Summary
Advances in the care of neonatal hyperbilirubinemia have decreased the incidence of kernicterus. However, neonatal exposure to high levels of bilirubin continues to cause severe motor symptoms and cerebral palsy (CP). Exposure to moderate levels of unconjugated bilirubin may also cause damage to the developing central nervous system, specifically the basal ganglia and cerebellum. Brain lesions identified using magnetic resonance imaging following extreme hyperbilirubinemia have been linked to dyskinetic CP. Newer imaging techniques, such as diffusion tensor imaging or single-photon emission computed tomography, allow quantification of more subtle white matter injury following presumed exposure to unbound bilirubin, and may explain more subtle movement disorders. New categories of bilirubin-induced neurologic dysfunction, either independently or characterized by subtle bilirubin encephalopathy, following moderate hyperbilirubinemia have been implicated in long-term motor function. Further research is needed to identify subtle impairments resulting from moderate to severe neonatal hyperbilirubinemia, understand the influence of perinatal risk factors on bilirubin toxicity, and develop neuroprotective treatment strategies to prevent movement disorders due to bilirubin toxicity.
Keywords: Kernicterus, Neonatology, Cerebral palsy, Gait, Preterm infants, Neurodevelopment
1. Introduction
Advances in the care of infants with severe hyperbilirubinemia have greatly reduced the incidence of classic kernicterus and possibly of bilirubin-induced neurologic dysfunction (BIND) [1,2]. Despite significant progress, subtle neurological impairments and motor disorders resulting from severe hyperbilirubinemia continue to occur in children around the world. A recent rise in the incidence of kernicterus, after decades of improvement, may be attributable to relaxed standards for initiation of phototherapy, or due to earlier infant discharge from the hospital [1–3]. Furthermore, the subtler symptoms of BIND may be under-recognized and contribute to increased risk of motor impairment such as developmental coordination disorder and learning disabilities [4]. The incidence of kernicterus in the USA is estimated to be 1 in 40,000 births, with about 1 in 650 to 1000 neonates born >35 weeks' postmenstrual age (PMA) experiencing transient hyperbilirubinemia (>25 mg/dL) [5]. Bilirubin has a neurotoxic effect on brain regions including the globus pallidus and subthalamic nuclei, which result in motor-related sequelae ranging from lack of coordination to severe movement disorders such as dyskinetic cerebral palsy (CP). Clinical manifestations of kernicterus vary in form and severity depending on degree of prematurity and stage of regional brain development, degree, and duration of hyperbilirubinemia, as well as other perinatal risk factors such as sepsis, hypoalbuminemia, and genetic predispositions. Regional brain development and vulnerability determine periods during which moderate hyperbilirubinemia could result in auditory versus motor-predominant symptoms of BIND. These manifestations are distinct form those associated with exposure to high levels of neonatal total serum/plasma bilirubin (TB) that are known to cause severe neonatal motor symptoms and long-term sequelae such as dyskinetic CP. This article describes the etiology of the spectrum of motor symptoms and presents emerging evidence on the range of motor outcome that may result from exposure of the developing central nervous system (CNS) to low–moderate levels of TB. Understanding the etiology and scope of motor sequelae is essential to development of neuroprotective treatment protocols and early identification of motor disorders related to moderate–severe hyperbilirubinemia.
2. History of kernicterus and motor impairment
In the mid-twentieth century, the effects of bilirubin on the CNS were substantially unknown and methods to prevent kernicterus were limited. Physicians observed significant motor impairments following severe hyperbilirubinemia in neonates and subsequently began to learn how bilirubin specifically targeted motor systems in the developing brain [6]. Classic clinical symptoms of kernicterus included lethargy, high-pitched crying, seizures, eye weakness (ophthalmoplegia), and truncal arching (opisthotonus) [7,8]. Involvement of the CNS suggested that bilirubin was crossing the blood–brain barrier (BBB) and damaging neural structures related to movement. Identifying the causes of hyperbilirubinemia, and some of the related risk factors predisposing infants to kernicterus, helped improve the understanding of the pathophysiology of bilirubin toxicity in the CNS.
3. Neonatal bilirubin metabolism and kernicterus
As the pathobiology of kernicterus became clear, concurrent advances in treatments such as phototherapy helped prevent damage to the developing CNS. Pediatricians monitor TB levels to prevent infants from excess or prolonged exposure to bilirubin. TB is composed of conjugated and unconjugated bilirubin, but in neonates, and particularly those preterm, the unconjugated form dominates due to low activity of the enzyme uridine 5′-diphospho-glucuronosyltransferase (UDPGT), or glucuronyltransferase [7]. As a water-insoluble compound, unconjugated bilirubin travels in the blood bound to proteins such as albumin. The level of albumin is low in the neonate and has a high degree of physiological variation, but increases with gestational age-at-birth and birth weight [9]. When the binding affinity of bilirubin–albumin is decreased, as occurs in medically unstable neonates, or the carrying proteins become saturated, bilirubin exists in its unbound (UB) or “free” toxic form and is able to cross the BBB [10,11]. Albumin-bound bilirubin cannot reach the brain unless the BBB becomes disrupted.
4. Clinical measures of hyperbilirubinemia and BIND
Measuring peak TB may not be the most predictive of outcome. Shapiro [8] and others have suggested that measuring free bilirubin in addition to TB may be important for identifying infants at higher risk for BIND and/or kernicterus. Though difficult, measuring UB may be more prognostic; Oh et al. demonstrated that, in extremely low birth weight (ELBW) neonates, UB is directly related to poor outcomes including CP [11]. Furthermore, UB concentrations are less dependent on clinical status, and thus may be more instructive for clinical decision-making [11]. Among clinically stable neonates, Oh et al. found an inverse relationship between peak TB and incidence of CP or death, suggesting that at moderate levels, bilirubin may have an antioxidant property that provides neuroprotection. More research is needed, however, to understand the possible neuroprotective value of low TB levels.
Measuring the total bilirubin/albumin molar ratio (BAMR) may be more clinically feasible and has been documented to be highly correlated with UB [12]. Furthermore, the BAMR was demonstrated to be more correlated with psychomotor outcome at four years compared to other measures of bilirubin [4]. However, another recent study by Hulzebos et al. [13] found that clinical use of BAMR, instead of TB thresholds, had no effect on neurodevelopmental outcome at 18–24 months of age [13].
Neonatal inflammation and sepsis are known to influence hyperbilirubinemia and subsequent sequelae [14]. During an acute inflammatory response such as neonatal sepsis, albumin production decreases [15]. Neonatal albumin levels have been demonstrated to have an inverse relationship with the inflammatory biomarker, C-reactive protein (CRP), as routinely measured over the first two weeks of life in neonates born preterm with very low birth weight (VLBW) [16], consistent with prior findings. Using diffusion tensor imaging (DTI), Rose et al. [16] also found an association between lower neonatal albumin levels and reduced microstructual development of the thalamus at near-term age, as measured with higher thalamus mean diffusivity (MD) on DTI [16]. Furthermore, lower neonatal albumin levels and higher near-term thalamus MD correlated with poorer motor performance as assessed with Bayley Scores of Toddler and Infant Development, 3rd edition (BSID-III) at 18–22 months of age, adjusted for prematurity (J. Rose et al., unpublished data). Figure 1 shows mean neonatal serum albumin levels in relation to near-term thalamus MD. Understanding these associations may provide neonatal clues to mechanisms that mediate later movement disorders related to bilirubin toxicity.
Fig. 1.
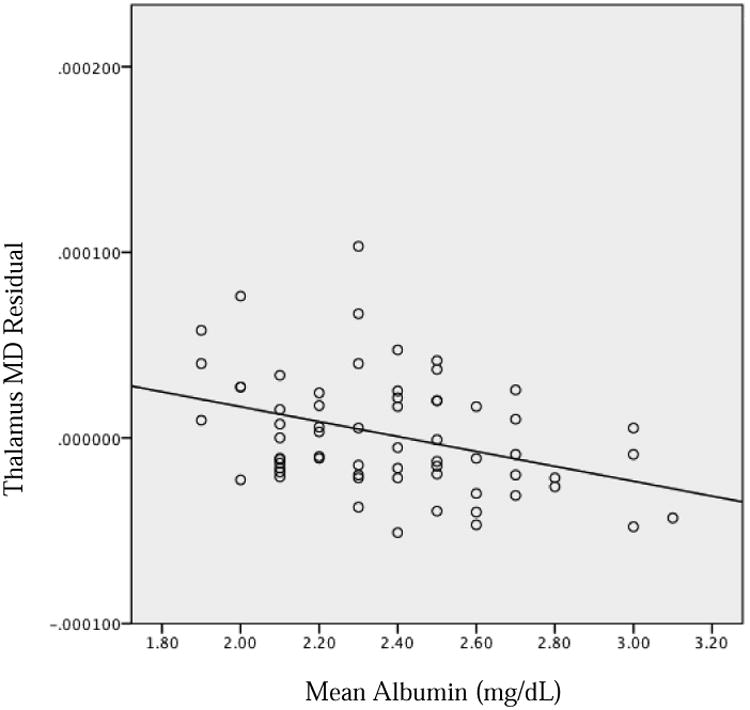
Near-term right thalamus mean diffusivity, controlling for postmentstrual age at scan, in relation to mean serum albumin, measured over the first two postnatal weeks. r = −368; P = 0.003.
The thalamus is known to be involved in basic motor function and is implicated in kernicterus following hyperbilirubinemia, so the connection between hypoalbuminemia, thalamic microstructural damage, and future motor impairment is plausible from a physiological perspective, and needs further study in larger populations.
5. Severity of hyperbilirubinemia
Whereas identification and treatment of hyperbilirubinemia have resulted in lower rates of severe kernicterus, infants with TB levels previously thought to be safe have been found to exhibit signs of neurological impairment. A safe TB level in neonates has not yet been established, as multiple factors may affect bilirubin–albumin binding and increase an infant's risk for BIND, with relatively low levels of TB. Unconjugated bilirubin is toxic to the developing brain and may be elevated even in the presence of relatively low TB. Several studies have observed increased risk for motor and cognitive impairments following moderate TB levels in neonates [17], though the association between minor neurological/behavioral problems and moderate bilirubin exposure continues to be debated [18].
Cerebral palsy and other movement disorders have been known to occur in children born preterm after exposure to relatively low–moderate levels of TB [3,19]. Though most infants experience a degree of hyperbilirubinemia due to impaired bilirubin–albumin binding, infants born preterm have even less albumin available to bind to conjugated bilirubin, thus causing a disproportionate amount of UB for a given level of bilirubin production. Further risk factors beyond prematurity include acidosis, sepsis, hypothermia, hematological/genetic abnormalities intraventricular hemorrhage, use of drugs that bind to albumin, and hypoxia [19]. Hypoxia may have additive toxic effects in combination with hyperbilirubinemia, as hypoxia before or at the time of elevated TB has been demonstrated to increase glial cell apoptosis and necrosis [20]. Acidosis may contribute to increased toxicity of bilirubin due to increased cerebral blood flow, and acid–base changes in the bilirubin molecule allowing it to be partially polar [21]. Exposure to any of these risk factors may lower the level at which bilirubin may be toxic to the developing brain.
6. Timing of hyperbilirubinemia
Studies have not yet identified exact time periods during which hyperbilirubinemia will result in auditory vs. motor-predominant symptoms of BIND, but since the auditory pathways generally develop before extrapyramidal motor pathways in the brain, earlier exposure to hyperbilirubinemia may result primarily in auditory symptoms. The neurons undergoing early differentiation are most vulnerable to cell damage and death from bilirubin neurotoxicity, whereas more highly matured neurons may be more likely spared from damage. This was demonstrated in a rat model, in which timing of bilirubin exposure determined degree of cerebellar neuronal damage [22]. Timing of the hyperbilirubinemia, and specifically the peak TB, may predict the accompanying symptoms [23]. For example, it has been hypothesized that preterm infants may be more likely to experience auditory impairments, whereas term infants may more frequently develop CP with associated motor abnormalities and cerebellar damage, due to the timing of the bilirubin toxicity in relation to the developing brain regions [7].
7. Neuroanatomy of bilirubin-associated motor impairment
Original autopsy studies of kernicterus identified yellow staining and necrosis of the basal ganglia, specifically in the globus pallidus, indicative of UB crossing the BBB. Other regions noted to have cellular damage after hyperbilirubinemia include the substantia nigra reticulata, subthalamic nuclei, vestibular and oculomotor nuclei, hippocampus, and cerebellar Purkinje cells. Loss of neurons, decreased myelination, and gliosis can be observed in the internal and external globus pallidus and subthalamic nuclei. These regions all have functions related to movement, balance, and posture regulation and appear to be selectively vulnerable. The globus pallidus may also serve to integrate inputs from multiple systems including somatosensory and motor pathways; thus, integration of this information may be damaged in patients with kernicterus [8]. By contrast, regions such as the striatum and thalamus generally appear to be spared following hyperbilirubinemia. This specific pattern of brain injury helps distinguish BIND from injury following hypoxic–ischemia, in which the caudate, putamen, posterior limb of the internal capsule, and cortex are more vulnerable. One theory for the selective vulnerability of the globus pallidus and subthalamic nuclei is their relatively high resting levels of neuronal activity observed in the neonatal brain, compared to nearby regions. Johnston et al. [24] observed that regions with higher neuronal activity are more vulnerable to oxidative stressors or toxins such as bilirubin. Lesions in these regions are observed in infants with classic athetotic CP. One hypothesis for the connection between globus pallidus injury and athetosis is that reduced activity of the globus pallidus results in decreased inhibitory input to the thalamus, resulting in its “dys-inhibition” and increased motor activity, consistent with athetosis [24].
Regional specificity of kernicterus and brain pathology resulting from mild, acute, and chronic bilirubin encephalopathies has been identified on magnetic resonance imaging (MRI). The globus pallidus, and specifically the posteromedial border, appears to be most sensitive to damage from bilirubin, possibly due to its location in the circuit of the basal ganglia [25]. Regulation of motor function involves basal ganglia circuits which receive input from the motor cortex to the caudate and putamen. The internal globus pallidus contains the output neurons of the basal ganglia, which project through the motor thalamus and back to the motor cortex [26]. Govaert et al. observed permanent damage to the globus pallidus, evident on T2 MRI, after preterm infants had been exposed to TB levels below suggested thresholds [9,21]. However, not all infants exposed to high levels of TB develop neurological symptoms or lasting globus pallidus lesions [25,27]. Lesions in the subthalamic nuclei may be more subtle and challenging to visualize on MRI [8].
There have been few studies on longitudinal microstructural changes in white and gray matter of the brain following hyperbilirubinemia in relation to motor disorders. Gkoltsiu et al. [19] described inconsistent patterns between early imaging abnormalities following (i) bilirubin exposure and (ii) motor outcome [19]. Among children in this cohort who developed CP, all demonstrated classic signs of kernicterus on MRI, i.e., high signal intensity in the globus pallidus on T2 images. Early images, however, were not reliable in their correlation with outcome. Shapiro et al. have found similar MRI patterns demonstrating acute and long-term changes on MRI, specifically bilateral hyperintense lesions in the globus pallidus, corresponding to dystonic kernicterus (Fig. 2) [7,28]. One explanation for the challenge of using early scans is the change of brain tissue following injury, and the resulting changes in appearance on MRI [19,28]. For example, signs of kernicterus on T1 are only visible in the short term following hyperbilirubinemia, but T2 hyperintensities are longer-lasting. Signal hyperintensities on T1 images are characteristic of the acute stages of bilirubin-induced astroglial necrosis, whereas the more permanent signals on T2 may reflect the long-term neuronal loss, demyelination, or gliosis [25,29]. Symmetric involvement of the globus pallidus on T1 has been found to be related to severity of hyperbilirubinemia, and severity of outcome may be predicted by the characteristic switch from hyperintensities visible on T1 to T2 [30]. Normalization of T1 imaging is common in chronic bilirubin encephalopathy.
Fig. 2.
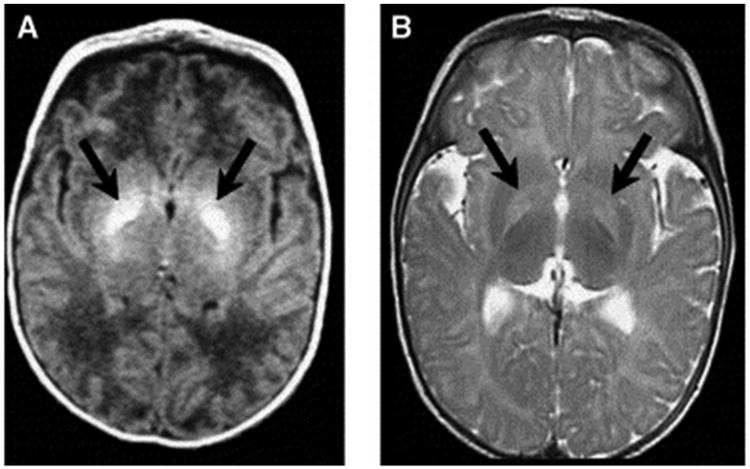
Axial magnetic resonance imaging (MRI) of bilateral hyperintense lesions in the globus pallidus in axial projections (arrows). (A) T1-weighted axial image of a six-day-old, 37-week gestation boy with peak total bilirubin of 34.6 mg/dL. At age seven years, this child was highly intelligent, but moderately to severely disabled with dystonic, athetoid kernicteric cerebral palsy; he ambulates with a walker. (B) Axial T2-weighted MRI of a two-year-old who has classic dystonic kernicterus. Note the increased intensity of the globus pallidus bilaterally (shown with arrows and dotted line on right side only). There were no abnormalities noted in brainstem or cerebellum. (Panel (A) reprinted with permission from Shapiro SM, Bilirubin toxicity in the developing nervous system. Pediatric Neurology 2003;29:414. Panel (B) and Fig. 2 caption reprinted from Shapiro et al. [28].)
Advanced methods have begun to be used to study the brains of infants with kernicterus. Okumura et al. [31] used single-photon emission computed tomography (SPECT) in three children (aged four to six years) born preterm with athetoid CP following kernicterus. All three demonstrated no brain abnormalities on ultrasound during the neonatal period. Though all had identifiable hyperintensities on MRI in the globus pallidus at five to nine months of age, abnormalities were mild in two of the infants, and in one of these two, abnormalities disappeared on follow-up MRI. SPECT was able to identify decreased blood flow to the basal ganglia region in all three patients, suggesting that SPECT may be more useful than MRI in identifying brain abnormalities in older children after neonatal kernicterus [31].
8. Classification of movement disorders due to bilirubin toxicity
Several classification systems have been proposed to describe the variety of motor symptoms that may follow severe neonatal hyperbilirubinemia. Shapiro has proposed a classification for BIND based on location of the injury, severity of symptoms, and timing of the peak of the TB levels [14]. For infants with kernicterus, characterization is based on the type of symptoms, specifically as “auditory predominant” or “motor predominant.” Generally, motor-predominant forms of kernicterus are due to lesions in the external and internal globus pallidus as well as in the subthalamic nuclei. Injury to the Purkinje cells of the cerebellum, and small lesions in the brainstem within the auditory, vestibular, and oculomotor nuclei, also are likely to be present, though current imaging techniques are limited and unable to visualize this small-scale damage. Classic motor symptoms caused by BIND are identified as the athetotic or dyskinetic form of CP and are related to lesions in the globus pallidus and subthalamic nuclei most prominently as well as the cerebellum and brainstem. Specific symptoms may also be involved due to damage to the vestibular nuclei, the interstitial nucleus of Cajal (responsible for upward gaze), and Dieter's nucleus (involved in truncal tone) [8,32,33]. Movement disorders associated with dyskinetic CP are distinct from those of spastic CP, which represents the majority of cases of CP, and involves symptoms of weakness, spasticity, short muscle tendon length, and impaired selective motor control characterized by flexor and extensor synergies. Spastic CP is associated with white matter injury to the corticospinal tract.
Mild kernicterus may manifest with motor symptoms including dystonia with or without athetosis and mild gross motor delays such as late developmental milestones such as age of initiation of walking. These infants generally can ambulate well on their own later in childhood and can speak with some clarity.
Moderate kernicterus may be accompanied by a moderate degree of hyperkinetic dystonia, also classified as athetoid CP. These children may have more difficulty ambulating without assistance due to the choreoathetoid movements. They may also have delayed speech but can benefit from therapy to improve quality of speech.
Severe “motor predominant” kernicterus is characterized by severe dystonia/athetosis preventing controlled voluntary movements including ambulation, speech, and self-feeding. This may also be accompanied by severe hypertonia and muscle cramping. Though some children may experience improvement in disability with time, classification of severity generally remains consistent over time.
Use of the term kernicterus, however, is debated as it has traditionally been used to describe infants exposed to very high TB levels (>20 mg/dL) who develop immediate clinical and neuroradiological signs of the disease. Infants exposed to lower TB levels, not severe enough to cause kernicterus, may have mild damage to the basal ganglia and cerebellum that may manifest as mild hypotonia, lack of coordination, or generalized clumsiness – not severe enough to be classified as a specific movement disorder.
BIND, a term introduced to include a wider spectrum of infants with clinical symptoms associated with moderate hyperbilirubinemia, has been associated with decreased sucking behavior, lethargy, hypotonia, or stupor [14]. Abnormal tone in the limbs is often observed during acute injury or as post-icteric sequelae; symptoms include alternating hypotonia and hypertonia, along with opisthotonus and retrocollis. Another classic sign is the “setting sun sign,” a term to describe the downward gaze of the eyes due to damage to the nuclei controlling upward gaze, which may be associated with a mask-like facies. This acute phase will likely correspond to hyperintensity in the globus pallidus on T1 images. After the first week of acute phase of injury, hypertonia will sometimes but not always predominate.
The transition to chronic sequelae is accompanied by a progression of symptomatology. Kernicterus is reserved for a diagnosis with a combination of clinical features and MRI findings. The classic diagnosis includes a tetrad of features: (1) dystonia and/or athetosis; (2) hearing impairment or deafness; (3) lack of upward gaze; and (4) dental enamel dysplasia, along with abnormal MRI findings in the globus pallidus and subthalamic nuclei. Other less common accompanying symptoms may include hypotonia or sensorimotor abnormalities, and oculomotor abnormalities may vary and include strabismus or other misalignment of the eyes [3,7,14,23].
Long-term outcomes of infants with kernicterus vary widely based on severity as well as a variety of risk factors described previously. Increased incidence of dyskinetic CP occurs following high bilirubin exposure in full-term infants or low–moderate levels of bilirubin exposure in preterm infants [3,19]. CP was most recently defined by an international committee as “a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain [34]. The motor disorders of CP are often accompanied by disturbances of sensation, perception, cognition, communication, behavior, by epilepsy, and by secondary musculoskeletal problems” [35,36].
The subtype of CP attributed to bilirubin neurotoxicity is best classified as dyskinetic CP to encompass the dystonic and athetotic movements that occur [3]. The definition and distinction between athetosis and dystonia has been heavily debated. Athetosis has been used to describe children with dyskinesia with involuntary movements as well as lack of postural control [3,7]. Przekop et al. suggests that “choreoathetosis” may be a better term to use to describe the athetotic movements observed in children, to distinguish it from athetosis in adults characterized by slow writhing, often in the fingers or toes. Athetosis in infants is characterized by “slow continuous writhing movements that prevent maintenance of a stable posture” [34], as defined by the 2008 Taskforce on Childhood Movement Disorders, whereas dystonia is more defined by hypertonic postures [6,34,37]. Shapiro has observed that infants with CP related to kernicterus rarely present initially with fixed postures or contractures. In addition, cognitive function is generally preserved due to limited injury to cortical and subcortical white matter pathways [14].
Activities of everyday life are often compromised in children with moderate–severe cerebral palsy. Speech is challenging, particularly for children with dyskinetic CP that also includes deafness or hearing impairment. Swallowing and eating can also be challenging due to spasms of pharyngeal muscles and involuntary movements of the tongue. Aspiration is a risk and children with severe CP may need gastrostomy tubes to supplement their caloric intake [3].
9. Therapies available for BIND-related movement disorders
There is currently no specific treatment for dyskinetic CP, but supportive therapy can be instituted to improve symptoms. Dystonia can be managed with a combination of physical, occupational, and speech therapies. The hypertonia generally disappears with sleep, and botulinum toxin (Botox) can be used to relax muscle tone. Orthopedic procedures, including tendon-lengthening as typically used to treat patients with spastic CP, are not indicated for dyskinetic CP, and medication and Botox injections are preferred [8]. For severe forms of dystonia, baclofen pumps may be useful in reducing muscle tone and severity of muscle spasms.
Due to hypertonia and hyperkinetic activity, infants with dyskinetic CP may have higher caloric requirements, complicated by frequent challenges in swallowing or self-feeding. Decreased or abnormal motility of gastrointestinal muscles can complicate digestion, creating further challenges with adequate nutrition.
Based on success of deep-brain stimulation (DBS) in other movement disorders such as Parkinson disease, DBS has been suggested as a possible therapy for dyskinetic CP. Stimulators placed in the globus pallidus internus may help control dystonia, but a limited number of trials has demonstrated only small improvements in motor symptoms. Results following DBS in patients with dystonia have been most effective in certain subgroups, such as individuals with genetic-based dystonia caused by a DYT1 mutation, but less effective in individuals with secondary dystonia [38]. Kim et al. demonstrated improvements in dystonia in adults with CP, but little improvement in overall disability measures [39].
10. Conclusion
The link between exposure to elevated, neurotoxic TB levels and severe neonatal motor symptoms and dyskinetic CP is well established. However, the influence of exposure to low– moderate levels of TB on the developing CNS is not well understood. Further research is needed to identify the range of motor impairments that may result from neonatal hyperbilirubinemia, to understand the interplay between perinatal risk factors and bilirubin toxicity, and to develop improved neuroprotective treatment for motor disorders related to hyperbilirubinemia.
Practice points
Though classic kernicterus has become rare, BIND still occurs in developed countries due to relaxed standards and earlier neonatal discharge.
Moderate levels of neonatal bilirubin exposure may contribute to more mild movement disorders such as developmental coordination disorder.
Imaging modalities such as MRI, DTI, and SPECT can help identify brain abnormalities following bilirubin exposure and may correlate with functional outcome.
Research directions
Albumin and inflammatory proteins may mediate exposure of the developing brain to free bilirubin; further investigation may clarify their role as neonatal physiological risk factors for BIND and determine implications for neuroprotective treatment.
Systematic and longitudinal imaging of the neonatal brain following moderate–severe bilirubin exposure would improve understanding of temporal changes of brain architecture and microstructure, and may inform delivery of neuroprotective treatment.
Acknowledgments
We thank Dr David K. Stevenson and Dr Susan R. Hintz for valuable discussions.
Funding sources: This material is based upon work supported by the Mary Baracchi Research Fund, Lucile Packard Children's Hospital Stanford, by the NIH Clinical and Translational Science Award UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) and for the Stanford Center for Clinical Informatics and Stanford Translational Research Integrated Database Environment (STRIDE), and by the Lucile Packard Foundation for Children's Health.
Footnotes
Conflict of interest statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the jaundiced neonate. New York: McGraw-Hill Medical; 2012. [Google Scholar]
- 2.Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr. 2002;140:396–403. doi: 10.1067/mpd.2002.123098. [DOI] [PubMed] [Google Scholar]
- 3.Przekop A, Sanger TD. Birth-related syndromes of athetosis and kernicterus. Handb Clin Neurol. 2011;100:387–95. doi: 10.1016/B978-0-444-52014-2.00030-6. [DOI] [PubMed] [Google Scholar]
- 4.Johnson L, Bhutani VK. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol. 2011;35:101–13. doi: 10.1053/j.semperi.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Bhutani VK, Johnson L. Kernicterus in the 21st century: frequently asked questions. J Perinatol. 2009;29:S20–S24. doi: 10.1038/jp.2008.212. [DOI] [PubMed] [Google Scholar]
- 6.Foley J. The athetoid syndrome. A review of a personal series. J Neurol Neurosurg Psychiatry. 1983;46:289–98. doi: 10.1136/jnnp.46.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro SM. Bilirubin toxicity in the developing nervous system. Pediatr Neurol. 2003;29:410–21. doi: 10.1016/j.pediatrneurol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro SM. Kernicterus. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the jaundiced neonate. New York: McGraw-Hill Medical; 2012. [Google Scholar]
- 9.Ahlfors CE. Criteria for exchange transfusion in jaundiced newborns. Pediatrics. 1994;93:488–94. [PubMed] [Google Scholar]
- 10.Cashore WJ, Oh W, Brodersen R. Reserve albumin and bilirubin toxicity index in infant serum. Acta Paediatr Scand. 1983;72:415–19. doi: 10.1111/j.1651-2227.1983.tb09739.x. [DOI] [PubMed] [Google Scholar]
- 11.Oh W, Stevenson D, Tyson J, et al. Influence of clinical status on the association between plasma total and unbound bilirubin and death or adverse neurodevelopmental outcomes in extremely low birth weight infants. Acta Paediatr. 2010;99:673–8. doi: 10.1111/j.1651-2227.2010.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato Y, Morioka I, Miwa A, et al. Is bilirubin/albumin ratio correlated with unbound bilirubin concentration? Pediatr Int. 2012;54:81–5. doi: 10.1111/j.1442-200X.2011.03457.x. [DOI] [PubMed] [Google Scholar]
- 13.Hulzebos CV, Dijk PH, van Imhoff DE, et al. the bilirubin albumin ratio in the management of hyperbilirubinemia in preterm infants to improve neurodevelopmental outcome: a randomized controlled trial – BARTrial. In: Carlo WA, editor. PLoS ONE. Vol. 9. 2014. p. e99466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND) J Perinatol. 2005;25:54–9. doi: 10.1038/sj.jp.7211157. [DOI] [PubMed] [Google Scholar]
- 15.Black S. C-reactive Protein. J Biol Chem. 2004;279:48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 16.Rose J, Vassar R, Cahill-Rowley K, et al. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. NeuroImage Clin. 2014;5:169–77. doi: 10.1016/j.nicl.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soorani-Lunsing I, Woltil HA, Hadders-Algra M. Are moderate degrees of hyperbilirubinemia in healthy term neonates really safe for the brain? Pediatr Res. 2001;50:701–5. doi: 10.1203/00006450-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Lunsing RJ, Pardoen WFH, Hadders-Algra M. Neurodevelopment after moderate hyperbilirubinemia at term: a prospective, case–control study. Pediatr Res. 2013;73:655–60. doi: 10.1038/pr.2013.28. [DOI] [PubMed] [Google Scholar]
- 19.Gkoltsiou K, Tzoufi M, Counsell S, Rutherford M, Cowan F. Serial brain MRI and ultrasound findings: relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Hum Dev. 2008;84:829–38. doi: 10.1016/j.earlhumdev.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Falcão AS, Silva RFM, Fernandes A, Brito MA, Brites D. Influence of hypoxia and ischemia preconditioning on bilirubin damage to astrocytes. Brain Res. 2007;1149:191–9. doi: 10.1016/j.brainres.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Govaert P, Lequin M, Swarte R, et al. Changes in globus pallidus with (pre)term kernicterus. Pediatrics. 2003;112(6 Pt 1):1256–63. doi: 10.1542/peds.112.6.1256. [DOI] [PubMed] [Google Scholar]
- 22.Conlee JW, Shapiro SM. Development of cerebellar hypoplasia in jaundiced Gunn rats: a quantitative light microscopic analysis. Acta Neuropathol (Berl) 1997;93:450–60. doi: 10.1007/s004010050639. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157–63. doi: 10.1016/j.siny.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Johnston MV, Hoon AH. Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J Child Neurol. 2000;15:588–91. doi: 10.1177/088307380001500904. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz Y, Alper G, Kilicoglu G, Celik L, Karadeniz L, Yilmaz-Degirmenci S. Magnetic resonance imaging findings in patients with severe neonatal indirect hyperbilirubinemia. J Child Neurol. 2001;16:452–5. doi: 10.1177/088307380101600615. [DOI] [PubMed] [Google Scholar]
- 26.Nolte J. The human brain: an introduction to its functional anatomy. 6th. Philadelphia, PA: Mosby/Elsevier; 2009. [Google Scholar]
- 27.Coskun A, Yikilmaz A, Kumandas S, Karahan OI, Akcakus M, Manav A. Hyperintense globus pallidus on T1-weighted MR imaging in acute kernicterus: is it common or rare? Eur Radiol. 2005;15:1263–7. doi: 10.1007/s00330-004-2502-2. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro SM, Bhutani VK, Johnson L. Hyperbilirubinemia and kernicterus. Clin Perinatol. 2006;33:387–410. doi: 10.1016/j.clp.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Martich-Kriss V, Kollias SS, Ball WS. MR findings in kernicterus. Am J Neuroradiol. 1995;16(4 Suppl):819–21. [PMC free article] [PubMed] [Google Scholar]
- 30.Mao J, Fu J, Chen L, Wang X, Xue X. Changes of globus pallidus in the newborn infants with severe hyperbilirubinemia. Chin J Pediatr. 2007;45:24–9. [PubMed] [Google Scholar]
- 31.Okumura A, Hayakawa F, Maruyama K, Kubota T, Kato K, Watanabe K. Single photon emission computed tomography and serial MRI in preterm infants with kernicterus. Brain Dev. 2006;28:348–52. doi: 10.1016/j.braindev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Shaia WT, Shapiro SM, Heller AJ, Galiani DL, Sismanis A, Spencer RF. Immunohistochemical localization of calcium-binding proteins in the brainstem vestibular nuclei of the jaundiced Gunn rat. Hear Res. 2002;173:82–90. doi: 10.1016/s0378-5955(02)00631-7. [DOI] [PubMed] [Google Scholar]
- 33.Spencer RF, Shaia WT, Gleason AT, Sismanis A, Shapiro SM. Changes in calcium-binding protein expression in the auditory brainstem nuclei of the jaundiced Gunn rat. Hear Res. 2002;171:129–41. doi: 10.1016/s0378-5955(02)00494-x. [DOI] [PubMed] [Google Scholar]
- 34.Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25:1538–49. doi: 10.1002/mds.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 36.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 37.Foley J. Dyskinetic and dystonic cerebral palsy and birth. Acta Paediatr. 1992;81:57–60. doi: 10.1111/j.1651-2227.1992.tb12079.x. discussion 93–94. [DOI] [PubMed] [Google Scholar]
- 38.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010;81:1383–9. doi: 10.1136/jnnp.2010.207993. [DOI] [PubMed] [Google Scholar]
- 39.Kim AR, Chang JW, Chang WS, Park ES, Cho SR. Two-year outcomes of deep brain stimulation in adults with cerebral palsy. Ann Rehabil Med. 2014;38:209. doi: 10.5535/arm.2014.38.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]


