Abstract
Catestatin is a peptide which is a potent inhibitor of catecholamine secretion and played essential functions in the cardiovascular system. Previous research found that dramatic changes of catestatin were associated with hemodynamics in acute myocardial infarction (AMI) during the first week after the AMI symptoms onset, but whether catestatin is also involved in the pathophysiological progression after AMI and then a predictor for outcomes is not clear. The aim of this study is to determine the correlation of plasma catestatin levels at different time points and the prognosis of AMI. 100 participants recruited were all patients with AMI, all of who received successful primary percutaneous coronary intervention (PCI) within 12h from the AMI symptom onset in our center; the concentrations of plasma catestatin were evaluated from blood samples of those 100 participants. Subsequent 65 months' follow-up was performed after discharging to evaluate cardiac adverse events and the association between catestatin levels and prognosis of AMI was examined. We confirmed the dramatic change of catestatin concentrations in the first week of AMI, and the levels of catestatin on D3 were much higher in adverse events group than those in non-adverse events group (p<0.0001), but the ratio of D7/D3 was significantly lower. In addition, the Kaplan-Meier analysis showed that the groups in which the levels on D3 were higher (p<0.0001) and the ratios of D7/D3 were lower (p<0.0001), patients trended to be more susceptive to adverse events after AMI. Furthermore, according to the analysis, we surmised catestatin level on D3 as an appropriate predictor for outcomes in patients with AMI with good specificity as well as sensitivity. All of the evidence confirmed that catestatin plays an important role in the progress of AMI, and may act as a promising target for prognostic prediction.
Introduction
Myocardial infarction is a major cause of morbidity and mortality worldwide. More than 3 million people each year are estimated to have an acute ST-elevation myocardial infarction (STEMI), with more than 4 million having a non-ST-elevation myocardial infarction (NSTEMI) [1]. Although deaths due to acute AMI have decreased rapidly in these days because of timely revascularization and multiple drug treatments, the adverse events such as heart failure and malignant arrhythmia that occur after AMI are still major causes of the poor long-term prognosis of patients with AMI [2,3]. Therefore, evaluation of adverse events is essential in predicting the prognosis of patients with AMI and methods to evaluate these processes are of great clinical importance.
AMI is associated with a complex pattern of neurohumoral activation in which catecholamines play an important role in both onset and course of AMI [4,5]. Catestatin is a 21-amino acid residue, cationic and hydrophobic peptide which is generated endogenously by proteolytic cleavage of its precursor chromogranin A (CHGA) [6], a protein found in secretory granules of chromaffin cells, postganglionic sympathetic neurons [7] and heart cells itself [8], where it was co-stored and co-released with catecholamines. Catestatin can inhibit catecholamine release from chromaffin cells and noradrenergic neurons [9,10] and induce desensitization of catecholamine release induced by nicotine [11]. In recent years, catestatin has been found to exert effects on cardiovascular system, for example, it displayed potent vasodilatory effect in rate which was mediated, at least in part, by augmented release of histamine [12], the possible mechanism of which involves inhibitory G proteins [13]. Another research in humans also proved that increasing concentrations of catestatin resulted in dose dependent vasodilation [14]. Meng et al. recently demonstrated that the plasma catestatin levels after the onset of AMI might be associated with left ventricular remolding (LVR) [15], those with LVR had a significantly higher level of plasma catestatin on admission, on day 3 and day 7. Furthermore, our study before proved its predictive effect to detect stage B failure [16]. Taken together, these data point to catestatin as a novel regulator of blood pressure and cardiac function.
On the basis of these cardiovascular effects of catestatin, we keep to ask whether catestatin exerts cardioprotective influence under Ischemia/Reperfusion (I/R) condition. Cardioprotection includes endothelial and adrenergic components [17], and these were proved to be affected by catestatin via anti-endothelian-1/pro-nitric oxide and anti-adrenergic actions, respectively [18,19]. Following this hypothesis, researchers used Langendorff reperfused rat heart and isolated cardiomyocytes and demonstrated the cardioprotective role for catestatin, which appears mainly due to a direct reduction of post-ischemic myocardial damages and dysfunction, rather than to an involvement of adrenergic terminals and/or endothelium [20], other researches also found that catestatin reduced myocardial I/R injury involving PI3K/AKT, PKCs, mitochodrial KATP channels and ROS signaling [21], and it can also increase the expression of anti-apoptotic and pro-angiogenetic factors in the post-ischemic hypertrophied heart of spontaneously hypertensive rats [22]. Due to the multiple and important functions of catestatin in the cardiac system, it provides a rationale for the hypothesis that catestatin might be a good marker or predictive factor in the pathophysiological process of AMI, especially for the outcomes. Since the role of catestatin in this aspect is yet to be addressed, in this study, we aimed to evaluate the plasma catestatin levels in patients with AMI within the first week, and determine the association between plasma catestatin levels and the adverse outcomes after AMI in our 65 months' follow-up study.
Materials and Methods
Study population
This study was approved by the ethics review boards of Peking University Health Science Center. Written informed consent was obtained from the study population. A total 117 consecutive patients with the first AMI admitted to the Department of Cardiology, Peking University Third Hospital, from June 2008 to April 2009 was included, and 17 of them were lost to follow-up, so we just analyzed the other 100 patients in the rest of the study. All patients received successful PCI within 12h from the AMI symptom onset. The STEMI was diagnosed according to the American College of Cardiology/American Heart Association guideline in 2004 [23]. The study excluded patients with chronic obstructive pulmonary disease, significant kidney or hepatic diseases, tumor, and infectious disease. During the same study period, all 30 subjects who were admitted to the same hospital because of atypical chest pain but with normal coronary arteries confirmed by coronary angiography were included as controls. Resting blood pressure and heart rates (HRs) were measured at the same time when blood samplings were performed in triplicate in the supine position, using an oscillometric device (Philips IntelliVue MP20, Germany).
Blood sampling
At the acute phase of AMI, blood samples were obtained from an antecubital vein without stasis in all patients immediately after admission to the emergency room (ER), and in the morning of the third (D3) and seventh day (D7) after AMI (n = 100). In the control subjects, blood samples were obtained from the antecubital vein in the morning of the same day when angiography was performed (n = 30). The blood samples, anticoagulated with ethylenediaminetetraacetic acid (EDTA), were immediately centrifuged at 3000rpm for 10min at 4°C. An aliquot of the EDTA plasma was stored at −80°C till analysis. Repeated freeze-thaw cycles were avoided.
Assays
Plasma levels of catestatin were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instruction (Cat. # EK-053-29, ELISA kit, Phoenix Pharmaceutical Inc., Burlingame, CA). The minimal detection limits for catestatin was 0.06ng/ml. These assays were performed by an investigator blinded to the sources of the samples.
Follow-up measurements and end points
The cardiac adverse events were measured at follow-up, within 65 months after study participation. 17 patients were lost to follow-up. The end points including death from cardiovascular causes, readmission with ACS or admission with CHF were evaluated within 65 months (54.98±19.20 months). Information on end points was collected from telephone interviews with patients or relatives of patients, hospital databases and patient case notes. The definition of readmission with ACS is: (1) STEMI, ST elevation>1 mm in two limb leads or>2 mm in leads V1–V6 or new left bundle branch block; (2) NSTEMI, no ST elevation on ECG despite elevated troponin T>0.01μg/ml; and (3) unstable angina, ischemic chest pain lasting more than 30 min with no evidence of myocyte necrosis or ST elevation. CHF was defined as hospitalization for a clinical syndrome involving at least two of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary crackles, third heart sound, and cardiomegaly or pulmonary edema on chest X-ray. These clinical signs and symptoms must have represented a clear change from the normal clinical status, requiring intravenous diuretics, inotropic support or vasodilator therapy.
Statistics
All results are expressed as the mean ± SD. Student's unpaired t-tests were used to compare the catestatin levels between control subjects and patients with AMI on ER, D3 and D7, as well as the differences between adverse events group and non-adverse events group. A chi-square test was used to determine differences between groups. On multivariable Cox proportional hazard analyses, the main models were adjusted for age, gender, and variables considered might reflect the severity of AMI at baseline. Multivariable Cox proportional hazard were performed as stepwise regressions with backward elimination. Kaplan-Meier analysis was performed on the non-adverse events occurrence rates stratified into 2 groups based on the median value, and the differences between the curves were analyzed by long-rank test. The sensitivity and specificity of D3, D7 and D7/D3 for predicting adverse events were determined, and receiver operating characteristics (ROC) curves were constructed. Spearman or Pearson correlation was used to identify the bivariate correlations. A value of p<0.05 was considered significant.
Results
Baseline clinical characteristics
The clinical characteristics and laboratory findings of patients with AMI and control subjects are summarized in Table 1. Totally, 100 patients with AMI (age, 62.1±14.0years; sex, 84.0% male) and 30 control subjects (age, 60.0±10.4years; sex, 76.6% male) were included. Risk factor profiles and most baseline clinical parameters were compared between the two groups: the heart rate, Hs-CRP and fasting glucose of AMI patients were significantly higher than those of control subjects, but other parameters stayed the same such as age, gender, blood pressure etc.
Table 1. Patient characteristics and laboratory findings at baseline.
| AMI | Controls | ||
|---|---|---|---|
| Variables | n = 100 | n = 30 | p value |
| Age (years) | 62.1±14.0 | 60.0±10.4 | 0.41 |
| Male (%) | 84.0 | 76.6 | 0.41 |
| Body mass index (kg/m2) | 25.5±3.4 | 27.0±5.7 | 0.16 |
| Systolic blood pressure (mmHg) | 134.3±17.9 | 130±13.5 | 0.21 |
| Diastolic blood pressure (mmHg) | 77.1±13.1 | 75.0±7.4 | 0.41 |
| Heart rates (beats/min) | 82.3±11.8 | 70.4±8.7 | <0.0001 |
| Diabetes mellitus (%) | 22.0 | 23.3 | 1.00 |
| Hypertension (%) | 62.0 | 70.0 | 0.52 |
| Hypercholesterolemia (%) | 56.0 | 50.0 | 0.68 |
| Current smoking (%) | 56.0 | 36.7 | 0.09 |
| Beta-blocker treatment before (%) | 6.0 | 10.0 | 0.43 |
| Hs-CRP (mg/l) | 19.94±24.83 | 1.68±2.05 | <0.0001 |
| Creatinine (μmol/l) | 97.0±17.5 | 101.0±23.3 | 0.26 |
| Fasting glucose (mmol/l) | 6.5±2.3 | 5.0±1.7 | 0.002 |
| Total cholesterol (mmol/l) | 4.83±1.18 | 4.73±1.08 | 0.70 |
| LDL cholesterol (mmol/l) | 3.12±0.87 | 3.05±1.03 | 0.74 |
Values represent mean ± SD or the percent of the patients and control subjects.
Hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
Time course of the plasma catestatin levels
Compared with the control group (21.8±6.3ng/ml, n = 30), plasma catestatin concentrations were significantly decreased on ER (p<0.0001). Among the three time points (ER, D3 and D7), the highest concentrations of catestatin in patients with AMI were found on D3 (30.9±12.1ng/ml), which were higher than those obtained on ER (16.7±5.4ng/ml, p<0.0001), whereas the concentrations remained low at 1 week (13.9±5.2ng/ml, p<0.0001), which were also lower than the values on ER (p = 0.0003) (Fig 1).
Fig 1. Plasma catestatin levels in controls and patients during the first week after AMI.
Box plots showing the distribution of concentrations of plasma catestatin at different time points (n = 100). Boxes specify inter-quartile ranges; the horizontal bars inside the boxes indicate medians; the upper and lower whiskers get to the most distant value within the 95% of the distribution. The p-values for the comparison of the groups are also indicated. Control (21.8±6.3ng/ml); ER: emergency room (16.7±5.4ng/ml); D3: the third day after AMI (30.9±12.1ng/ml); D7: the seventh day after AMI (13.9±5.2ng/ml).
Patient Characteristics according to the occurrence of adverse events
Patients were stratified into two groups (the adverse events group and the non-adverse events group) according to whether patients recruited showed up cardiac adverse events during our study. Table 2 summarizes and compares patient characteristics from these two groups. During the follow-up of 65 months (54.98±19.20 months), 26 patients had adverse events. The levels of catestatin on ER and D3 were significantly higher in adverse events group (19.4±6.7ng/ml, 44.6±13.0ng/ml, respectively) than those in non-adverse events group (15.8±4.5ng/ml, p = 0.003, 26.1±7.5ng/ml, p<0.0001, respectively), but the ratio of D7/D3 was much lower in adverse events group (0.31±0.13) than that in non-adverse events group (0.59±0.25, p<0.0001) (Fig 2), because the p values of D3 and D7/D3 were lower than that of ER, it’s reasonable to hypothesize that the differences of them are more closed to the real, so we chose these two parameters to conduct the subsequent analysis. For other factors that might have influence on the outcome of AMI, the differences of body mass index, LDL cholesterol, percentage of diabetes mellitus and current smokers are significant (Table 2).
Table 2. Patient characteristics according to occurrence of adverse events.
| Variables | Non-adverse events group (n = 74) | Adverse events group (n = 26) | p value |
|---|---|---|---|
| Age (years) | 61.8±14.8 | 63.0±11.8 | 0.703 |
| Gender (M/F) | 62/12 | 22/4 | 1.000 |
| Body mass index (kg/m2) | 24.9±2.2 | 26.9±3.7 | 0.012 |
| HRs | 83±13 | 82±9 | 0.788 |
| Systolic blood pressure (mmHg) | 133.0±18.4 | 138.2±16.0 | 0.202 |
| Diastolic blood pressure (mmHg) | 75.7±13.5 | 81.0±11.3 | 0.079 |
| Creatinine (μmol/l) | 97.7±18.9 | 95.0±12.9 | 0.504 |
| Fasting glucose (mmol/l) | 6.6±2.4 | 6.4±2.1 | 0.713 |
| LDL cholesterol (mmol/l) | 3.01±0.82 | 3.43±0.92 | 0.029 |
| LVEDD (cm) | 49.4±4.3 | 49.0±6.2 | 0.700 |
| LVEF (%) | 53.3±10.2 | 50.5±9.8 | 0.244 |
| Lesion Region-LAD, n (%) | 24 (32.4) | 4 (15.4) | 0.096 |
| Lesion Region-LCX n (%) | 24 (32.4) | 10 (38.5) | 0.577 |
| Lesion Region-RCA n (%) | 26 (35.2) | 12 (46.1) | 0.319 |
| Diabetes mellitus, n (%) | 22 (29.7) | 0 (0) | 0.040 |
| Hypertension, n (%) | 42 (56.8) | 20 (76.9) | 0.068 |
| Hypercholesterolemia, n (%) | 44 (59.5) | 12 (46.2) | 0.240 |
| Current Smoking, n (%) | 46 (62.2) | 10 (38.5) | 0.036 |
| Beta-blocker treatment, n (%) | 72 (97.3) | 26 (100) | 0.974 |
| ACEI/ARB treatment, n (%) | 56 (75.7) | 22 (84.6) | 0.344 |
| Aspirin treatment, n (%) | 68 (91.9) | 25 (96.2) | 0.464 |
| Plavix treatment, n (%) | 65 (87.8) | 23 (88.5) | 0.933 |
| Catestatin-ER (ng/ml) | 15.8±4.5 | 19.4±6.7 | 0.003 |
| Catestatin-D3 (ng/ml) | 26.1±7.5 | 44.6±13.0 | <0.0001 |
| Catestatin-D7 (ng/ml) | 14.4±5.8 | 12.6±2.6 | 0.135 |
| Catestatin-D7/D3 | 0.59±0.25 | 0.31±0.13 | <0.0001 |
Values represent mean ± SD or the percent of the patients.
LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery
Fig 2. Comparison of the catestatin levels between adverse events group and non-adverse events group.
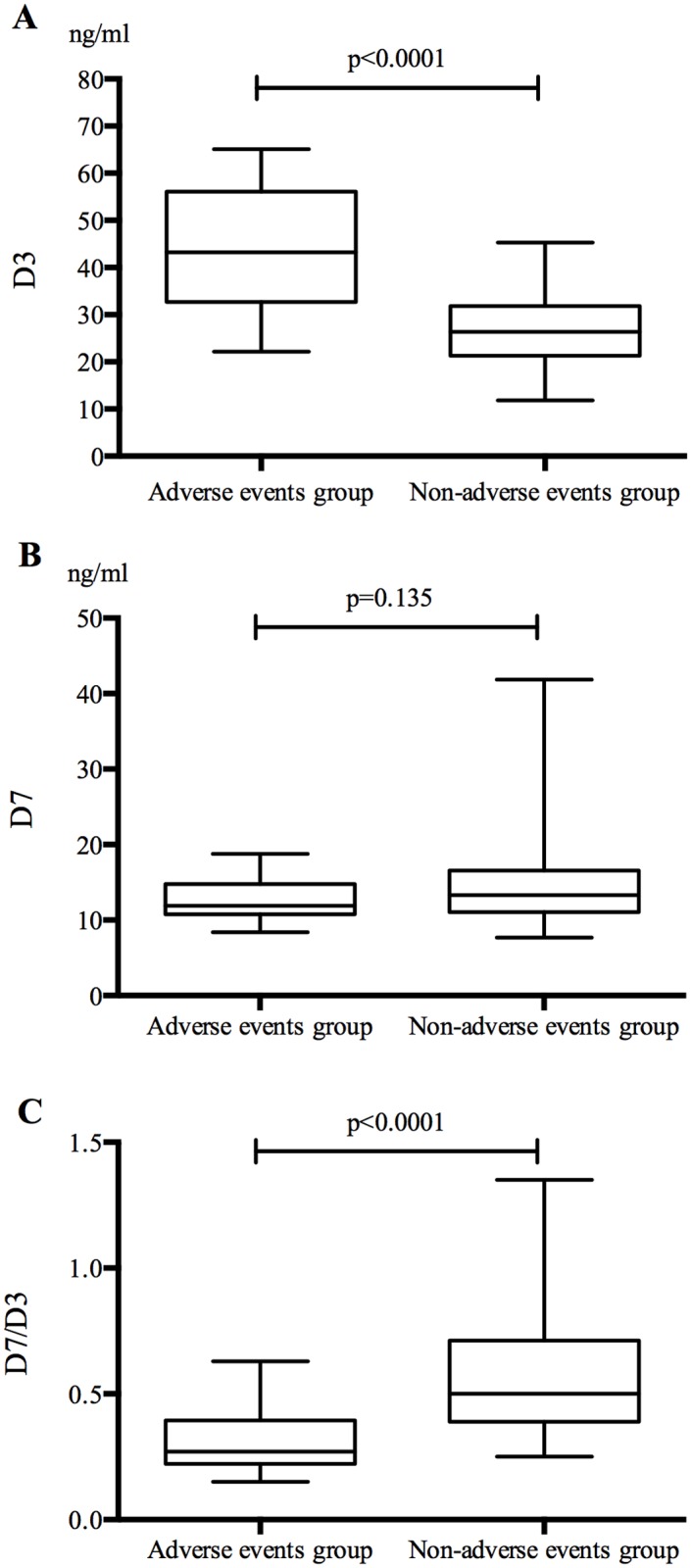
Box plots showing the distribution of concentrations of plasma catestatin in two groups (n1 = 26, n2 = 74) on ER (A), D3 (B) and D7 (C). Boxes specify inter-quartile ranges; the horizontal bars inside the boxes indicate medians; the upper and lower whiskers get to the most distant value within the 95% of the distribution. The p-values for the comparison of the groups are also indicated.
Difference of outcomes according to the value of D3, D7 and D7/D3 in patients with AMI
Patients were divided into 2 groups based on the median values of catestatin on D3 (29.2ng/ml), D7 (12.835ng/ml) and D7/D3 (0.46), event-free survival rates were measured using long-rank test, as shown with Kaplan-Meier survival curves, we found that the occurrence rate of adverse events was higher (p<0.0001) in the group in which the values of D3 were higher (>29.2ng/ml), but in the group in which the ratios of D7/D3 were lower (<0.46), but again, we didn’t see significant difference when analyzing values on D7 (Fig 3).
Fig 3. Kaplan-Meier survival curves.
According to the median value of catestatin levels on D3 (A), D7 (B) and the ratio of D7/D3 (C), patients were separated into two groups respectively to analyze the event-free survival rate.
Univariate and Multivariable Predictors of adverse events: Comparison of catestatin concentrations on D3, D7 and D7/D3 and other possible parameters
Eight clinical variables were analyzed using univariate and stepwise multivariable Cox proportional hazard regression analyses (Table 3). On stepwise multivariable analyses, only the value of logD3 was significantly independent predictor (p<0.0001). ROC curves of values on D3, D7 and D7/D3 demonstrating adverse events risks after AMI are shown in Fig 4. The area under curve of D3 is 0.888 (AUC = 0.888), the cut off level for D3 was determined as 32.95ng/ml, giving a sensitivity of 76.9% and specificity of 82.4%; the area under curve of D7 is 0.601 (AUC = 0.601), the cut off level for D7 was determined as 12.43ng/ml, giving a sensitivity of 61.54% and specificity of 60.81%; and the area under curve of D7/D3 is 0.860 (AUC = 0.860), the cut off level for D7/D3 was determined as 0.39, giving a sensitivity of 76.9% and specificity of 74.3%.
Table 3. Univariate and Multivariate Predictors of adverse events: comparison of catestatin concentrations on D3 and D7/D3 and other possible parameters.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Chi-square | p value | Chi-square | p value | |
| Age (years) | 0.092 | 0.761 | ||
| Gender (male = 1) | 0.051 | 0.882 | ||
| LVEDD (cm) | 0.045 | 0.702 | ||
| LVEF (%) | 1.460 | 0.227 | ||
| Creatinine (μmol/l) | 0.486 | 0.486 | ||
| logD3 | 52.852 | <0.0001 | 52.852 | <0.0001 |
| logD7 | 2.404 | 0.121 | ||
| log(D7/D3) | 26.814 | <0.0001 | ||
Fig 4. Receiver operating characteristic curves.
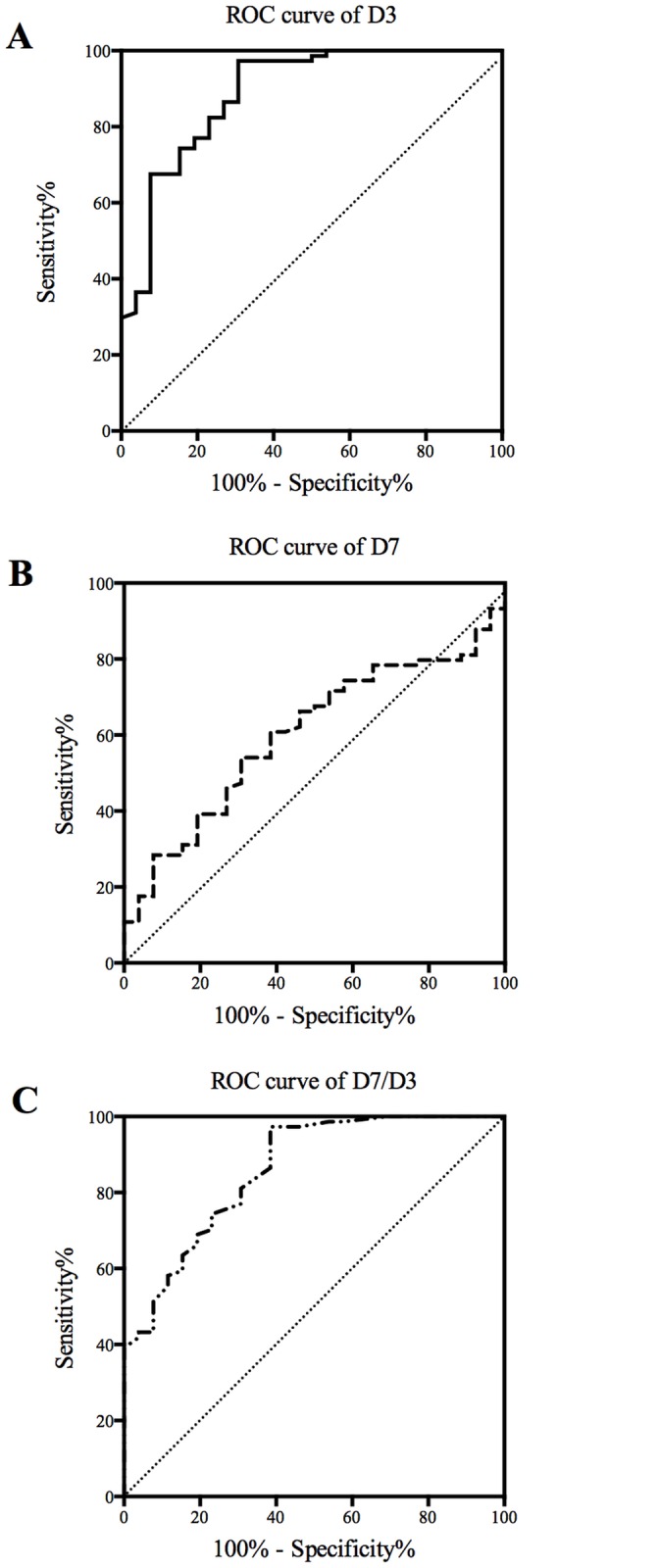
The ability of catestatin values on D3 (A), D7 (B) and D7/D3 (C) to predict adverse events in patients with AMI was evaluated and their specificity as well as sensitivity were determined. The diagonal was shown in each diagram.
Discussion
Activation of the sympathetic nervous system, as assessed by circulating concentrations of catecholamines, is associated with reduced survival in patients with chronic heart failure [24]; greatly elevated plasma catecholamine levels in the acute and subacute phase after AMI also signify a poor prognosis [25], however, measurement of circulating catecholamines is rarely used for risk stratification, probably because of a cumbersome sampling procedure that involves insertion of an intravenous cannula and a period of rest in the supine position before blood collection [26]. Thus, a simple and reliable index that is less prone to rapid fluctuations in circulating levels, and that has similar or better prognostic value as catecholamines, would be useful.
The current study demonstrated the changes of plasma catestatin levels at certain time points during the first week in patients with AMI who underwent successful PCI within 12 hours from onset: catestatin levels decreased on admission, increased significantly on D3, but remained decreased on D7. In the 65 months' follow-up study, we found that circulating levels of catestatin in the first week after onset of AMI were associated with long-term adverse cardiac events. And it is also an independent prognostic indicator with high sensitivity and specificity. Since catestatin can be measured in serum or plasma collected with standard equipment, stored for prolonged period, and analyzed without tedious extraction procedures, developing the clinical use of catestatin might be of great importance.
Among many peptides derived from CHGA, catestatin was the only one responsible for inhibition of catecholamine release caused by nicotinic stimulation [9]. In recent years, CHGA has been found to be a sensitive marker of prognosis of morbidity and mortality in acute coronary syndromes (ACS), the mechanism for the predictive effects could probably contribute to the downstream product catestatin [27]. It is well established that catecholamine activation in patients with AMI, as measured by plasma adrenaline and noradrenaline, showed an early and rapid increase in the first 3 days and was restricted to the first 5–7 days [28], so the time points for blood sampling in our study were chosen according to the changes of catecholamines, which play an important role in a vicious circle that increases myocardial irritability and damage in AMI. In our study, it is speculated that the decreased catestatin levels observed at admission may be the response to the increased catecholamines levels, which might inhibit the release of catestatin; we also found the catestatin levels on D3 were the highest, which gave us the clue that the increased sympathoadrenal activities might increase catestatin release to promote negative feedback; moreover, when the catecholamines returned to normal levels within 5–7 days, the corresponding decrease of catestatin on D7 might further indicate the compensation mechanism of catecholaminergic activation, which might reduce the harmful effect of catecholamine. And dramatic change in patients with AMI indicates its possible involvement in the progress of AMI.
Initially identified as the most potent endogenous antagonist of nicotinic-cholinergic receptor, catestatin exerted antagonistic inhibition of nicotine-evoked catecholamines secretion in a non-competitive way [9], and it has been proved to be a multifunctional peptide with different mechanisms in the last decade, for example, its vasoactive and relevant anti-hypertensive properties have been extensively reviewed by a lot of researchers (e.g. [29]). In this study, the results indicated that the occurrence rate of cardiac adverse events in patients with AMI increased significantly as the catestatin levels elevated on D3, also, patients with higher catestatin levels seemed to have higher probability to develop adverse events. Here we surmised the increase of catestatin was the compensatory response of the sympathetic nerve activity, which release catecholamine and do harm to the myocardium, so the catestatin levels during the acute phase of AMI could reflect the degree of sympathetic nervous system activation, which gives its role to be a good prognostic factor of adverse events. Furthermore, there should be a balance between catecholamines and catestatin, but it might be insufficiently compensatory at a higher level (acute phase of AMI here), and this is still not enough to compensate the damage to the body caused by sympathetic and catecholamine toxicity.
Though most evidences showed catestatin is related with cardiovascular diseases, whether it is a protective factor and the underlying mechanisms are still not well elucidated. Previous research indicated that in isolated murine heart, catestatin (75nM) could significantly reduce the infarction size after ischemia and improve the post-ischemia left ventricular systolic and diastolic function [20]. But opposite to the effect mentioned above, some evidences showed both wild type (WT) and Pro370Leu (P370L) variant catestatin (100nM) increased infarction size, through inhibiting phosphorylation of Akt and its downstream targets, BAD and FoxO1, increasing the cell death [30]. The inconformity of those studies might be a result of different animal models, variants of catestatin and different concentration of catestatin. Since most damage happened during reperfusion after ischemia, this period is crucial for prognosis. In our study, we failed to reveal obvious relationship between catestatin and peak CK-MB or LVEF (S1 File), whether there is a correlation between catestatin and infarct size of heart remains to be elucidated in a relatively larger sample size, and more researches about the function of catestatin are needed to provide more clues about the role of catestatin in AMI and post-ischemia reperfusion.
There were still some notable limitations in this study. First, only 100 AMI patients had been recruited for the follow-up research, which is a relatively small group; second, this is also only one center study; third, there are some papers [31,32] which demonstrated the relationship between lipid mobilization and catestatin, but in our study, the mean value of BMI of participants was high and we also lost some of the BMI data, which made it confused to analyze; At last, a careful echocardiographic analysis at longer time might provide more useful information about the prognostic value of catestatin.
In conclusion, plasma catestatin levels changed dramatically during the first week in patients with AMI, suggesting an important role of catestatin in the progress of AMI. In addition, patients who had adverse events after AMI showed higher catestatin levels on D3, and when separating into 2 groups according to the median value on D3, the group with higher levels had more adverse events, at last, the multivariable Cox proportional hazard analyse also suggested that the level on D3 was an independent predictor, all of which determined the predictive role of catestatin level on D3 in AMI, but the particular underlying mechanism should also be studied further.
Supporting Information
The correlation between catestatin levels on D3 and peak CK-MB as well as LVEF were determined using Spearman or Pearson correlation analyses, the p values were 0.999, 0.128, respectively.
(TIFF)
(XLS)
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 81200167) and the Ph.D. Programs Foundation of the Ministry of Education of China (No. 20120001120131), and the International cooperation projects of the Ministry of Science and Technology (No. 2013DFG32940). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. White HD, Chew DP. Acute myocardial infarction. The Lancet. 2008; 372: 570–584. 10.1016/S0140-6736(08)61237-4 [DOI] [PubMed] [Google Scholar]
- 2. Husebye T, Eritsland J, Müller C, Sandvik L, Arnesen H, Seljeflot I, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. European journal of heart failure. 2013; 15: 565–572. 10.1093/eurjhf/hfs215 [DOI] [PubMed] [Google Scholar]
- 3. Jons C, Jacobsen UG, Joergensen RM, Olsen NT, Dixen U, Johannessen A, et al. The incidence and prognostic significance of new-onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: a CARISMA substudy. Heart Rhythm. 2011; 8: 342–348. 10.1016/j.hrthm.2010.09.090 [DOI] [PubMed] [Google Scholar]
- 4. Sigurdsson A, Held P, Swedberg K. Short-and long-term neurohormonal activation following acute myocardial infarction. American heart journal. 1991; 126: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 5. Foy SG, Crozier IG, Richards AM, Nicholls MG, Turner JG, Frampton CM, et al. Neurohormonal changes after acute myocardial infarction Relationships with haemodynamic indices and effects of ACE inhibition. European heart journal. 1995; 16: 770–778. [DOI] [PubMed] [Google Scholar]
- 6. Taylor CV, Taupenot L, Mahata SK, Mahata M, Wu H, Yasothornsrikul S, et al. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A Determination of proteolytic cleavage sites in hormone storage granules. Journal of Biological Chemistry. 2000; 275: 22905–22915. [DOI] [PubMed] [Google Scholar]
- 7. Helle K, Corti A, Metz-Boutigue M-H, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cellular and Molecular Life Sciences. 2007; 64: 2863–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, et al. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. European Heart Journal. 2007; 28: 1117–1127. [DOI] [PubMed] [Google Scholar]
- 9. Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, et al. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. Journal of Clinical Investigation. 1997; 100: 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahata SK, Mahata M, Wakade AR, O’Connor DT. Primary Structure and Function of the Catecholamine Release Inhibitory Peptide Catestatin (Chromogranin A344− 364): Identification of Amino Acid Residues Crucial for Activity. Molecular Endocrinology. 2000; 14: 1525–1535. [DOI] [PubMed] [Google Scholar]
- 11. Mahata SK, Mahata M, Parmer RJ, O’Connor DT. Desensitization of Catecholamine Release THE NOVEL CATECHOLAMINE RELEASE-INHIBITORY PEPTIDE CATESTATIN (CHROMOGRANIN A344–364) ACTS AT THE RECEPTOR TO PREVENT NICOTINIC CHOLINERGIC TOLERANCE. Journal of Biological Chemistry. 1999; 274: 2920–2928. [DOI] [PubMed] [Google Scholar]
- 12. Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998; 19: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 13. Krüger P-G, Mahata SK, Helle KB. Catestatin (CgA 344–364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regulatory peptides. 2003; 114: 29–35. [DOI] [PubMed] [Google Scholar]
- 14. Fung MM, Salem RM, Mehtani P, Thomas B, Lu CF, Perez B, et al. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clinical and experimental hypertension. 2010; 32: 278–287. 10.3109/10641960903265246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng L, Wang J, Ding W-h, Han P, Yang Y, Qi LT, et al. Plasma catestatin level in patients with acute myocardial infarction and its correlation with ventricular remodelling. Postgraduate medical journal. 2013; 89: 193–196. 10.1136/postgradmedj-2012-131060 [DOI] [PubMed] [Google Scholar]
- 16. Zhu D, Wang F, Yu H, Mi L, Gao W. Catestatin is useful in detecting patients with stage B heart failure. Biomarkers. 2011; 16: 691–697. 10.3109/1354750X.2011.629058 [DOI] [PubMed] [Google Scholar]
- 17. Heusch G, Boengler K, Schulz R. Cardioprotection nitric oxide, protein kinases, and mitochondria. Circulation. 2008; 118: 1915–1919. 10.1161/CIRCULATIONAHA.108.805242 [DOI] [PubMed] [Google Scholar]
- 18. Herrero CJ, Alés E, Pintado AJ, López MG, García-Palomero E, Mahata SK, et al. Modulatory mechanism of the endogenous peptide catestatin on neuronal nicotinic acetylcholine receptors and exocytosis. The Journal of neuroscience. 2002; 22: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, et al. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008; 149: 4780–4793. 10.1210/en.2008-0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penna C, Alloatti G, Gallo MP, Cerra MC, Levi R, Tullio F, et al. Catestatin improves post-ischemic left ventricular function and decreases ischemia/reperfusion injury in heart. Cellular and molecular neurobiology. 2010; 30: 1171–1179. 10.1007/s10571-010-9598-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perrelli M-G, Tullio F, Angotti C, Cerra MC, Angelone T, Tota B, et al. Catestatin reduces myocardial ischaemia/reperfusion injury: involvement of PI3K/Akt, PKCs, mitochondrial KATP channels and ROS signalling. Pflügers Archiv-European Journal of Physiology. 2013; 465: 1031–1040. [DOI] [PubMed] [Google Scholar]
- 22. Penna C, Pasqua T, Amelio D, Perrelli M-G, Angotti C, Tullio F, et al. Catestatin increases the expression of anti-apoptotic and pro-angiogenetic factors in the post-ischemic hypertrophied heart of SHR. PloS one. 2014. August 6 10.1371/journal.pone.0102536 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Journal of the American College of Cardiology. 2004; 44: 671–719. [DOI] [PubMed] [Google Scholar]
- 24. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. New England journal of medicine. 1984; 311: 819–823. [DOI] [PubMed] [Google Scholar]
- 25. Jewitt DE, Reid D, Thomas M, Mercer C, Valori C, Shillingford JP, et al. Free noradrenaline and adrenaline excretion in relation to the development of cardiac arrhythmias and heart-failure in patients with acute myocardial infarction. The Lancet. 1969; 293: 635–641. [DOI] [PubMed] [Google Scholar]
- 26. Omland T, Dickstein K, Syversen U. Association between plasma chromogranin A concentration and long-term mortality after myocardial infarction. The American journal of medicine. 2003; 114: 25–30. [DOI] [PubMed] [Google Scholar]
- 27. Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, et al. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. European heart journal. 2009; 30:25–32. 10.1093/eurheartj/ehn513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petersen CL, Nielsen JR, Petersen BL, Kjaer A. Catecholaminergic activation in acute myocardial infarction: time course and relation to left ventricular performance. Cardiology. 2003; 100: 23–28. [DOI] [PubMed] [Google Scholar]
- 29. Mahata SK, Mahata M, Fung MM, O'Connor DT. Reprint of: catestatin: a multifunctional peptide from Chromogranin A. Regulatory peptides. 2010; 165: 52–62. 10.1016/j.regpep.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brar BK, Helgeland E, Mahata SK, Zhang K, O'Connor DT, Helle KB, et al. Human catestatin peptides differentially regulate infarct size in the ischemic–reperfused rat heart. Regulatory peptides. 2010; 165: 63–70. 10.1016/j.regpep.2010.07.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ, et al. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. Journal of hypertension. 2002; 20: 1335–1345. [DOI] [PubMed] [Google Scholar]
- 32. Durakoğlugil ME, Ayaz T, Kocaman SA, Kırbaş A, Durakoğlugil T, Erdoğan T, et al. The relationship of plasma catestatin concentrations with metabolic and vascular parameters in untreated hypertensive patients: Influence on high-density lipoprotein cholesterol. Anadolu kardiyoloji dergisi: AKD = the Anatolian journal of cardiology. 2014. July 17 10.5152/akd.2014.5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The correlation between catestatin levels on D3 and peak CK-MB as well as LVEF were determined using Spearman or Pearson correlation analyses, the p values were 0.999, 0.128, respectively.
(TIFF)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.




