Abstract
A novel polyene compound NPP identified in a rare actinomycetes, Pseudonocardia autotrophica KCTC9441, was shown to contain an aglycone identical to nystatin but to harbor a unique di-sugar moiety, mycosaminyl-(α1-4)-N-acetyl-glucosamine, which led to higher solubility and reduced hemolytic activity. Although the nppDI was proved to be responsible for the transfer of first polyene sugar, mycosamine in NPP biosynthesis, the gene responsible for the second sugar extending glycosyltransferase (GT) as well as NPP post-PKS tailoring mechanism remained unknown. Here, we identified a NPP-specific second sugar extending GT gene named nppY, located at the edge of the NPP biosynthetic gene cluster. Targeted nppY gene deletion and its complementation proved that nppY is indeed responsible for the transfer of second sugar, N-acetyl-glucosamine in NPP biosynthesis. Site-directed mutagenesis on nppY also revealed several amino acid residues critical for NppY GT function. Moreover, a combination of deletions and complementations of two GT genes (nppDI and nppY) and one P450 hydroxylase gene (nppL) involved in the NPP post-PKS biosynthesis revealed that NPP aglycone is sequentially modified by the two different GTs encoded by nppDI and nppY, respectively, followed by the nppL-driven regio-specific hydroxylation at the NPP C10 position. These results set the stage for the biotechnological application of sugar diversification for the biosynthesis of novel polyene compounds in actinomycetes.
Introduction
Invasive fungal infections are becoming a serious problem in human health. The mortality rates for fungal infections with three most common species of pathogens are Candida albicans of 20%-40%, Aspergillus fumigatus of 50%-90%, and Cryptococcus neoformans of 20%-70% [1–3]. These diseases usually occur in the setting of immunocompromised patients as a result of HIV infection, anticancer therapy, or immunosuppressive therapy after organ transplantation [1]. Azoles and polyenes have been classified as two major classes of antifungal drugs [4–6]. Although azoles inhibit the fungal cytochrome P450 (CYP)-dependent enzyme involved in ergosterol biosynthesis, the emergence of Candida resistance to azoles has become an increasing concern [7]. Meanwhile, polyene macrolides including nystatin and amphotericin exhibit their antifungal activities through direct binding to fungal membrane ergosterol [8, 9]. Although broad-spectrum fungicidal activity and low incidence of fungal resistance were considered as main benefits of polyenes, high toxicity and low water solubility limit their broad clinical application [10, 11]. In recent years, genetic engineering has become a promising strategy for design of new analogues of polyene with improved pharmacological properties [9, 12–15].
Polyene macrolides are usually biosynthesized by large modular type I polyketide synthases (PKSs), followed by several sequential post-PKS modifications. During post-PKS modification, a unique cytochrome P450 hydroxylase (CYP) regio-specifically transfers a hydroxyl group to a specific carbon position of the polyene backbone, and a polyene glycosyltransferase (GT) catalyzes addition of mycosamine, a deoxyaminosugar derived from GDP-d-mannose. GTs are an important class of enzymes and are essential for the biosynthesis of glycosylated natural products [16–18], and this glycosylation reaction often converts a non-bioactive intermediate into a biologically active compound [19]. Although most polyene compounds contain a single sugar mycosamine attached, a few polyenes naturally contain a disaccharide with an additional sugar residue to mycosamine. 67-121C, a disaccharide-modified aromatic heptaene, has been isolated from Actinoplanes caeruleus [20]. Another example, nystatin P1 containing a disaccharide mycosamine-glucose was also identified in the Pseudonocardia P1 strain collected from Acromyrmex octospinosus garden worker ants [21]. Although the genes for the second sugar extending GTs in 67-121C and nystatin P1 were proposed as pegA and nypY, respectively [20, 21], their functional confirmation and post-PKS mechanisms have been poorly understood.
Previously, a rare actinomycetes called Pseudonocardia autotrophica was determined to contain a cryptic polyene biosynthetic gene cluster through a polyene CYP-specific genome screening strategy [22], and then a novel nystatin-like polyene (NPP) containing a disaccharide, mycosamine (α 1–4)-N-acetyl-2-aminoglucose was identified (Fig 1) [23]. Interestingly, NPP harboring a disaccharide moiety showed significantly higher solubility and lower hemolytic activity than nystatin which contains a single sugar mycosamine only, suggesting that NPP could be a promising candidate for further development into a pharmacokinetically-improved, less-cytotoxic polyene antifungal antibiotic. The first GT involved in NPP biosynthetic pathway, NppDI was confirmed to be homologous to a previously-identified NysDI, which is responsible for the transfer of the first sugar, mycosamine to the C19 position of nystatin in S. noursei ATCC11455. Although the additional sugar, N-acetyl-glucosamine present in NPP is believed to be the major reason for the increased solubility and the reduced toxicity [23], the gene for this NPP-specific second sugar extending GT was not found in the previously-reported biosynthetic gene cluster. In this report, we identified a NPP-specific second sugar extending GT through P. autotrophica genome mining, targeted gene disruption, and complementation. In addition, NPP post-PKS biosynthetic modification steps including regio-specific hydroxylation and two glycosylations were completely elucidated in P. autotrophica.
Fig 1. Chemical structures of nystatin A1 and NPP.
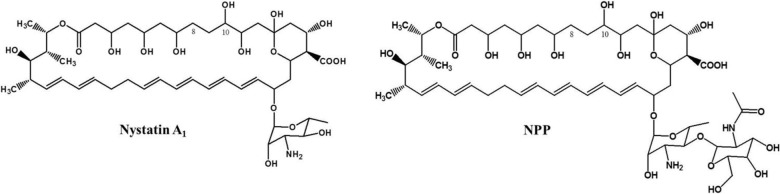
These structurally related compounds share the same aglycone with mono-sugar, mycosamine. NPP include additional sugar moiety, N-acetylglucosamine.
Materials and Methods
Bacterial Cultures
The bacterial strains and plasmids used in the present study are described (Table 1). The rare actinomycetes Pseudonocardia autotrophica KCTC 9441 was purchased from the Korean Collection for Type Cultures. The P. autotrophica strain was maintained on ISP2 agar medium containing (grams per liter) 4g glucose, 4g yeast extract, and 10g malt extract at 28°C for the preparation of spore [22], and was cultured in YEME liquid medium composed of 3g yeast extract, 5g peptone, 3g malt extract, 10g glucose, 340g sucrose, 2mL MgCl2 6H2O (2.5M) in 1L of distilled water for NPP production [23]. Cultivations of S. noursei strains were performed as described previously [24]. All Escherichia coli strains were incubated at 37°C in Luria-Bertani broth or on Luria-Bertani agar supplemented with the appropriate antibiotics when needed [25].
Table 1. Bacterial strains and plasmids used in this study.
| Strain of plasmid | Characteristic a | Source or reference |
|---|---|---|
| Strain | ||
| Escherichia coli | ||
| DH5α | General cloning host | |
| ET12567 / pUZ8002 | Strain for intergeneric conjugation; Kmr, Cmr | [37] |
| Pseudonocardia autotrophica | ||
| KCTC9441 | Wild-type, NPP producing strain | KCTC |
| ESK601 | nppDI mutant producing 10-deoxyaglycone | [23] |
| ESK602 | nppDI-complemented ESK601 | [23] |
| ESK6011 | nppY mutant producing 10-deoxynystatin | This work |
| ESK6012 | nppY-complemented ESK6011 | This work |
| ESK60111 | ESK6011 harboring plasmid pYM1 | This work |
| ESK60112 | ESK6011harboring plasmid pYM2 | This work |
| ESK60113 | ESK6011 harboring plasmid pYM3 | This work |
| ESK60114 | ESK6011 harboring plasmid pYM4 | This work |
| ESK60115 | ESK6011 harboring plasmid pYM5 | This work |
| ESK60116 | ESK6011 harboring plasmid pYM6 | This work |
| ESK60117 | ESK6011 harboring plasmid pYM7 | This work |
| ESK60118 | ESK6011 harboring plasmid pYM8 | This work |
| ESK60119 | ESK6011 harboring plasmid pYM9 | This work |
| ESK6021 | nppL mutant producing C10 deoxy NPP | This work |
| ESK6022 | nppL-complemented ESK6021 | This work |
| ESK611 | nppDI nppY double mutant producing 10-deoxyaglycone | This work |
| ESK612 | nppDI-complemented ESK611 | This work |
| ESK613 | nppY-complemented ESK611 | This work |
| ESK614 | nppDI nppY-double complemented ESK611 | This work |
| ESK621 | nppDI nppL double mutant producing 10-deoxyaglycone | This work |
| ESK622 | nppDI-complemented ESK621 | This work |
| ESK623 | nppL-complemented ESK621 | This work |
| ESK624 | nppDI nppY-double complemented ESK621 | This work |
| ESK631 | nppY nppL double mutant producing 10-deoxynystatin | This work |
| ESK632 | nppY-complemented ESK631 | This work |
| ESK633 | nppL-complemented ESK631 | This work |
| ESK634 | nppY nppL-double complemented ESK631 | This work |
| ESK641 | nppDI nppY nppL triple mutant producing 10-deoxyaglycone | This work |
| ESK642 | nppDI-complemented ESK641 | This work |
| ESK643 | nppDI nppY-complemented ESK641 | This work |
| ESK644 | nppDI nppY nppL-complemented ESK641 | This work |
| Plasmid | ||
| pTY | Template plasmid for nppY mutation, Amr | This work |
| pDELY | Plasmid for deletion nppY gene, Aprr | This work |
| pDELL | Plasmid for deletion nppL gene, Aprr | This work |
| ermE*pSET152 | E. coli-P. autotrophica conjagative vector with constitutive promoter, ermE*, Aprr | [38] |
| pMJPDI | Plasmid for nppDI complementation, Aprr | [23] |
| pPY | Plasmid for nppY complementation, Aprr | This work |
| pPL | Plasmid for nppL complementation, Aprr | This work |
| pPDIY | Plasmid for nppDI nppY complementation, Aprr | This work |
| pPDIL | Plasmid for nppDI nppL complementation, Aprr | This work |
| pPYL | Plasmid for nppY nppL complementation, Aprr | This work |
| pYM1 | pPY with additional 7 amino acids (FARSYTP), Hygr | This work |
| pYM2 | pPY with A37N mutation, Hygr | This work |
| pYM3 | pPY with E197K mutation, Hygr | This work |
| pYM4 | pPY with R200N mutation, Hygr | This work |
| pYM5 | pPY with I201Q mutation, Hygr | This work |
| pYM6 | pPY with R219V mutation, Hygr | This work |
| pYM7 | pPY with R247E mutation, Hygr | This work |
| pYM8 | pPY with I385Y mutation, Hygr | This work |
| pYM9 | pPY combined with C-terminal domain of NppDI, Hygr | This work |
aKmr, kanamycin resistance; Cmr, chloramphenicol resistance; Amr, ampicillin resistance; Aprr, apramycin resistance; Hygr, hygromycin resistance.
Production and Purification of NPP
The large-scale fermentation was performed in YEME medium (3L). NPP producing strain, P. autotrophica spores were inoculated to ISP medium 2 (6 x 50mL) incubated (28°C; 220 rpm) for 72hr. All precultures were added to YEME autoclaved at 121°C for 20 min and 150g Amberlite XAD16 resin was supplied after 48hr cultivation. After 24hr, the mycelia and resin from production media were isolated and extracted two times with 600ml butanol. The organic phase was concentrated using a vacuum evaporator. The raw extract was dissolved in methanol and loaded onto column packed with C18 reversed-phase silica gel (Daiso) with methanol-water (30:70, v/v) to remove sugar originating from production media. The fractions were separated and purified using fraction collector (Interchim, France) with solvent A (water) and solvent B (methanol) following gradient: 30% B (v/v) (0 to 10 min), 100% B (v/v) (100 min) at a flow rate of 15ml/min. The fractions containing NPP with >90% purity were judged by HPLC analysis with detection at 305nm.
HPLC analysis of polyene analogues
Polyene macrolides were extracted with an equal volume of butanol, followed by concentration and dissolving in methanol. HPLC analysis of these extract were carried out using a Shimadzu SPD M10A (Shimadzu, Japan) with a Zorbax SB-C18 column (5 μm particles, 4.6 x150 mm, Agilent). The column was equilibrated with 50% solvent A (0.05 M ammonium acetate, pH 6.5) and 50% solvent B (methanol), followed by development using the following gradient: 50% B (0 min), 75% B (3 min), 100% B (30 min), 50% B (33 min), and 50% B (40 min) at a flow rate of 1.0 ml/min and UV/Vis detection at 305 nm [26]. NPP quantification was performed with HPLC using the authentic Nystatin A1 (Sigma Aldrich) as a standard, of which the method was also described elsewhere [12, 22, 23]
Targeted gene deletion
nppY-deletion mutant, ESK6011 and nppL-deletion mutant, ESK6021 were constructed by double homologous recombination (Fig 2A). To construct both double knock-out mutants (ESK611, ESK621, ESK631) in P. autotrophica, an identical strategy for genetically targeted gene deletion was applied using bi-functional E. coli-Streptomyces and temperature-sensitive plasmid, pKC1139. This plasmid contained two 1.5 kb fragments homologous to upstream and downstream of targeted gene to be deleted. Each of the fragments was individually amplified using the polymerase chain reaction (PCR) (BioRad, USA). All PCR primer sequences used in this work are listed (S1 Table). Amplified fragments were sequenced and ligated into pKC1139 digested with HindIII-EcoRI. The constructed plasmid was introduced into P. autotrophica using conjugation. The spores containing pKC1139 derivative were selected and sub-cultured three times in broth media with apramycin at 37°C. The collected spores were cultivated in broth media without apramycin at 30°C After three repeats of sub-culture, several double cross-over apramycin-sensitive recombinants were identified via PCR amplification of the deleted-junction.
Fig 2. Genetic manipulations for targeted gene disruption and complementation.
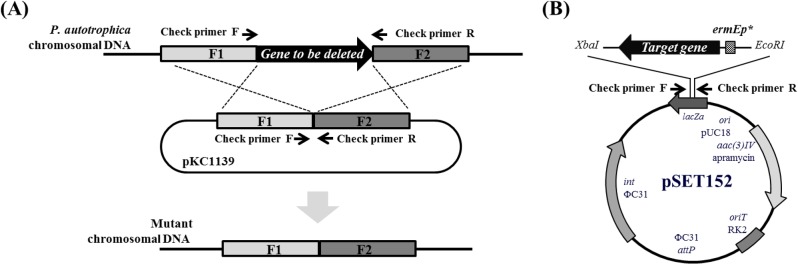
(A) A scheme representation of the in-frame deletion mutant by double homologous recombination. (B) Plasmid map of the Streptomyces integrative vector, pSET152 for expression of target genes under the control of strong constitutive promoter, ermE*.
Functional expression and complementation
For complementation of gene deleted mutant and functional gene expression, the integrative plasmid pSET152 harboring the strong constitutive promoter, ermE* was utilized (Fig 2B). Oligonucleotide sequences and templates applied for amplifying targeted gene using PCR were shown (S1 Table). Amplified genes were cloned into pSET152 using In-Fusion HD Cloning Kit (Clontech, Japan) and were sequenced. The resulting plasmids were introduced into P. autotrophica via intergeneric conjugation and apramycin was used for selection [27].
Mutagenesis of NPP-specific glycosyltransferase (NppY) and cytochrome P450 (NppL)
pTY, in which the nppY gene had been subcloned into T&A cloning vector (RBC) was used as the template for site-directed mutagenesis and construction of the hybrid fusion protein. The site-directed mutations were introduced by using the Q5 Site-Directed Mutagenesis Kit (New England BioLabs Inc.) and hybrid fusion proteins were constructed using In-Fusion HD Cloning Kit (Clontech, Japan). All PCR primer pairs are listed in S1 Table. About 1.5kb fragment emcompassing the modified nppY coding sequences were excised with BamHI/XbaI, and ligated with the integrative plasmid pSET152 containing ermE* promoter. The resulting recombinant plasmids were introduced into the ESK6011 strain. The DNA sequence of nppY was deposited in GenBank under the accession number KP419743.
Results
Identification of a NPP-specific extending GT
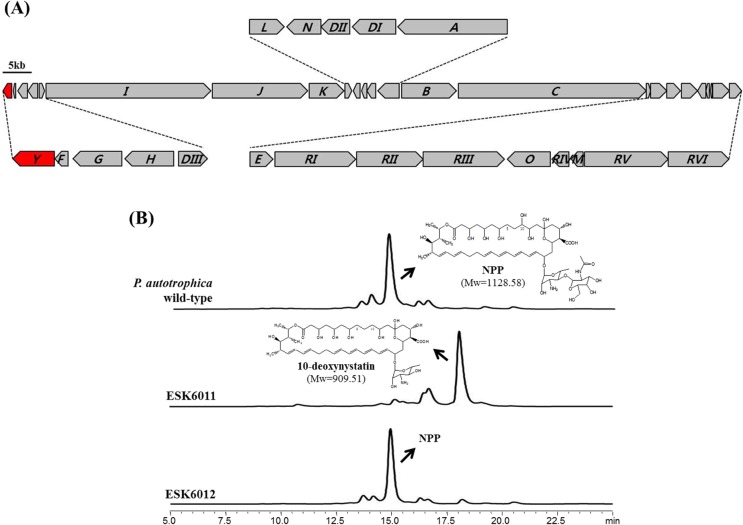
To isolate a NPP-specific extending GT responsible for the second sugar transfer, the P. autotrophica KCTC9441 genome was sequenced by GenoTech, Korea. The draft genome sequence of P. autotrophica comprised 9,977,725 bases, assembled into 1,016 contigs (>500bp), and it had a GC content of 69.9%. Further, there were 96 predicted tRNAs sequences along with 10,581 protein-coding sequences (CDSs) in the genome sequence. Specifically, the coding percentage was 70.5%, and 7,466 CDSs showed functional predictions. Using COG (Clusters of Orthologous Group) functional assignment, the majority of predicted proteins were classified into 25 COG categories. Using in silico analysis of amino acid sequences performed by GenoTech Prokaryotic Genome Automatic Annotation System, a total of 112 putative glycosyltransferase (GT) gene was identified from P. autotrophica whole genome (S2 Table). Among P. autorhophica GT genes, one GT gene located in the next nppF coding phosphopantetheinyl transferase in NPP biosynthesis, was identified (Fig 3A). The translational product of that gene exhibited 42% identity to NppDI which is responsible for catalyzing the transfer of first sugar, mycosamine during NPP biosynthesis (S3 Table). Interestingly, this gene product also displayed 83% amino acid identity to NypY, which adds a hexose to the mycosamine of a nystatin in Pseudonocardia sp. P1 strain [28]. Since the nypY, present in the similar location in the nystatin P1 cluster, was recently proposed to encode a GT responsible for second sugar transfer [29], this gene was accordingly named as nppY encoding a putative NPP-specific extending GT. NppY also showed 51% identity to PegA which is the GT that transfers the second sugar to biosynthesize 67-121C polyene macrolide in Couchioplanes caeruleus.
Fig 3. NPP biosynthetic gene cluster and disruption-complementation of nppY.
(A) Organization of the NPP biosynthetic gene cluster. The nppY gene (red highlight) is located immediately upstream of the nppF gene. NppF, phosphopantetheinyl transferase; NppG and NppH, ABC transporter; NppDIII, GTP-mannose-4,6-dehydratase; NppI, NppJ, NppK, NppA, NppB, and NppC, Type I PKS; NppL and NppN, P450 mono oxygenase; NppDII, aminotransferase; NppDI, glycosyltransferase; NppE, thioesterase; NppRI, NppRII, NppRIII, NppRIV, NppRV, and NppRVI, regulatory enzyme; NppO, decarboxylase; NppM, ferredoxin. (B) HPLC profiles and chemical structures of culture extracts from P. autotrophica wild-type, ESK6011, and ESK6012.
Deletion and Complementation of NPP-specific second sugar extending GT
To verify the function of putative NPP-specific second sugar extending GT, deletion of the nppY was performed using a temperature-sensitive suicide plasmid, pKC1139 (Fig 2A). Construction of the nppY-deleted mutant, ESK6011 was confirmed by PCR analysis (S1A Fig) and HPLC-MS analysis (Fig 3B). Since the additional sugar present in NPP is the only difference between NPP and nystatin, ESK6011 was predicted to produce nystatin with the expected mass of 926.09. Interestingly, however, the polyene compound produced in ESK6011 was a 10-deoxynystatin with the mass of 909.51, indicating that the polyene analogue accumulated in ESK6011 lacked both the second sugar moiety and the C10 hydroxyl group. Through co-injection experiment with the 10-deoxynystatin produced by the nysL inactivation mutant [29], we could confirm that the polyene derivative resulted from the nppY deletion mutant is indeed 10-deoxynystatin (S1B Fig). When nppY gene was functionally expressed in a pSET152 vector under the ermE* promoter in the ESK6011 (named ESK6012), the NPP production was restored (Fig 3B and S1B Fig). Considering the fact that the functional NppL responsible for C10 hydroxylation is still present in the ESK6011, these results suggest that the NPP C10 hydroxylation might be catalyzed after the completion of NppY-driven second glycosylation (see more below).
Molecular Dissection of NPP-specific Extending GT
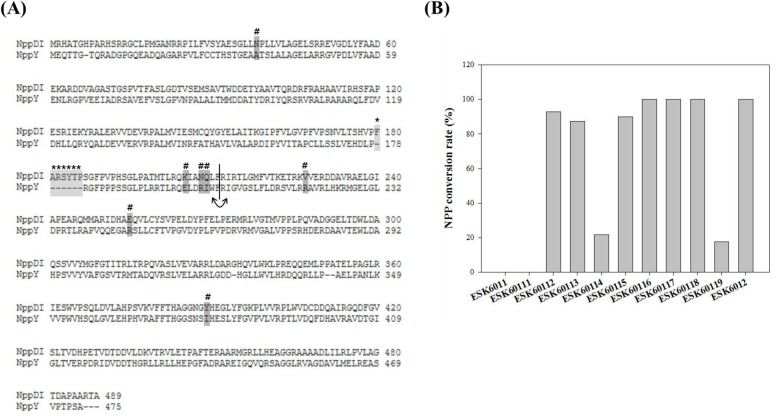
To increase understanding of the NPP-specific second sugar extending GT, further investigation of the key amino acid residues of NppY was pursued. We analyzed the amino acid sequence of NppY in specialized BLAST based on the TDP-vancosaminyltransferase GtfD involved in the final stage of vancomycin biosynthesis (S2 Fig) [30]. Through comparing amino acids with GtfD, we deduced putative active sites, acceptor binding sites, and activated NDP-sugar donor binding sites of the NppY protein. Alignment of the amino acid between NppY and NppDI would also be the clue to find donor interacting domain, because these proteins could dedicate the transfer of different sugar moiety for NPP biosynthesis in P. autotrophica (Fig 4A). To verify which amino acid residues are essential for function of NppY, we substituted some amino acid residues of NppY with those of NppDI at the predicted critical position. In addition, consecutive amino acids (FARSYTP) present only in the NppDI were inserted into the corresponding position in NppY. A fusion protein between NppY and NppDI was also generated. HPLC analyses showed that NPP productions in ESK60116 (R219V), ESK60117 (R247E), and ESK60118 (I385Y) had no significant change compared to the control, which is the ESK6011 complemented with the intact nppY gene (ESK6012). ESK60112 (A37N), ESK60113 (E197K), and ESK60115 (I201Q) led to reductions of the NPP production by approximately 20%. More than 80% decreases of NPP production in ESK60114 (R200N) and ESK60119 (fusion between N-terminal of NppY and C-terminal of NppDI) were observed. Moreover, addition of seven amino acid residues (FARSYTP) present only in NppDI into the corresponding sites of NppY (ESK60111) led to a complete loss of NppY enzyme activity (Fig 4B).
Fig 4. Amino acid sequence alignment and mutagenesis of NppY.
(A) Amino acid sequence alignment between NppDI and NppY glycosyltransferases in NPP biosynthesis. Regions for mutagenesis are marked; crosshatch, site-directed mutagenesis (dark gray); asterisk, addition of 7 consecutive amino acids (FARSYTP) from NppDI (light gray); arrow, N-terminal of NppY and C-terminal of NppDI were combined based on straight line. (B) NPP conversion rate through expression of various modified nppY genes in ESK6011.
Post-PKS Tailoring Steps of NPP
Although three genes including two GT genes (nppDI and nppY) and one CYP gene (nppL) are now proposed to be required in NPP post-PKS biosynthesis, the NPP post-PKS tailoring cascade including the order of these three steps is not clearly understood. Based on the results of nppY disruption-&-complementation, one could hypothesize that the NppL might not be able to hydroxylate the deoxynystatin at the C10 position as a substrate, despite of the high degree sequence homology between NysL and NppL (68% identity). To elucidate the NPP post-PKS tailoring steps, single knockout mutants of, ESK601(ΔnppDI), ESK6011(ΔnppY), and ESK6021(ΔnppL) as well as double knockout mutants ESK611(ΔnppDI ΔnppY), ESK621(ΔnppDIΔnppL), and ESK631(ΔnppYΔnppL) were generated (Table 2). Subsequently, each mutant was complemented by the expression construct containing either single (nppDI, nppY, and nppL) or double (nppDI/nppY and nppDI/nppL) genes (Table 2).
Table 2. Generated P. autotrophica mutant strains and compounds through both gene deletion and complementation.
| Deletion | Complementation | ||
|---|---|---|---|
| Mutant strain | Resulted compound | Complementing gene | Resulted compound |
| ESK601(ΔnppDI) | 10-deoxyaglycone | nppDI(ESK602) | NPP |
| ESK6011(ΔnppY) | 10-deoxynystatin | nppY(ESK6012) | NPP |
| ESK6021(ΔnppL) | 10-deoxyNPP | nppL(ESK6022) | NPP |
| ESK611(ΔnppDIΔnppY) | 10-deoxyaglycone | nppDI(ESK612) | 10-deoxynystatin |
| nppY(ESK613) | 10-deoxyaglycone | ||
| nppDI&nppY(ESK614) | NPP | ||
| ESK621(ΔnppDIΔnppL) | 10-deoxyaglycone | nppDI(ESK622) | 10-deoxyNPP |
| nppL(ESK623) | 10-deoxyaglycone | ||
| nppDI&nppL(ESK624) | NPP | ||
| ESK631(ΔnppYΔnppL) | 10-deoxynystatin | nppY(ESK632) | 10-deoxyNPP |
| nppL(ESK633) | 10-deoxynystatin | ||
| nppY&nppL(ESK634) | NPP | ||
| ESK641(ΔnppDIΔnppYΔnppL) | 10-deoxyaglycone | nppDI(ESK642) | 10-deoxynystatin |
| nppDI&nppY(ESK643) | 10-deoxyNPP | ||
| nppDI&nppY&nppL(ESK644) | NPP | ||
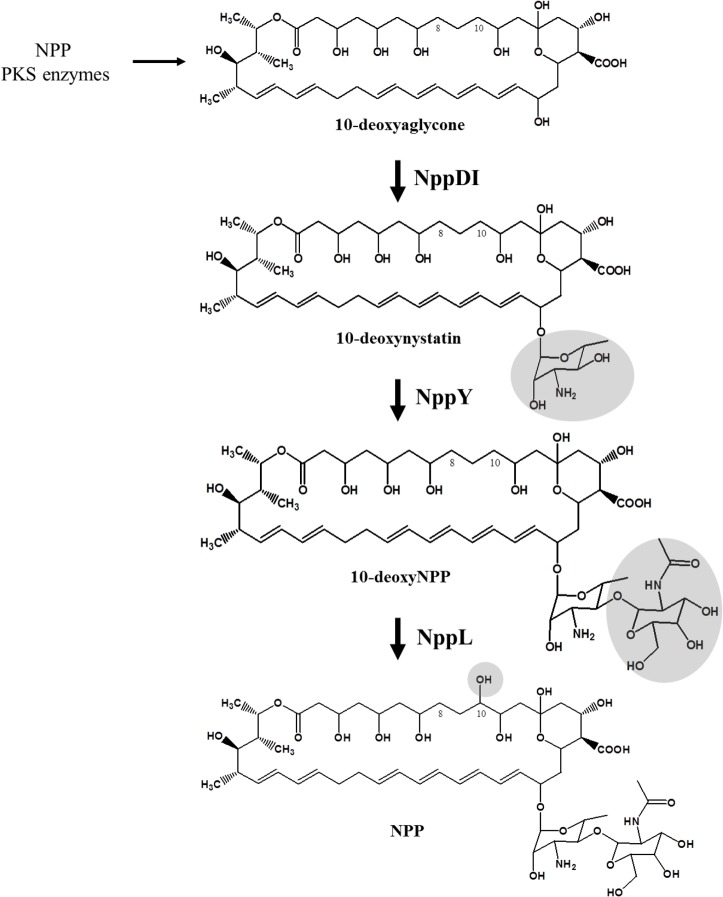
The nppL gene in the P. autotrophica chromosome is located downstream of nppK encoding the last module of the NPP PKS. Since the experimental evidence for NysL which leads to nystatin production from 10-deoxynystatin was suggested [29], we could predict the role of NppL in production of NPP. The nppL gene was inactivated via double homologous recombination in P. autotrophica chromosomal DNA. The polyene compound produced in ESK6021 was confirmed to be 10-deoxyNPP using HPLC-MS analysis (S3 Fig). The functional expression of nppL gene under the control of the constitutive promoter, ermE* in the ESK6021 restored NPP production, suggesting that NppL-driven hydroxylation should be the last step converting 10-deoxyNPP to NPP (S3 Fig). When the nppY gene was functionally expressed under the ermE* promoter in the double knock-out mutant ESK631, the 10-deoxyNPP was produced (S4 Fig). In addition, the expression of the nppDI gene in the double knock-out mutant ESK621 also generated 10-deoxyNPP. All these results also indicate that NppY catalyzes the transfer of second sugar, N-acetylglucosamine first, and then the NppL-driven regio-specific hydroxylation is the final post-PKS modification step in NPP biosynthesis (Fig 5). The proposed post-PKS tailoring steps of NPP biosynthesis was re-confirmed by a series of complementation in a triple knockout mutant (ΔnppDIΔnppYΔnppL) (Table 2 and S4 Fig).
Fig 5. A proposed post-PKS modification pathway in the NPP biosynthesis.
Discussion
Polyene macrolides such as amphotericin B and nystatin are very effective broad spectrum antifungal antibiotic that is active against human immunodeficiency virus and prion diseases. Unfortunately, the therapeutic application of polyene macrolides is restricted by severe side effects that can be moderated by structural alteration or liposomal formulation [31, 32]. Using the genetic information of various polyene biosynthetic gene clusters, a number of approaches have been used to address the problems associated with their medical use, such as generation of both semisynthetic and genetically-engineered polyene analogues. Despite several promising results, no new polyene-based antifungal agent has been applied in the clinic, except for in lipid and liposomal formulations [33], suggesting that the search for novel polyene macrolide antifungals for human use remains a challenging task. Recently, a rare actinomycetes, P. autotrophica KCTC9441, was shown to contain genes likely to encode compounds involved in polyene biosynthesis and were named NPP. These structural studies revealed that the aglycone of NPP is identical to the aglycone of nystatinolide. However, we have shown that NPP includes a unique disaccharide moiety, mycosamine (α1–4)-N-acetyl-glucosamine, linked at the C-19 hydroxyl position. In order to examine the unusual sugar moiety, nppDI was investigated using gene inactivation and complementation with the nppDI and nysDI genes. Surprisingly, nppDI could be substituted by nysDI, which can be explained by the existence of an alternative glycosyltransferase (GT) capable of attaching N-acetyl-glucosamine to nystatin. The alternative GT gene could be crucial for further investigation of the other polyenes with disaccharide moieties.
In this present study, we demonstrated that NPP-specific second sugar extending GT, NppY was responsible for the transfer of the second sugar, N-acetylglucosamine to 10-deoxynystatin through P. autotrophica genome mining, targeted-gene inactivation and complementation. The genetic deletion of nppY in P. autotrophica chromosomal DNA completely blocked not only NPP production but 10-deoxyNPP biosynthesis, indicating that the loss of N-acetylglucosamine has an impact on the downstream post-PKS modification process. When the ESK631 (ΔnppYΔnppL) was complemented by introduction of the nppY gene, the 10-deoxyNPP production was restored, suggesting that the hydroxylation at the C10 position was performed as the final step of NPP biosynthesis after the completion of two glycosylation reactions. This observation was also proved through the expression of the nppDI gene in the ESK621 (ΔnppDIΔnppL) mutant, leading to the production of 10-deoxyNPP (S4 Fig).
Recently, nypY from Pseudonocardia sp. P1 was heterologously expressed in various strains of S. nodosus allowed conversion of amphotericin A, B and 7-oxo-amphotericin B to disaccharide-modified forms, yet the conversion yield of these polyene analogues were extremely low [28]. Similarly, we also attempted to introduce nppY to generate the disaccharide forms of nystatin analogues in S. noursei. However, the undetectable levels of nystatin-like polyene derivatives were observed, even though the nppY gene was cloned into pSET152 vector under the control of the constitutive promoter, ermE* promoter (S5 Fig). Based on the NPP post-PKS tailoring steps that we propose here, we could hypothesize that NppY recognizes 10-deoxynystatin as a preferred substrate than nystatin itself in S. noursei.
To deduce the critical regions of NppY, various site-directed mutagenesis analysis were pursued. In general, GT reveals high structure similarity and are classified as a GT-A or GT-B fold. Interestingly, most of the GTs involved in natural product biosynthesis is known to employ a GT-B fold [34]. These proteins are composed of N-terminal domain responsible for the acceptor binding and the C-terminal domain for the sugar donor binding [16, 35, 36]. Through site-directed mutagenesis, arginine at the position of 200 and seven consecutive amino acids in N-terminal were proposed to play significant roles in NppY enzyme activity. In addition, the fusion protein containing the N-terminal half of NppY (presumably involved in 10-deoxynystatin binding) and the C-terminal half of the NppDI (presumably involved in mycosamine transfer) also induced significant decrease of NPP production, implying that the C-terminal domain in NppY might be better suitable for the N-acetyl-glucosamine transfer.
Through combination of deletions and complementations of nppDI, nppY, and nppL, the rational post-PKS tailoring pathway of the disaccharide-containing polyene such as NPP has been proposed for the first time. A NPP polyene aglycone is first produced by PKS enzymes, and then sequentially modified by two separate GT enzymes, followed by the regio-specific hydroxylation at the NPP C10 position by the specific P450 hydroxylase. The results shown in this paper not only increased our understanding on polyene post-PKS biosynthetic mechanism, but also provided the second sugar extending GT-driven application for the biosynthesis of novel toxicity-reduced polyene compounds in various actinomycetes.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ011296), Rural Development Administration, Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Butts A, Krysan DJ. Antifungal Drug Discovery: Something Old and Something New. PLoS Pathogens. 2012; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai CC, Tan CK, Huang YT, Shao PL, Hsueh PR. Current challenges in the management of invasive fungal infections. J Infect Chemother. 2008; 14: 77–85. 10.1007/s10156-007-0595-7 [DOI] [PubMed] [Google Scholar]
- 3. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009; 23: 525–530. 10.1097/QAD.0b013e328322ffac [DOI] [PubMed] [Google Scholar]
- 4. Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009; 49: 39–59. 10.1111/j.1600-0757.2008.00291.x [DOI] [PubMed] [Google Scholar]
- 5. Williams D, Lewis M. Pathogenesis and treatment of oral candidosis. Journal of Oral Microbiol. 2011; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellepola ANB, Samaranayake LP. Oral candidal infections and antimycotics. Crit Rev Oral Biol Med. 2000; 11: 172–198. [DOI] [PubMed] [Google Scholar]
- 7. Wakieć R, Prasad R, Morschhäuser J, Barchiesi F, Borowski E, Milewski S. Voriconazole and multidrug resistance in Candida albicans . Mycoses. 2007; 50: 109–115. [DOI] [PubMed] [Google Scholar]
- 8. Baginski M, Czub J, Sternal K. Interaction of amphotericin B and its selected derivatives with membranes: Molecular modeling studies. Chem Rec. 2006; 6: 320–332. [DOI] [PubMed] [Google Scholar]
- 9. Brautaset T, Sletta H, Degnes KF, Sekurova ON, Bakke I, Volokhan O, et al. New nystatin-related antifungal polyene macrolides with altered polyol region generated via biosynthetic engineering of Streptomyces noursei . Appl Environ Microbiol. 2011; 77: 6636–6643. 10.1128/AEM.05780-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baginski M, Czub J. Amphotericin B and its new derivatives—Mode of action. Curr Drug Metab. 2009; 10: 459–469. [DOI] [PubMed] [Google Scholar]
- 11. Zotchev SB. Polyene macrolide antibiotics and their applications in human therapy. Curr Med Chem. 2003; 10: 211–223. [DOI] [PubMed] [Google Scholar]
- 12. Bruheim P, Borgos SEF, Tsan P, Sletta H, Ellingsen TE, Lancelin JM, et al. Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC. Antimicrob Agents Chemother. 2004; 48: 4120–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carmody M, Murphy B, Byrne B, Power P, Rai D, Rawlings B, et al. Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J Biol Chem. 2005; 280: 34420–34426. [DOI] [PubMed] [Google Scholar]
- 14. Murphy B, Anderson K, Borissow C, Caffrey P, Griffith G, Heam J, et al. Isolation and characterisation of amphotericin B analogues and truncated polyketide intermediates produced by genetic engineering of Streptomyces nodosus . Org Biomol Chem. 2010; 8: 3758–3770. 10.1039/b922074g [DOI] [PubMed] [Google Scholar]
- 15. Power P, Dunne T, Murphy B, Lochlainn LN, Rai D, Borissow C, et al. Engineered Synthesis of 7-Oxo- and 15-Deoxy-15-Oxo-Amphotericins: Insights into Structure-Activity Relationships in Polyene Antibiotics. Chem Biol. 2008; 15: 78–86. 10.1016/j.chembiol.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 16. Hansen SF, Bettler E, Rinnan A, Engelsen SB, Breton C. Exploring genomes for glycosyltransferases. Mol BioSyst. 2010; 6: 1773–1781. 10.1039/c000238k [DOI] [PubMed] [Google Scholar]
- 17. Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyl transferases: Structures, functions, and mechanisms. Annu Rev Biochem. 2008; 77: 521–555. 10.1146/annurev.biochem.76.061005.092322 [DOI] [PubMed] [Google Scholar]
- 18. Thibodeaux CJ, Melançon CE, Liu HW. Unusual sugar biosynthesis and natural product glycodiversification. Nature. 2007; 446: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 19. Weymouth-Wilson AC. The role of carbohydrates in biologically active natural products. Nat Prod Rep. 1997; 14: 99–110. [DOI] [PubMed] [Google Scholar]
- 20. Stephens N, Rawlings B, Caffrey P. Versatility of enzymes catalyzing late steps in polyene 67-121C biosynthesis. Biosci Biotechnol Biochem. 2013; 77: 880–883. [DOI] [PubMed] [Google Scholar]
- 21. Barke J, Seipke RF, Grüschow S, Heavens D, Drou N, Bibb MJ, et al. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus . BMC Biol. 2010; 8: 109 10.1186/1741-7007-8-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim BG, Lee MJ, Seo JY, Hwang YB, Lee MY, Han K et al. Identification of functionally clustered nystatin-like biosynthetic genes in a rare actinomycetes, Pseudonocardia autotrophica . J Ind Microbiol Biotechnol. 2009; 36: 1425–1434. 10.1007/s10295-009-0629-5 [DOI] [PubMed] [Google Scholar]
- 23. Lee MJ, Kong D, Han KB, Sherman DH, Bai L, Deng Z, et al. Structural analysis and biosynthetic engineering of a solubility-improved and less-hemolytic nystatin-like polyene in Pseudonocardia autotrophica . Appl Microbiol Biotechnol. 2012; 95: 157–168. 10.1007/s00253-012-3955-x [DOI] [PubMed] [Google Scholar]
- 24. Sekurova O, Slettaa H, Ellingsen TE, Valla S, Zotchev S. Molecular cloning and analysis of a pleiotropic regulatory gene locus from the nystatin producer Streptomyces noursei ATCC11455. FEMS Microbiol Lett. 1999; 177: 297–304 [DOI] [PubMed] [Google Scholar]
- 25. Hopwood DA. Genetic contributions to understanding polyketide synthases. Chem Rev. 1997; 97: 2465–2497. [DOI] [PubMed] [Google Scholar]
- 26. Kim HJ, Kim MK, Kim YW, Kim ES. Effect of antibiotic down-regulatory gene wblA ortholog on antifungal polyene production in rare actinomycetes Pseudonocardia autotrophica . J Microbiol Biotechnol. 2014; 25:143 [DOI] [PubMed] [Google Scholar]
- 27. Choi SU, Lee CK, Hwang YI, Kinoshita H, Nihira T. Intergeneric conjugal transfer of plasmid DNA from Escherichia coli to Kitasatospora setae, a bafilomycin B1 producer. Arch Microbiol. 2004; 181: 294–298. [DOI] [PubMed] [Google Scholar]
- 28. De Poire E, Stephens N, Rawlings B, Caffrey P. Engineered biosynthesis of disaccharide-modified polyene macrolides. Appl Environ Microbiol. 2013; 79: 6156–6159. 10.1128/AEM.02197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Volokhan O, Sletta H, Ellingsen TE, Zotchev SB. Characterization of the P450 monooxygenase NysL, responsible for C-10 hydroxylation during biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei . Appl Environ Microbiol. 2006; 72: 2514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mulichak AM, Lu W, Losey HC, Walsh CT, Garavito RM. Crystal structure of vancosaminyltransferase GtfD from the vancomycin biosynthetic pathway: interactions with acceptor and nucleotide ligands. Biochemistry. 2014; 43: 5170–80. [DOI] [PubMed] [Google Scholar]
- 31. Byrne B, Carmody M, Gibson E, Rawlings B, Caffrey P. Biosynthesis of deoxyamphotericins and deoxyamphoteronolides by engineered strains of Streptomyces nodosus . Chem Biol. 2003; 10: 1215–24. [DOI] [PubMed] [Google Scholar]
- 32. Caffrey P, Lynch S, Flood E, Finnan S, Oliynyk M. Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes. Chem Biol. 2001; 8: 713–23. [DOI] [PubMed] [Google Scholar]
- 33. Nosanchuk JD. Current status and future of antifungal therapy for systemic mycoses. Recent Pat Antiinfect Drug Discov. 2006; 1: 75–84. [DOI] [PubMed] [Google Scholar]
- 34. Luzhetskyy A, Méndez C, Salas JA, Bechthold A. Glycosyltransferases, important tools for drug design. Curr Top Med Chem. 2008; 8: 680–709. [DOI] [PubMed] [Google Scholar]
- 35. Breton C, Šnajdrová L, Jeanneau C, Koca J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006; 16: 29–37R. [DOI] [PubMed] [Google Scholar]
- 36. Luzhetskyy A, Weiss H, Charge A, Welle E, Linnenbrink A. Vente A, et al. A strategy for cloning glycosyltransferase genes involved in natural product biosynthesis. Appl Microbiol Biotechnol. 2007; 75: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 37. MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992; 111: 61–68. [DOI] [PubMed] [Google Scholar]
- 38. Bierman M, Logan R, O’Brien K, Seno ET, Rao RN, Schoner BE. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992; 116: 43–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.