Abstract
Catecholamines are host stress hormones that can induce the growth of many bacteria by facilitating iron utilization and/or regulate the expression of virulence genes through specific hormone receptors. Whether these two responsive pathways are interconnected is unknown. In our previous study, it was found that catecholamines can regulate the expression of a great number of genes of Actinobacillus pleuropneumoniae, an important swine respiratory pathogen. However, bacterial growth was not affected by catecholamines in rich medium. In this study, it was discovered that catecholamines affected A. pleuropneumoniae growth in chemically defined medium (CDM). We found that serum inhibited A. pleuropneumoniae growth in CDM, while epinephrine, norepinephrine and dopamine promoted A. pleuropneumoniae growth in the CDM containing serum. The known bacterial hormone receptor QseC didn’t play roles in this process. Ion-supplementation and transcriptome analysis indicated that serum addition resulted in iron-restricted conditions which were alleviated by the addition of catecholamines. Transferrin, one of the components in serum, inhibited the growth of A. pleuropneumoniae in CDM, an effect reversed by addition of catecholamines in a TonB2-dependent manner. Our data demonstrate that catecholamines promote A. pleuropneumoniae growth by regulating iron-acquisition and metabolism, which is independent of the adrenergic receptor QseC.
Introduction
In recent years, studies in microbial endocrinology have discovered that host stress-related neuroendocrine catecholamine hormones can activate pathogen responses [1], providing an important bridge between infectious disease and stress. In 1992, the first study confirming the direct effect of catecholamines on bacterial growth was reported [2]. After that, many studies have discovered that catecholamines can stimulate the growth of bacteria [3–7]. Meanwhile, other studies have reported that in different bacterial species, catecholamines can regulate the expression of lots of genes including those involved in virulence [8–13]. Bacterial colonists of the gut, oral cavity and respiratory tract are exposed and can respond to catecholamines [1]. In some bacteria, catecholamines can facilitate the capture of iron from host transferrin (Tf) and lactoferrin (Lf) and promote bacterial growth via an enterobactin uptake system [14,15]. Norepinephrine (NE) can form complexes with the iron within Tf / Lf and reduce Fe3+ to Fe2+, resulting in release of the iron from the siderophores [16]. In other studies, the sensor kinases QseC and QseE, which are members of bacterial two component signal transduction systems (TCSTS), have been identified as adrenergic receptors in Escherichia coli O157:H7 [17–19]. QseC, QseE and their cognate regulators form complicated signaling cascades, linking host hormone responses to E. coli virulence [17–19]. However, it is still uncertain that whether there is any connection between the catecholamine- induced growth and QseC/E dependent signaling pathways.
Actinobacillus pleuropneumoniae, a member of the family Pasteurellaceae, is the etiologic agent of porcine contagious pleuropneumonia causing substantial global economic losses in the pig industry [20]. Stress factors including crowding, transportation, movement of pigs and adverse climatic conditions, contribute to A. pleuropneumoniae infection and transmission [21]. Following such stresses, the morbidity and mortality of disease are consequently affected [21]. Iron-acquisition systems are important factors involved in A. pleuropneumoniae infection [22]. A. pleuropneumoniae can use various iron sources from the host. Porcine Tf and haem compounds, but not porcine Lf, can be used by A. pleuropneumoniae as a sole iron source [23–26]. No siderophore has been found in A. pleuropneumoniae, but this bacterium can use exogenously supplied hydroxamate and catechol siderophores to promote growth [27]. A. pleuropneumoniae has two TonB systems encoded by tonB1-exbB-exbD and tonB2-exbB2-exbD2 [28]. TonB2 has been confirmed to be essential for infection in the host [28]. afuABC encoding a ferric uptake ABC transporter [29], hbpA encoding a hemoglobin binding protein [30], and fhuABCD encoding a ferrichrome transporter [31] have also been reported in A. pleuropneumoniae. Genome sequencing has identified at least 55 genes in the A. pleuropneumoniae genome that are involved in iron acquisition and metabolism [32]. The iron-acquisition and metabolism genes of A. pleuropneumoniae, including the two tonB gene systems, are up-regulated when bacteria are grown under iron-restriction [28,33].
Our previous study found that A. pleuropneumoniae can actively respond to the host stress hormones epinephrine (Epi) and NE [34]. The two hormones can affect expression of a great number of genes involved in A. pleuropneumoniae infection and metabolic processes. One of the genes regulated by the hormones was qseC, encoding the known adrenergic receptor QseC. The effects on selected virulence determinants were also found. However, in our previous study, catecholamines had no effect on A. pleuropneumoniae growth in rich medium. To further understand the response of A. pleuropneumoniae to catecholamines, in this study, we investigated the response of the bacterium to stress hormones when grown in chemically defined medium (CDM). Investigations included the iron-utilization mechanisms involved and whether they were dependent on the QseC pathway.
Materials and Methods
Bacterial strains and culture conditions
A. pleuropneumoniae 4074 (serovar 1 reference strain) and its mutants ΔqseB, ΔqseC, ΔqseBC (constructed by deletion inactivation using a sucrose counter-selectable marker system as described previously [35]) and ΔtonB1, ΔtonB2 (reported in previous study [28]) were used in this study. Tryptic Soy Broth (TSB) (Difco Laboratories, USA) or Tryptic Soy Agar (TSA) (Difco Laboratories, USA) supplemented with 10 μg/ml of nicotinamide adenine dinucleotide (NAD) and 10% (v/v) filtered cattle serum was used as rich medium. For iron-restricted conditions in rich medium, 2,2-dipyridyl at 100μM was added into the TSB medium [36]. The chemically defined medium (CDM) was prepared as previously described [37]. Filtered cattle serum, FeCl3, MgCl2, ZnCl2, apo-transferrin (ATF) or holo-transferrin (HTF) (Sigma, USA) at various concentrations was added into the CDM when necessary. For detection of the effects of catecholamines and/or their antagonists on bacterial growth, Epi, NE and dopamine (DA), α-adrenergic receptor antagonist phentolamine (PE), β- adrenergic receptor antagonist propranolol (PO) and the non-selective dopaminergic receptor antagonist haloperidol (Hal) (Sigma, USA) [38] were supplemented into the medium. To assess bacterial growth, bacteria were cultured aerobically at 37°C with rotation at 200 rpm and optical density at 600nm (OD600nm) and/or bacterial viable numbers were recorded at selected time points.
Microarray construction
The microarray used in this study has been described previously [34]. In brief, the microarray consists of 15744 60-mer oligonucleotide probes synthesized in situ by Agilent Technologies. The probes were designed based on the genome sequences of A. pleuropneumoniae 4074 (serovar 1), JL03 (serovar 3) and L20 (serovar 5) (GenBank accession numbers: AACK00000000, CP000687, CP000569) including 2132 ORFs. Each probe with the same sequence for a given gene was repeated twice on the array.
Microarray experiments and data analysis
The bacterial culture samples were collected from mid-log phase cultures (7 hours after culturing with an inoculum of 106 CFU/ml in CDM). Three independent biological replicates were performed. Total RNA was extracted using RNA-Solv Reagent (Promega, USA) according to the manufacturer’s instructions. Hybridization and scanning were conducted according to the Agilent microarray single channel experiment protocols (Agilent, USA).
The signal intensities were normalized using Feature Extraction Software (Agilent, USA) and transformed into log2 values. The genes with positive signals (flags = P or M) in all hybridizations were selected to be further analyzed. The genes with fold change ≥ 2 and P < 0.05 were selected as differentially expressed genes. Gene annotations and functional classification were conducted according to our previous studies [34,39]. All the data are MIAME compliant and the raw data has been deposited in the NCBI GEO database under the number GSE61054.
Real-time quantitative RT-PCR
RNA was extracted as described above and reverse-transcribed into cDNA using Superscript II reverse transcriptase (Invitrogen, USA). Real time quantitative RT-PCR (qRT-PCR) was performed using ABI Power SYBR Green PCR Master Mix (ABI, USA) and the 7900 HT Sequence Detection System (ABI, USA) at 50°C, 5 min; 95°C, 10 min; 40 cycles of 95°C, 15 s; 60°C, 1 min. The primers used for real-time qRT-PCR are listed in S1 Table. The relative transcription level of each gene was determined by normalization to that of the kdsB gene which displayed no changes in the present microarray analysis using the 2-ΔCtΔCt method [40].
Statistical methods
The bacterial densities revealed by OD600nm values or bacterial numbers at different time-points during growth were compared by using a two-tailed paired student’s t-test. Microarray data were analyzed using the two class paired t-test in SAM (significance analysis of microarray) inserted into the software TM4. A two-tailed paired student’s t-test was used to analyze the results of qRT-PCR. The correlation between the results of microarray and qRT-PCR was determined by calculating R2 using the mean log2 ratios.
Results
Serum inhibited A. pleuropneumoniae growth in CDM
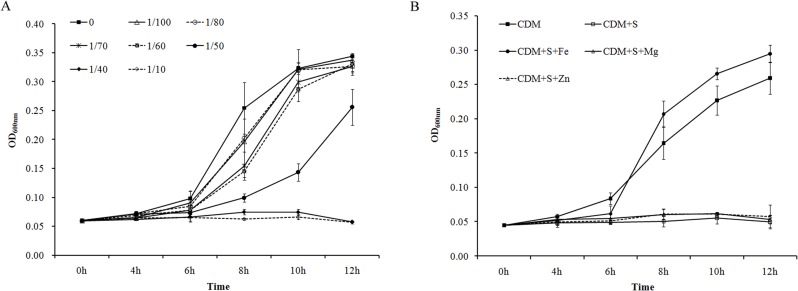
In our previous studies, it was found that cattle serum promoted the growth of A. pleuropneumoniae in the rich medium TSB, and catecholamines had no detectable effect on A. pleuropneumoniae growth [34,41]. To investigate the effect of catecholamines on growth under iron-limiting conditions, 2,2-dipyridyl was added to chelate the iron in the TSB medium. There was impairment of A. pleuropneumoniae growth when 2,2-dipyridyl was added into TSB in both the absence and presence of supplemented catecholamines (data not shown). In CDM,A. pleuropneumoniae grew slowly, and cattle serum was added to facilitate bacterial growth. However, unexpectedly, serum inhibited A. pleuropneumoniae growth in CDM in a concentration-dependent manner (Fig 1A). Since the concentration-dependent inhibition was similar to that resulting from addition of 2,2-dipyridyl to rich medium, iron (in the form of FeCl3) was added into the serum supplemented CDM to identify the possible reason of growth inhibition. Compared with MgCl2 and ZnCl2, only FeCl3 addition reversed serum-induced growth inhibition in CDM. Various concentrations of metal ions were tested and the similar results were observed. The influence of metal ions at a concentration of 40 μM on A. pleuropneumoniae growth are shown in Fig 1B.
Fig 1. The effect of serum on A. pleuropneumoniae growth in chemically defined medium (CDM).
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM at a dose of 104 CFU/ml. Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. (A) Various concentrations of serum (0–1/10; V/V) were added into CDM; (B) Serum at the concentration of 1/40 was added into CDM (CDM + S). FeCl3, MgCl2 and ZnCl2 were supplemented into the serum-containing medium respectively (CDM + S + Fe/Mg/Zn). A. pleuropneumoniae cultured in CDM without any supplementation was used as a control (CDM). Data are shown as means ± SD from four independent replications.
Catecholamines stimulated A. pleuropneumoniae growth in serum-supplemented CDM
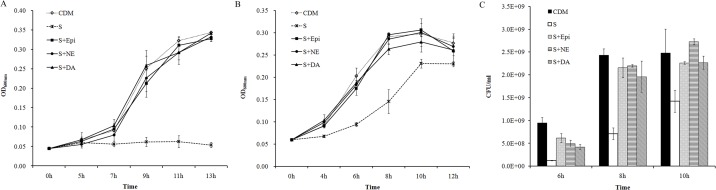
The catecholamines Epi, NE and DA at the concentration of 50 μM were added into serum-supplemented CDM and growth curves obtained. The three hormones stimulated A. pleuropneumoniae growth significantly (Fig 2). With an initial inoculum of 104 CFU/ml, A. pleuropneumoniae could hardly grow in the serum-supplemented CDM, but addition of catecholamines induced bacterial growth (Fig 2A). With a starting inoculum of 106 CFU/ml, A. pleuropneumoniae grew in serum medium but reached a lower bacterial density in log-phase compared to that in medium containing catecholamines (Fig 2BC). Furthermore, various concentrations, ranging from 0.1 μM to 50 μM, of catecholamines were tested to detect the minimum effective concentration. The results showed that catecholamines at the concentrations higher than 0.5 μM could induce growth (S1 Fig).
Fig 2. Catecholamine induced A. pleuropneumoniae growth in CDM containing serum.
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM containing 1/40 of serum (S) using an inoculation dose of 104 CFU/ml (A) or 106 CFU/ml (BC). Epi, NE and DA at 50μM were supplemented into the serum-containing medium (S + Epi/NE/DA). A. pleuropneumoniae cultured in CDM without any supplementation was used as control (CDM). Optical densities of bacterial cultures (OD600nm) were recorded at selected time points (AB). Bacterial viable numbers were recorded after sub-culturing using 106 CFU/ml at 6 h, 8h and 10h, respectively (C). Data are shown as means ± SD from four independent replications.
Stimulation of growth was not mediated by known adrenergic receptors
To explore the cause of growth promotion, the known adrenergic receptors in mammalian as well as in bacteria were investigated. The eukaryotic α-adrenergic receptor antagonist PE, β-adrenergic receptor antagonist PO and the non-selective dopaminergic receptor antagonist Hal were added into the medium to determine whether these antagonists could block the growth-inducing effect of catecholamines or not. A range of concentrations from 10–100 μM were used, but the antagonists did not block the growth induction caused by Epi, NE and DA. The results using concentration of 50μM are shown in S2 Fig. These observations demonstrate that the mechanism of growth promotion by catecholamines did not involve the eukaryotic adrenergic signaling pathways investigated.
The TCSTS sensor kinases QseC and QseE are known as adrenergic receptors in E. coli. qseC and its cognate regulator qseB are present in the A. pleuropneumoniae genome and are regulated by Epi and NE [34]. Thus, the deletion mutants ΔqseC, ΔqseB and ΔqseBC were used to determine whether growth induction was mediated by this two component system or not. However, the growth of the three mutants did not show any difference to that of the parental strain (S3 Fig), indicating that the induced growth by catecholamines in A. pleuropneumoniae was not mediated by the known bacterial adrenergic receptor QseC.
Iron availability and metabolism contributed to the growth stimulation
To further investigate the mechanism of serum-inhibited and catecholamine-induced growth of A. pleuropneumoniae in CDM, the gene expression profiles of the bacteria grown in CDM, CDM supplemented with serum and CDM supplemented with serum plus Epi/NE/DA were compared. The microarray data showed that 363 genes (172 induced and 191 repressed) were differentially expressed in CDM supplemented with and without serum. These genes will be described as serum-regulated. Meanwhile, 255 genes (139 induced and 116 repressed), 723 genes (336 induced and 387 repressed) and 716 genes (339 induced and 377 repressed) were differentially expressed after addition of Epi, NE and DA into the serum-supplemented CDM, respectively. These genes will be described as hormone-regulated. Five genes (three genes regulated by serum and hormones, and two genes which were not regulated) were selected to conduct the qRT-PCR to validate the microarray results. The two methods showed high correlation with R2 = 0.903 (S4 Fig). The functions of all the differentially expressed genes can be divided into 21 categories (S5 Fig). Only a few genes involved in cell division and cell cycle were differentially expressed, while a large amount of regulated genes were metabolism-related. Among the serum-, Epi-, NE- and DA-regulated genes, an important proportion are involved in inorganic ion transport and metabolism, and 21 of these genes were found to be involved in iron metabolism. Twenty out of 21 of these iron metabolism genes were up-regulated by supplementation of serum, but down-regulated by addition of Epi, NE or DA into the serum-containing medium (Table 1). Genes encoding the two TonB systems involved in iron uptake systems of A. pleuropneumoniae, the TonB1-ExbB-ExbD and TonB2-ExbB2-ExbD2, were differentially expressed. In addition, genes encoding proteins involved in acquisition of various forms of iron including the Tf, hemoglobin, heme and ferrichrome were regulated.
Table 1. Differentially expressed genes encoding iron metabolism proteins regulated by serum, Epi, NE and DA.
| Gene locus_tag | Name | Function | Serum | Epi | NE | DA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fold change | p-value | Fold change | p-value | Fold change | p-value | Fold change | p-value | |||
| APJL_0076 | tonB2 | TonB energy transducing protein | 13.33 | 1.62E-05 | 0.14 | 3.68E-02 | 0.07 | 1.76E-02 | 0.06 | 2.36E-02 |
| APJL_0077 | exbD2 | biopolymer transport protein | 11.80 | 2.37E-04 | 0.10 | 3.41E-03 | 0.10 | 9.62E-03 | 0.08 | 1.64E-02 |
| APJL_0078 | exbB2 | biopolymer transport protein | 12.75 | 7.75E-07 | 0.09 | 3.50E-03 | 0.08 | 9.77E-03 | 0.07 | 8.86E-03 |
| APJL_0128 | yfeD | iron (chelated) transport system membrane protein | 2.16 | 2.51E-04 | 0.43 | 3.02E-04 | 0.22 | 3.85E-03 | 0.20 | 2.72E-04 |
| APJL_0286 | frpB | iron-regulated outer membrane protein | 7.88 | 4.39E-05 | 0.09 | 5.10E-03 | 0.11 | 2.12E-02 | 0.10 | 1.27E-02 |
| APJL_0554 | cirA | outer membrane receptor proteins, mostly Fe transport | 3.42 | 1.68E-03 | 0.32 | 3.94E-03 | 0.35 | 7.76E-03 | 0.27 | 4.58E-03 |
| APJL_0665 | - | high-affinity Fe2+/Pb2+ permease | 5.71 | 2.00E-02 | 0.13 | 1.74E-02 | 0.21 | 1.75E-02 | 0.17 | 1.03E-02 |
| APJL_1065 | hgbA | hemoglobin and hemoglobin haptoglobin-binding protein 4 | 3.22 | 1.42E-03 | 0.27 | 1.33E-03 | 0.21 | 1.36E-03 | 0.21 | 1.82E-03 |
| APJL_1066 | hugZ | heme iron utilization protein | 44.51 | 1.52E-04 | 0.01 | 1.88E-02 | 0.02 | 9.44E-06 | 0.01 | 1.20E-04 |
| APJL_1312 | - | iron-regulated outer membrane protein | 11.63 | 4.10E-04 | 0.08 | 1.96E-02 | 0.15 | 1.84E-02 | 0.11 | 1.01E-02 |
| APJL_1597 | tbpA1 | transferrin-binding protein 1 | 4.00 | 1.03E-02 | 0.29 | 3.98E-02 | 0.23 | 5.24E-03 | 0.25 | 8.63E-03 |
| APJL_1598 | tbpB1 | transferrin-binding protein 2 | 5.12 | 7.87E-04 | 0.18 | 3.36E-04 | 0.17 | 2.97E-03 | 0.20 | 9.98E-04 |
| APJL_1599 | exbD | biopolymer transport protein | 6.26 | 3.84E-03 | 0.12 | 2.19E-03 | 0.14 | 1.49E-04 | 0.11 | 5.66E-04 |
| APJL_1600 | exbB | biopolymer transport protein | 6.38 | 1.21E-04 | 0.16 | 1.59E-04 | 0.14 | 6.34E-04 | 0.11 | 1.19E-03 |
| APJL_1601 | tonB1 | periplasmic protein | 5.81 | 9.66E-04 | 0.18 | 2.00E-02 | 0.18 | 1.76E-02 | 0.22 | 4.21E-07 |
| APJL_1922 | - | outer membrane receptor proteins, mostly Fe transport | 0.17 | 4.12E-03 | 2.27 | 1.68E-02 | 3.20 | 7.60E-03 | 3.68 | 5.09E-06 |
| APJL_2000 | - | hemoglobin receptor precursor | 12.63 | 9.85E-06 | 0.12 | 2.19E-02 | 0.09 | 7.86E-04 | 0.08 | 2.39E-05 |
| APJL_2060 | hbpA2 | heme-binding protein A | 4.53 | 1.21E-04 | 0.22 | 1.25E-02 | 0.28 | 2.66E-04 | 0.23 | 3.04E-02 |
| APJL_2064 | fhuD | ferrichrome-binding periplasmic protein | 2.74 | 2.61E-03 | 0.41 | 3.27E-03 | 0.36 | 2.31E-03 | 0.32 | 1.68E-03 |
| APJL_2065 | fhuB | ferrichrome uptake protein | 4.30 | 2.76E-03 | 0.21 | 6.56E-04 | 0.21 | 8.77E-04 | 0.17 | 3.32E-03 |
| APJL_2066 | fhuA | outer membrane ferric hydroxamate receptor | 3.22 | 2.69E-04 | 0.38 | 4.97E-02 | 0.26 | 2.66E-02 | 0.24 | 6.53E-03 |
Transferrin contributed to the growth stimulation by catecholamines
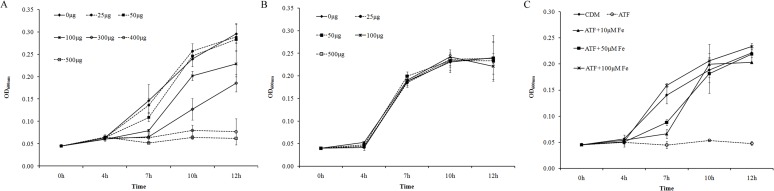
Tf has been identified as the key component in serum that stimulates the growth of E. coli [42]. A. pleuropneumoniae can also use Tf as an iron source [28]. Thus, both ATF and HTF were added into CDM (instead of the serum) to further identify the cause of growth promotion by catecholamines in A. pleuropneumoniae. ATF inhibited A. pleuropneumoniae growth in a concentration-dependent manner while the HTF had no effect (Fig 3AB). ATF contains no iron but HTF is saturated with iron. Hence, we added both ATF and FeCl3 into the CDM. The growth curves showed that with the increased amount of FeCl3, the inhibition effect of ATF on A. pleuropneumoniae growth disappeared (Fig 3C). Hence, the inhibition of ATF on A. pleuropneumoniae growth was due to iron availability.
Fig 3. The effect of transferrin on A. pleuropneumoniae growth in CDM).
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM at an inoculation dose of 104 CFU/ml. Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. Various concentrations of apo-transferrin (ATF) (A) or holo-transferrin (HTF) (B) were added into CDM. (C). ATF at 400μg/ml was added into CDM (ATF) and FeCl3 at different concentrations were supplemented into the medium containing ATF (ATF + Fe). A. pleuropneumoniae cultured in CDM without any supplementation was used as a control (CDM). Data are shown as means ± SD from four independent replications.
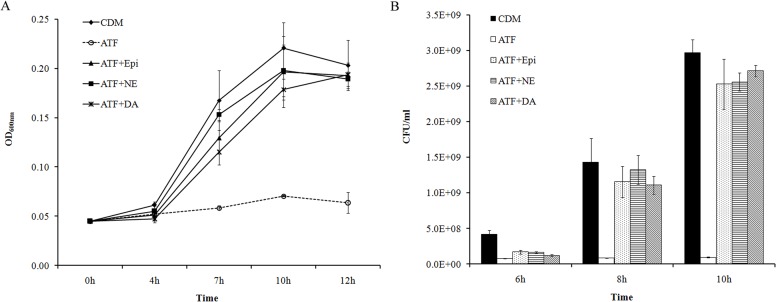
When catecholamines were added into CDM containing 400μg/ml ATF, the growth of A. pleuropneumoniae was totally inhibited. When the hormones Epi, NE or DA were added into CDM containing 400μg/ml ATF, the growth of A. pleuropneumoniae was promoted (Fig 4). In contrast, catecholamines did not show any effect on bacterial growth in the CDM containing HTF (data not shown). Furthermore, under such growth conditions, the eukaryotic adrenergic receptor antagonists did not block the growth stimulation, and the A. pleuropneumoniae ΔqseC, ΔqseB and ΔqseBC mutants showed the same growth characteristics as their parental strain (data not shown). Thus, it can be concluded that Tf is one of the components in serum that contributes to catecholamine-induced growth of A. pleuropneumoniae. Again, this growth stimulation is not mediated by the known adrenergic receptors investigated.
Fig 4. Catecholamine induced A. pleuropneumoniae growth in CDM containing ATF.
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM using an inoculation dose of 104 CFU/ml. ATF at 400μg/ml was added into CDM (ATF). Epi, NE and DA at 50μM were supplemented into the medium containing ATF (ATF + Epi/NE/DA). A. pleuropneumoniae cultured in CDM without any supplementation was used as a control (CDM). (A). Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. (B). Bacterial viable numbers were recorded at 6 h, 8h and 10h. Data are shown as means ± SD from four independent replications.
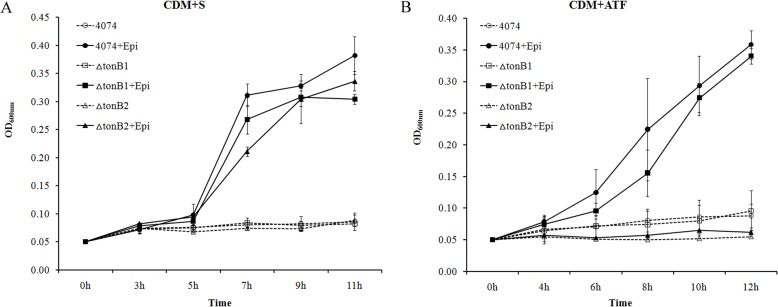
TonB2 contributed to growth stimulation of A. pleuropneumoniae by catecholamines
According to the results of microarray analysis, multiple genes involved in iron-acquisition, including tonB1 and tonB2, were regulated by serum and catecholamines. ΔtonB1 and ΔtonB2 mutants were used to discover if either or both TonB systems have a role in growth induction by catecholamines. In serum-containing CDM, the growth of both ΔtonB1 and ΔtonB2 mutants were inhibited as the parental strain. The growths of the two mutants were also promoted by addition of Epi in serum-containing CDM (Fig 5A). When ATF was used to replace serum, the ΔtonB1 mutant had similar growth features as that of the parental strain, whereas the growth of the ΔtonB2 mutant was not induced by Epi (Fig 5B). NE and DA displayed the same effect as Epi with both mutants (data not shown). Therefore, the TonB2 system has an important role in catecholamine-induced growth in medium containing ATF.
Fig 5. The effects of catecholamines on growth of A. pleuropneumoniae ΔtonB1 and ΔtonB2 mutants.
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM using an inoculation dose of 104 CFU/ml. A. pleuropneumoniae 4074 and the mutants were sub-cultured in CDM containing 50μM of Epi and 1/40 of serum (S in figure A) or 400μg/ml ATF (figure B). Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. Data are shown as means ± SD from four independent replications.
Discussion
In our previous study, we found that A. pleuropneumoniae alters virulence gene expression and infection-related behaviour in response to the host stress hormones Epi and NE [34]. In that work, we used the rich medium TSB to support the growth of A. pleuropneumoniae. Catecholamines had no effect on growth in the TSB. According to previous studies, bacterial growth can be induced by catecholamines as a result of iron-acquisition [14]. Therefore, in the present study, we investigated the effect of catecholamines on A. pleuropneumoniae growth under iron-restricted conditions. The addition of the Fe-chelator 2,2-dipyridyl was used to induce iron-restriction in rich medium but addition of catecholamine had no effect on A. pleuropneumoniae growth (data not shown). The use of CDM in this study allowed the effects of catecholamines on A. pleuropneumoniae to be determined. Initially, it was unexpected that, in contrast to that observed with the rich medium TSB, addition of serum to CDM resulted in growth inhibition of A. pleuropneumoniae. Analysis of the growth characteristics of A. pleuropneumoniae in media supplemented with ions in combination with gene expression changes in response to supplementation of serum and serum plus catecholamines to CDM indicated that addition of serum resulted in iron restricted conditions in CDM for A. pleuropneumoniae. In CDM plus serum, bacterial growth was inhibited and iron-acquisition genes were up-regulated. The addition of catecholamines removed the iron-restriction, hence bacterial growth was induced and iron-acquisition genes were down-regulated. Similar discoveries have also been reported in previous studies with Salmonella enterica, in which 36 genes involved in iron-utilization were down-regulated after the exposure to NE, confirming the iron-restricted environment resulted from the serum-SAPI medium used in that study [15].
In E. coli O157, regulation of virulence genes by catecholamines are dependent on the QseC/B and QseE/F two component systems [18]. There are no homologous genes to qseE/F in A. pleuropneumoniae. Homologous qseC/B genes are found in A. pleuropneumoniae which are regulated by Epi and NE [34]. However, mutations of qseC/B did not change catecholamine-induced growth of A. pleuropneumoniae, and the expression of qseC/B were neither regulated by serum nor catecholamines in CDM. Therefore, under the iron-restricted conditions tested in this study, catecholamines-stimulated growth of A. pleuropneumoniae was independent of QseC/B. Most previous studies investigating growth promotion effects of catecholamines have been carried out in medium containing serum or Tf [4–6,15], but those characterizing QseC/B and QseE/F signaling cascades have used different media [18,19]. The systems used by bacteria to respond to host catecholamines may vary dependent on growth conditions.
In this study, the ATF-supplemented CDM may capture iron in the medium to establish iron-restricted condition for A. pleuropneumoniae. As shown in previous study, catecholamines can form a complex with transferrin-bound iron and release the iron for the bacterium to utilize [16]. Hence the growth of A. pleuropneumoniae could be promoted by catecholamines. A. pleuropneumoniae has two TonB systems in which TonB2 is essential for survival in vivo [28]. In this study, we found TonB2 played an important role in catecholamine-induced growth in ATF-supplemented CDM. This TonB2 dependent adjustment of growth might be important for A. pleuropneumoniae infection in the host during stress. In Bordetella bronchiseptica, another respiratory pathogen, catecholamine-induced growth is also TonB-dependent [43]. In B. bronchiseptica, NE can release iron from transferrin and deliver it directly to bacterial cells or shuttle iron to siderophores. The newly characterized NE receptors and a specific siderophore receptor of B. bronchiseptica are required, respectively [43]. Similar mechanisms present in B. bronchiseptica enabling catecholamine-induced growth may also exist in A. pleuropneumoniae. It has been reported that A. pleuropneumoniae produce uncharacterized siderphores and can use exogenously supplemented hydroxamate and catechol siderophores [27]. TonB2 is crucial for the acquisition of iron in the form of hemin, hemoglobin, ferrichrome/hydroxamate and transferrin [28]. The genes encoding proteins for binding and uptake of these forms of irons have been found in the A. pleuropneumoniae genome [32]. In a recent study, genes encoding a putative enterobactin receptor system and a cirA like siderophore have been identified to be up-regulated under iron-restricted conditions [33,44]. These known and/or putative iron-acquisition systems might have roles in catecholamine-induced growth of A. pleuropneumoniae.
The results suggest that, in the CDM containing serum, the induced growth of A. pleuropneumoniae is TonB2 independent. There are three possible reasons: (1) Tf was not the sole component in serum that catecholamines bind and induce bacterial growth; (2) in the medium containing serum, TonB1 has the same function as TonB2; and (3) unknown iron-uptake system(s) independent of TonB have roles in iron-acquisition in medium containing serum (but not ATF). In fact, A. pleuropneumoniae is equipped with lots of genes encoding various iron-acquisition systems [32]. Many of these genes were differentially expressed after supplementation of serum and Epi/NE/DA (Table 1). Perhaps growth in serum-containing medium involves more than one iron uptake system.
Conclusion
In conclusion, the effect of the addition of catecholamines on A. pleuropneumoniae growth was tested in CDM. In CDM, serum and ATF inhibited A. pleuropneumoniae growth in a concentration-dependent manner. This growth inhibition was reversed by the addition of the catecholamines Epi, NE and DA in a bacterial adrenergic receptor QseC-independent manner. The underlying mechanism found was that the addition of serum or ATF to CDM resulted in iron-restricted conditions and this was reversed by addition of catecholamines. TonB2 of A. pleuropneumoniae was essential for catecholamine-induced growth of A. pleuropneumoniae in the CDM containing ATF. Thus, the results show that catecholamines induce growth of A. pleuropneumoniae through iron-acquisition in a QseC-independent manner. This response may contribute to disease development caused by A. pleuropneumoniae.
Supporting Information
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM using an inoculation dose of 104 CFU/ml. Catecholamines at concentrations ranging from 0.1μM to 50μM were added into CDM containing 1/40 of serum. Optical densities of bacterial cultures (OD600nm) were recorded at early stationary phase (12 hours after sub-culture). Data are from one test out of three similar results.
(TIF)
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM containing 1/40 of serum (S) using an inoculation dose of 104 CFU/ml. Epi (A), NE (B) and DA (C) at 50μM were supplemented into the serum-containing medium. The eukaryotic α-adrenergic receptor antagonist phentolamine (PE), β- adrenergic receptor antagonist propranolol (PO) and the non-selective dopaminergic receptor antagonist haloperidol (Hal) at the concentration of 50μM were separately added into the serum medium containing different catecholamines. A. pleuropneumoniae cultured in CDM without any supplementation was used as a control (CDM). Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. Data are shown as means ± SD from three independent replications.
(TIF)
A. pleuropneumoniae parental strain 4074 and the mutants (ΔqseB and ΔqseC) were cultured in TSB medium overnight and then sub-cultured in CDM containing 1/40 of serum with or without 50μM of different catecholamines (+ Epi/NE/DA). The inoculation dose was 104 CFU/ml for sub-culture. Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. Data are shown as means ± SD from three independent replications.
(TIF)
Mean log2 ratios obtained from microarray results are plotted against the mean log2 ratios obtained from qRT-PCR. 1–4: gene tonB2 regulated by serum, Epi, NE and DA; 5–8: gene tonB1 regulated by serum, Epi, NE and DA, 9–12: gene tbpA1 regulated by serum, Epi, NE and DA; 13–16: gene qseC regulated by serum, Epi, NE and DA; 17–20: gene fur regulated by serum, Epi, NE and DA.
(TIF)
Up or down means up or down-regulated by serum/Epi/NE/DA. Gene functions were sorted according to COG categories: C: Energy production and conversion; D: Cell cycle control, cell division, chromosome partitioning; E: Amino acid transport and metabolism; F: Nucleotide transport and metabolism; G: Carbohydrate transport and metabolism; H: Coenzyme transport and metabolism; I: Lipid transport and metabolism; J: Translation, ribosomal structure and biogenesis; K: Transcription; A, RNA processing and modification; L: Replication, recombination and repair; M: Cell wall/membrane/envelope biogenesis; O: Posttranslational modification, protein turnover, chaperones; P: Inorganic ion transport and metabolism; Q: Secondary metabolites biosynthesis, transport and catabolism; R: General function prediction only; T: Signal transduction mechanisms; U: Intracellular trafficking, secretion, vesicular transport; V: Defense mechanisms; S/N: Function unknown in COG.
(TIF)
(DOCX)
Acknowledgments
We are grateful to Professor Paul R. Langford (Imperial College, London) for kind donation of A. pleuropneumoniae mutants ΔtonB1 and ΔtonB2 and manuscript modification. We thank Dr. Huasheng Xiao from National Engineering Center for Biochip at Shanghai for technical assistance in microarray analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31201932) (http://www.nsfc.gov.cn/, National Basic Research Program of China (973 Program, 2012CB518802) (http://www.973.gov.cn/Default_3.aspx, Special Fund for Agro-scientific Research in the Public Interest (201303034-11) (http://www.moa.gov.cn/) and Fundamental Research Funds for the Central Universities (2662014BQ021) (http://www.hzau.edu.cn/2014/ch/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Freestone PP, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16: 55–64. 10.1016/j.tim.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 2. Lyte M. The role of catecholamines in gram-negative sepsis. Med Hypotheses. 1992;37: 255–258. [DOI] [PubMed] [Google Scholar]
- 3. Kinney KS, Austin CE, Morton DS, Sonnenfeld G. Catecholamine enhancement of Aeromonas hydrophila growth. Microb Pathog. 1999;26: 85–91. [DOI] [PubMed] [Google Scholar]
- 4. Burton CL, Chhabra SR, Swift S, Baldwin TJ, Withers H, Hill SJ, et al. The growth response of Escherichia coli to neurotransmitters and related catecholamine drugs requires a functional enterobactin biosynthesis and uptake system. Infect Immun. 2002;70: 5913–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belay T, Aviles H, Vance M, Fountain K, Sonnenfeld G. Catecholamines and in vitro growth of pathogenic bacteria: enhancement of growth varies greatly among bacterial species. Life Sci. 2003;73: 1527–1535. [DOI] [PubMed] [Google Scholar]
- 6. Anderson MT, Armstrong SK. The Bordetella bfe system: growth and transcriptional response to siderophores, catechols, and neuroendocrine catecholamines. J Bacteriol. 2006;188: 5731–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freestone PP, Haigh RD, Lyte M. Specificity of catecholamine-induced growth in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica . FEMS Microbiol Lett. 2007;269: 221–228. [DOI] [PubMed] [Google Scholar]
- 8. Lyte M, Erickson AK, Arulanandam BP, Frank CD, Crawford MA, Francis DH. Norepinephrine-induced expression of the K99 pilus adhesin of enterotoxigenic Escherichia coli . Biochem Biophys Res Commun. 1997;232: 682–686. [DOI] [PubMed] [Google Scholar]
- 9. Lyte M, Arulanandam BP, Frank CD. Production of Shiga-like toxins by Escherichia coli O157:H7 can be influenced by the neuroendocrine hormone norepinephrine. J Lab Clin Med. 1996;128: 392–398. [DOI] [PubMed] [Google Scholar]
- 10. Bansal T, Englert D, Lee J, Hegde M, Wood TK, Jayaraman A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun. 2007;75: 4597–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karavolos MH, Spencer H, Bulmer DM, Thompson A, Winzer K, Williams P, et al. Adrenaline modulates the global transcriptional profile of Salmonella revealing a role in the antimicrobial peptide and oxidative stress resistance responses. BMC Genomics. 2008;9: 458 10.1186/1471-2164-9-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dowd SE. Escherichia coli O157:H7 gene expression in the presence of catecholamine norepinephrine. FEMS Microbiol Lett. 2007;273: 214–223. [DOI] [PubMed] [Google Scholar]
- 13. Kendall MM, Rasko DA, Sperandio V. Global effects of the cell-to-cell signaling molecules autoinducer-2, autoinducer-3, and epinephrine in a luxS mutant of enterohemorrhagic Escherichia coli . Infect Immun. 2007;75: 4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freestone PP, Haigh RD, Williams PH, Lyte M. Involvement of enterobactin in norepinephrine-mediated iron supply from transferrin to enterohaemorrhagic Escherichia coli . FEMS Microbiol Lett. 2003;222: 39–43. [DOI] [PubMed] [Google Scholar]
- 15. Bearson BL, Bearson SM, Uthe JJ, Dowd SE, Houghton JO, Lee I, et al. Iron regulated genes of Salmonella enterica serovar Typhimurium in response to norepinephrine and the requirement of fepDGC for norepinephrine-enhanced growth. Microbes Infect. 2008;10: 807–816. 10.1016/j.micinf.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 16. Sandrini SM, Shergill R, Woodward J, Muralikuttan R, Haigh RD, Lyte M, et al. Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin. J Bacteriol. 2010;192: 587–594. 10.1128/JB.01028-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A. 2006;103: 10420–10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes DT, Clarke MB, Yamamoto K, Rasko DA, Sperandio V. The QseC adrenergic signaling cascade in Enterohemorrhagic E. coli (EHEC). PLoS Pathog. 2009;5: e1000553 10.1371/journal.ppat.1000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reading NC, Rasko DA, Torres AG, Sperandio V. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc Natl Acad Sci U S A. 2009;106: 5889–5894. 10.1073/pnas.0811409106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bosse JT, Janson H, Sheehan BJ, Beddek AJ, Rycroft AN, Kroll JS, et al. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect. 2002;4: 225–235. [DOI] [PubMed] [Google Scholar]
- 21. Chiers K, De Waele T, Pasmans F, Ducatelle R, Haesebrouck F. Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet Res. 2010;41: 65 10.1051/vetres/2010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacques M. Surface polysaccharides and iron-uptake systems of Actinobacillus pleuropneumoniae . Can J Vet Res. 2004;68: 81–85. [PMC free article] [PubMed] [Google Scholar]
- 23. Niven DF, Donga J, Archibald FS. Responses of Haemophilus pleuropneumoniae to iron restriction: changes in the outer membrane protein profile and the removal of iron from porcine transferrin. Mol Microbiol. 1989;3: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 24. Deneer HG, Potter AA. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae . Infect Immun. 1989;57: 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Archambault M, Labrie J, Rioux CR, Dumas F, Thibault P, Elkins C, et al. Identification and preliminary characterization of a 75-kDa hemin- and hemoglobin-binding outer membrane protein of Actinobacillus pleuropneumoniae serotype 1. Can J Vet Res. 2003;67: 271–277. [PMC free article] [PubMed] [Google Scholar]
- 26. D'Silva CG, Archibald FS, Niven DF. Comparative study of iron acquisition by biotype 1 and biotype 2 strains of Actinobacillus pleuropneumoniae . Vet Microbiol. 1995;44: 11–23. [DOI] [PubMed] [Google Scholar]
- 27. Diarra MS, Dolence JA, Dolence EK, Darwish I, Miller MJ, Malouin F, et al. Growth of Actinobacillus pleuropneumoniae is promoted by exogenous hydroxamate and catechol siderophores. Appl Environ Microbiol. 1996;62: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beddek AJ, Sheehan BJ, Bosse JT, Rycroft AN, Kroll JS, Langford PR. Two TonB Systems in Actinobacillus pleuropneumoniae: Their Roles in Iron Acquisition and Virulence. Infection and Immunity. 2004;72: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chin N, Frey J, Chang CF, Chang YF. Identification of a locus involved in the utilization of iron by Actinobacillus pleuropneumoniae . FEMS Microbiol Lett. 1996;143: 1–6. [DOI] [PubMed] [Google Scholar]
- 30. Srikumar R, Mikael LG, Pawelek PD, Khamessan A, Gibbs BF, Jacques M, et al. Molecular cloning of haemoglobin-binding protein HgbA in the outer membrane of Actinobacillus pleuropneumoniae . Microbiology. 2004;150: 1723–1734. [DOI] [PubMed] [Google Scholar]
- 31. Mikael LG, Pawelek PD, Labrie J, Sirois M, Coulton JW, Jacques M. Molecular cloning and characterization of the ferric hydroxamate uptake (fhu) operon in Actinobacillus pleuropneumoniae . Microbiology. 2002;148: 2869–2882. [DOI] [PubMed] [Google Scholar]
- 32. Xu Z, Zhou Y, Li L, Zhou R, Xiao S, Wan Y, et al. Genome biology of Actinobacillus pleuropneumoniae JL03, an isolate of serotype 3 prevalent in China. PLoS ONE. 2008;3: e1450 10.1371/journal.pone.0001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deslandes V, Nash JH, Harel J, Coulton JW, Jacques M. Transcriptional profiling of Actinobacillus pleuropneumoniae under iron-restricted conditions. BMC Genomics. 2007;8: 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li L, Xu Z, Zhou Y, Sun L, Liu Z, Chen H, et al. Global effects of catecholamines on Actinobacillus pleuropneumoniae gene expression. PLoS One. 2012;7: e31121 10.1371/journal.pone.0031121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bei W, He Q, Yan L, Fang L, Tan Y, Xiao S, et al. Construction and characterization of a live, attenuated apxIICA inactivation mutant of Actinobacillus pleuropneumoniae lacking a drug resistance marker. FEMS Microbiol Lett. 2005;243: 21–27. [DOI] [PubMed] [Google Scholar]
- 36. Li L, Xu Z, Zhou Y, Li T, Sun L, Chen H, et al. Analysis on Actinobacillus pleuropneumoniae LuxS regulated genes reveals pleiotropic roles of LuxS/AI-2 on biofilm formation, adhesion ability and iron metabolism. Microb Pathog. 2011;50: 293–302. 10.1016/j.micpath.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 37. Wagner TK, Mulks MH. A subset of Actinobacillus pleuropneumoniae in vivo induced promoters respond to branched-chain amino acid limitation. FEMS Immunol Med Microbiol. 2006;48: 192–204. [DOI] [PubMed] [Google Scholar]
- 38. Freestone PP, Haigh RD, Lyte M. Blockade of catecholamine-induced growth by adrenergic and dopaminergic receptor antagonists in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica . BMC Microbiol. 2007;7: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu Z, Chen X, Li L, Li T, Wang S, Chen H, et al. Comparative genomic characterization of Actinobacillus pleuropneumoniae . J Bacteriol. 2010;192: 5625–5636. 10.1128/JB.00535-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lone AG, Deslandes V, Nash JH, Jacques M, Macinnes JI. Modulation of gene expression in Actinobacillus pleuropneumoniae exposed to bronchoalveolar fluid. PLoS One. 2009;4: e6139 10.1371/journal.pone.0006139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li L, Zhou R, Li T, Kang M, Wan Y, Xu Z, et al. Enhanced biofilm formation and reduced virulence of Actinobacillus pleuropneumoniae luxS mutant. Microb Pathog. 2008;45: 192–200. 10.1016/j.micpath.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 42. Freestone PP, Lyte M, Neal CP, Maggs AF, Haigh RD, Williams PH. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J Bacteriol. 2000;182: 6091–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Armstrong SK, Brickman TJ, Suhadolc RJ. Involvement of multiple distinct Bordetella receptor proteins in the utilization of iron liberated from transferrin by host catecholamine stress hormones. Mol Microbiol. 2012;84: 446–462. 10.1111/j.1365-2958.2012.08032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klitgaard K, Friis C, Angen O, Boye M. Comparative profiling of the transcriptional response to iron restriction in six serotypes of Actinobacillus pleuropneumoniae with different virulence potential. BMC Genomics. 2010;11: 698 10.1186/1471-2164-11-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM using an inoculation dose of 104 CFU/ml. Catecholamines at concentrations ranging from 0.1μM to 50μM were added into CDM containing 1/40 of serum. Optical densities of bacterial cultures (OD600nm) were recorded at early stationary phase (12 hours after sub-culture). Data are from one test out of three similar results.
(TIF)
A. pleuropneumoniae was cultured in TSB medium overnight and then sub-cultured into CDM containing 1/40 of serum (S) using an inoculation dose of 104 CFU/ml. Epi (A), NE (B) and DA (C) at 50μM were supplemented into the serum-containing medium. The eukaryotic α-adrenergic receptor antagonist phentolamine (PE), β- adrenergic receptor antagonist propranolol (PO) and the non-selective dopaminergic receptor antagonist haloperidol (Hal) at the concentration of 50μM were separately added into the serum medium containing different catecholamines. A. pleuropneumoniae cultured in CDM without any supplementation was used as a control (CDM). Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. Data are shown as means ± SD from three independent replications.
(TIF)
A. pleuropneumoniae parental strain 4074 and the mutants (ΔqseB and ΔqseC) were cultured in TSB medium overnight and then sub-cultured in CDM containing 1/40 of serum with or without 50μM of different catecholamines (+ Epi/NE/DA). The inoculation dose was 104 CFU/ml for sub-culture. Optical densities of bacterial cultures (OD600nm) were recorded at selected time points. Data are shown as means ± SD from three independent replications.
(TIF)
Mean log2 ratios obtained from microarray results are plotted against the mean log2 ratios obtained from qRT-PCR. 1–4: gene tonB2 regulated by serum, Epi, NE and DA; 5–8: gene tonB1 regulated by serum, Epi, NE and DA, 9–12: gene tbpA1 regulated by serum, Epi, NE and DA; 13–16: gene qseC regulated by serum, Epi, NE and DA; 17–20: gene fur regulated by serum, Epi, NE and DA.
(TIF)
Up or down means up or down-regulated by serum/Epi/NE/DA. Gene functions were sorted according to COG categories: C: Energy production and conversion; D: Cell cycle control, cell division, chromosome partitioning; E: Amino acid transport and metabolism; F: Nucleotide transport and metabolism; G: Carbohydrate transport and metabolism; H: Coenzyme transport and metabolism; I: Lipid transport and metabolism; J: Translation, ribosomal structure and biogenesis; K: Transcription; A, RNA processing and modification; L: Replication, recombination and repair; M: Cell wall/membrane/envelope biogenesis; O: Posttranslational modification, protein turnover, chaperones; P: Inorganic ion transport and metabolism; Q: Secondary metabolites biosynthesis, transport and catabolism; R: General function prediction only; T: Signal transduction mechanisms; U: Intracellular trafficking, secretion, vesicular transport; V: Defense mechanisms; S/N: Function unknown in COG.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.