tirnitz-Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, Knight B. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52:291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, Gershwin ME, Gao B. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143:188–198. doi: 10.1053/j.gastro.2012.03.044. .e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, Wetsel RA, Erdei A, Lambris JD. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunol. 2004;173:747–754. doi: 10.4049/jimmunol.173.2.747. [DOI] [PubMed] [Google Scholar]
- Brooling JT, Campbell JS, Mitchell C, Yeoh GC, Fausto N. Differential regulation of rodent hepatocyte and oval cell proliferation by interferon gamma. Hepatology. 2005;41:906–915. doi: 10.1002/hep.20645. [DOI] [PubMed] [Google Scholar]
- Doles J, Storer M, Cozzuto L, Roma G, Keyes WM. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012;26:2144–2153. doi: 10.1101/gad.192294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jiang X, Gu P, Chen W, Zeng X, Gao X. Gsdma3 mutation causes bulge stem cell depletion and alopecia mediated by skin inflammation. Am J Pathol. 2012;180:763–774. doi: 10.1016/j.ajpath.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Goss RJ. The Natural History (and mystery) of Regeneration. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- Erlandsson A, Lin CH, Yu F, Morshead CM. Immunosuppression promotes endogenous neural stem and progenitor cell migration and tissue regeneration after ischemic injury. Exp Neurol. 2010;230:48–57. doi: 10.1016/j.expneurol.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development. 2009;136:2323–2327. doi: 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: an immunologic perspective. Dev Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel WA, Simeonova PP. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005;19:413–415. doi: 10.1096/fj.04-2421fje. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Li L, Yan B, Shi YQ, Zhang WQ, Wen ZL. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J Biol Chem. 2012;287:25353–25360. doi: 10.1074/jbc.M112.349126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci USA. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol. 2005;174:589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]


