Abstract
Objective:
To describe acute EEG findings in HIV-infected adults with new-onset seizure, assess baseline clinical characteristics associated with EEG abnormalities, and evaluate the relationship between EEG abnormalities and recurrent seizure.
Methods:
Eighty-one HIV-infected adults with new-onset seizure had EEG recordings during their index admission. Baseline characteristics assessed included HIV stage, seizure semiology, serum and CSF studies, neuroimaging, cognitive function based on the Zambian Mini-Mental State Examination and International HIV Dementia Scale, and psychiatric symptoms using the Shona Symptom Questionnaire. We evaluated the relationship between baseline characteristics and EEG abnormalities. Patients were followed for seizure recurrence, and the association between acute EEG abnormalities and seizure recurrence was assessed. Death was a secondary outcome.
Results:
Fifty-five patients had abnormal EEGs (68%): 18 (22%) had interictal spikes (12) or a recorded seizure (6). Among baseline clinical characteristics, more advanced HIV disease (p = 0.039) and any imaging abnormality (p = 0.027) were associated with abnormal EEGs. Cortical (p = 0.008) and white matter (p = 0.004) abnormalities were associated with slow posterior dominant rhythm. Patients were followed for a median of 303 days (interquartile range 103–560). Twenty-four (30%) died and 23 (28%) had recurrent seizures. EEG abnormalities were not associated with recurrent seizure. There was a nonsignificant association between seizures recorded during EEG and death (67% vs 26%, p = 0.051).
Conclusions:
EEG abnormalities are common in this population, particularly in patients with imaging abnormalities and advanced HIV. Acute EEG abnormalities were not associated with recurrent seizure, but high mortality rates during follow-up limited this analysis.
More-affordable equipment, increasing local expertise, and digital transmission for offsite interpretation are enhancing EEG access in resource-limited settings. Patients with HIV infection may especially benefit from improved EEG availability because they are at increased risk of seizures from CNS opportunistic infections (OIs) in addition to common, non-HIV–related factors such as metabolic disturbances.1 Hospital-based cohort studies suggest that 2% to 13% of HIV-infected (HIV+) adults present with new-onset seizure.2–5
Although some studies have reported EEG findings in HIV+ individuals in developed regions,5–7 data are limited for high HIV prevalence areas such as sub-Saharan Africa.8 Previous research has shown that patients with advanced HIV infection demonstrate more EEG abnormalities than asymptomatic patients7,9,10 and that EEG abnormalities correlate with underlying CNS dysfunction in HIV.11–13 As a result, EEG patterns in HIV+ African patients with new-onset seizure might differ from those in developed regions because of more advanced, prolonged immunosuppression and higher prevalence of endemic infections, such as tuberculosis. Abnormal EEGs have been reported in 24 of 37 (67%) HIV+ South Africans14 and 18 of 24 (75%) HIV+ Cameroonians8 with new-onset seizure, but specific patterns were neither described nor associated with long-term health outcomes. Whether EEG abnormalities predict future outcomes, such as recurrent seizure, remains unclear.15
To assess the value of EEG in a resource-limited setting with high HIV prevalence, we report the EEG findings from 81 HIV+ Zambian adults with new-onset seizure enrolled in the longitudinal Cohort Study of HIV-Associated Seizure and Epilepsy (CHASE). We assessed the association between baseline clinical characteristics and EEG abnormalities and evaluated whether acute EEG abnormalities are associated with subsequent seizure recurrence as well as death.
METHODS
From August 1, 2011, to June 19, 2013, we enrolled HIV+ adults who presented with new-onset seizure to inpatient and outpatient units at the University Teaching Hospital in Lusaka, Zambia. Inclusion criteria were age 18 years or older, HIV+, new-onset seizure within the last 2 weeks, and no seizure history except childhood febrile seizures. During initial recruitment, from August 1, 2011, to October 17, 2012, inclusion required a score ≥50 on the Karnofsky Performance Status Scale16 as well as lumbar puncture (LP) for CSF analyses. To optimize population representativeness, from October 18, 2012, until June 19, 2013, Karnofsky status and LP requirements were waived. The original goal was to enroll 100 patients based on recruitment feasibility, follow-up time, and cost, and one of the primary study goals was to acquire outcomes frequencies (regarding seizure recurrence and death) to determine the feasibility and planning parameters for a larger, more definitive study.
At enrollment, a study investigator (O.K.S., I.S.) documented patient demographic data, presenting clinical symptoms, medical history, and neurologic examination findings. HIV infection was staged using the World Health Organization (WHO) staging criteria17; stages III and IV were considered advanced HIV infection. Antiretroviral usage was documented. Seizure semiology was based on patient or witness event descriptions, and relevant neurologic findings (e.g., Todd paralysis).
We obtained 20- to 30-minute EEG recordings on a 16-channel Biologic CEEGraph, using the international 10–20 electrode placement. Two neurologists with EEG fellowship training (O.K.S., W.H.T.) interpreted records. EEGs were systematically scored on a data collection instrument incorporating the National Institute of Neurologic Disorders and Stroke Common Data Elements for EEG.18 An abnormal EEG was defined as the presence of one or more of the following: background slowing, generalized slowing, focal slowing, interictal focal discharges, or active seizure during recording.
Each patient had detailed clinical evaluations to identify underlying seizure etiology. Laboratory studies included serum testing for CD4+ T-cell count, sodium, glucose, malaria infection by a rapid diagnostic antigen test, and syphilis via a screening rapid plasma reagin test and confirmatory serum Treponema pallidum hemagglutination assay, if needed. When patients declined LP, serum cryptococcal antigen and serum toxoplasma antibody testing were performed. Toxoplasma antibody testing was also performed in patients with neuroimaging suspicious for a toxoplasma lesion. CSF testing included gram stain, cell count, differential, total protein, glucose, Venereal Disease Research Laboratory test for neurosyphilis, and cryptococcal antigen testing. CSF DNA PCR testing was conducted for Mycobacterium tuberculosis, Epstein-Barr virus, JC virus, varicella zoster virus, cytomegalovirus, herpes simplex virus type 1, herpes simplex virus type 2, and Toxoplasma gondii on a Rotor-Gene 6000 (Corbett Life Sciences, Sydney, Australia) real-time thermocycler as previously described.19 The presence of a CNS OI was defined as positive CSF or serum cryptococcal antigen, amplification of DNA from CSF PCR, or neuroimaging consistent with a CNS OI confirmed by serum studies and/or response to treatment.
Neuroimaging studies were obtained on patients who either had imaging as part of routine clinical care or did not have etiology established after initial clinical and laboratory evaluation. These were performed on a Siemens CT2007YS CT scanner or a Magnetom Essenza 1.5T MRI scanner (Siemens AG, Erlangen, Germany). CT protocols included 1.5-mm contiguous axial imaging from foramen magnum through vertex for 3-dimensional reconstruction with 4-mm oblique axial imaging pre- and postcontrast. MRI protocols included sagittal T1, axial T2, fluid-attenuated inversion recovery, diffusion-weighted imaging with apparent diffusion coefficients, T1 pre- and postcontrast, and coronal T2 images. Magnevist MRI contrast was administered by hand injection. Abnormal neuroimaging was defined as the presence of one or more of the following: cortical, white matter, deep structural, or posterior fossa abnormalities; masses or mass effect; fluid collections; intracranial bleeds; calcifications; abnormal ventricular size; abnormal brain volume; or contrast enhancement.
A trained neuropsychologist (L.K.) completed neuropsychiatric screening evaluations between 2 weeks and 2 months postdischarge from index admission. The Zambian Mini-Mental State Examination (zMMSE) and International HIV Dementia Scale (IHDS) were used to screen for cognitive dysfunction. The zMMSE is an adaptation of the standard MMSE with modifications to account for limited literacy.20–22 The IHDS is a widely used scale for HIV-associated dementia that assesses memory registration, recall, and psychomotor speed.20–22 The Shona Symptom Questionnaire (SSQ) is a 14-item instrument initially developed in neighboring Zimbabwe to identify symptoms of anxiety and depression.15,16 The SSQ was used to quantify psychiatric morbidity because SSQ score has been associated with increased mortality among HIV+ Zambian adults, even after controlling for HIV stage and socioeconomic status.20 These instruments have been used with other populations in Zambia, and standard cutoffs for abnormalities have been established (zMMSE score <22; IHDS score <10; SSQ score >4).20,21
After EEG, CHASE patients were followed until death, loss to follow-up, or study closure on December 18, 2013. Initial seizure care as well as treatment for underlying etiologies, such as CNS OIs, were managed according to hospital protocols. HIV infection and recurrent seizures during follow-up were managed using the Zambian National Guidelines23 and the WHO's Mental Health Gap Action Programme guidelines,24 respectively. The primary outcome of interest was recurrent seizures. A secondary outcome of interest was death.
For all primary analyses, participants were dichotomized based on EEG findings (abnormal vs normal). Baseline clinical characteristics were evaluated for any association with EEG abnormalities. The association between EEG abnormalities during the index admission and the primary outcome of seizure recurrence was evaluated. Death was a secondary outcome. Associations between EEG findings and baseline characteristics and EEG findings and outcomes were also performed using specific EEG findings.
To assess relationships among EEG abnormalities, baseline clinical characteristics, and outcomes during follow-up, 2-tailed t tests or χ2 tests were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC). The t tests were also performed using SPSS version 20 (IBM Corp., Armonk, NY) to conduct 2-tailed comparisons between EEG abnormalities and imaging findings. Lastly, logistic regression was used to examine the relationship between an abnormal EEG and seizure recurrence, controlling for clinical and demographic variables found to be associated with EEG abnormalities. A p value of <0.05 was considered significant.
Standard protocol approvals, registrations, and patient consents.
A study investigator sought written informed consent to participate in this study from patients or their health care proxies in the language of their choice (English, Nyanja, or Bemba). Before study initiation, the Michigan State University Biomedical Institutional Review Board and the University of Zambia Biomedical Research Ethics Committee provided ethical approval of the study.
RESULTS
Study population and baseline characteristics.
Ninety-five patients were recruited into CHASE. Eighty-one (85%) had an EEG (table 1); 13 died before EEG could be obtained and one withdrew from study participation. Thirty-five patients were recruited with the initial enrollment criteria and 46 after Karnofsky and LP requirements were waived. Patients with EEG did not differ on any demographic or clinical characteristic from those without EEG.
Table 1.
Clinical characteristics and acute EEG abnormalities in HIV+ adults with new-onset seizure (n = 81)
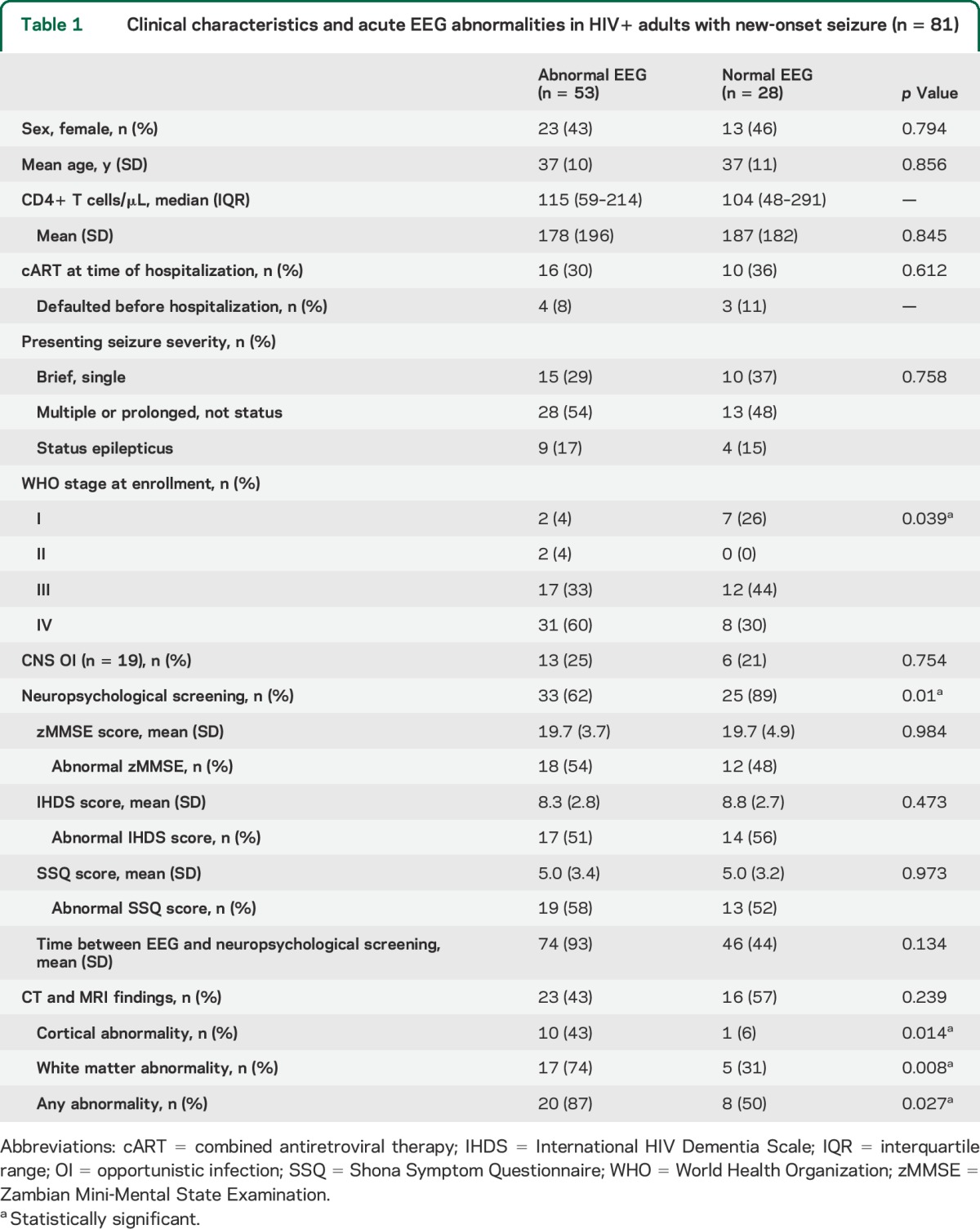
At the time of enrollment, 68 of 81 participants (84%) had advanced HIV infection. Twenty-eight (35%) were taking combined antiretroviral therapy (cART) at initial hospitalization. At etiologic assessment, 23 (28%) had a clear history of focal new-onset seizures. Fifty-four (68%) presented with a seizure lasting longer than 15 minutes, multiple seizures, or status epilepticus. An underlying CNS OI was identified in 19 (23%) and included detection of Cryptococcus neoformans, M tuberculosis, JC virus, varicella zoster virus, cytomegalovirus, and toxoplasmosis, both alone and in combinations. Epstein-Barr virus was also detected in the CSF although its pathogenic significance was unclear in the absence of viral copy number or antibody titers.
Fifty-eight patients (72%) completed neuropsychological screening a mean of 62 days (SD 75) after EEG. Of the 23 patients who had EEG but not neuropsychological screening, 17 died before testing could be conducted and 6 were lost to follow-up. Forty-seven patients (81%) had an abnormal score on at least one neuropsychological screen, whereas 16 (28%) had abnormal scores on all 3 screens (table 2). Thirty patients (52%) had cognitive impairment on the zMMSE. Neuroimaging was completed on 39 patients (48%) (37 CT and 2 MRI scans) (table 2). Twelve patients with neuroimaging (33%) had lateralized abnormalities and 6 (15%) had mass lesions.
Table 2.
EEG findings (n = 81)
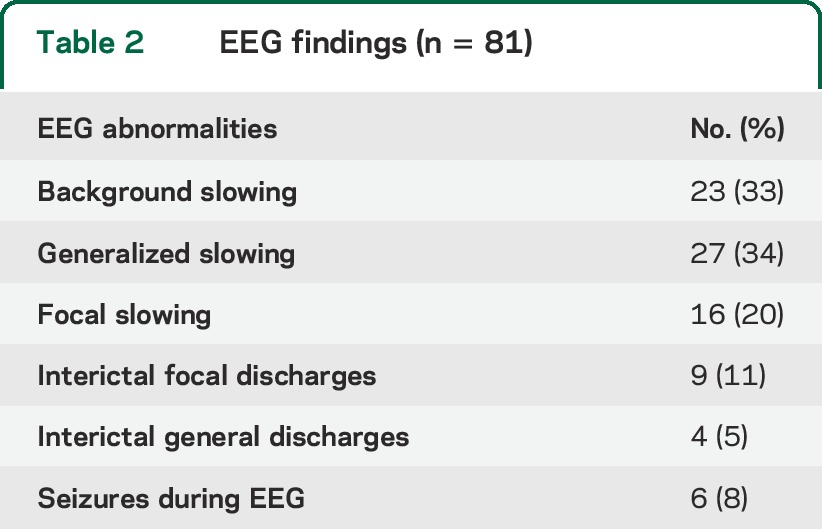
EEG findings.
EEG was obtained within a median of 8 days (interquartile range 6–11) of most recent seizure (table 3). Abnormal EEGs were noted in 53 patients (65%). Eighteen (22%) had interictal spikes (12), a recorded seizure (5), or both (1). Six patients had seizures on EEG: 1 partial, 4 generalized, and 1 nonconvulsive status epilepticus. Seventy-one (87%) had an identifiable posterior dominant rhythm, slow in 23 (33%). Twenty-seven (34%) demonstrated persistent or transient generalized slowing, and 16 (20%) persistent or transient focal slowing.
Table 3.
Acute EEG abnormalities, seizure recurrence, and death (n = 81)
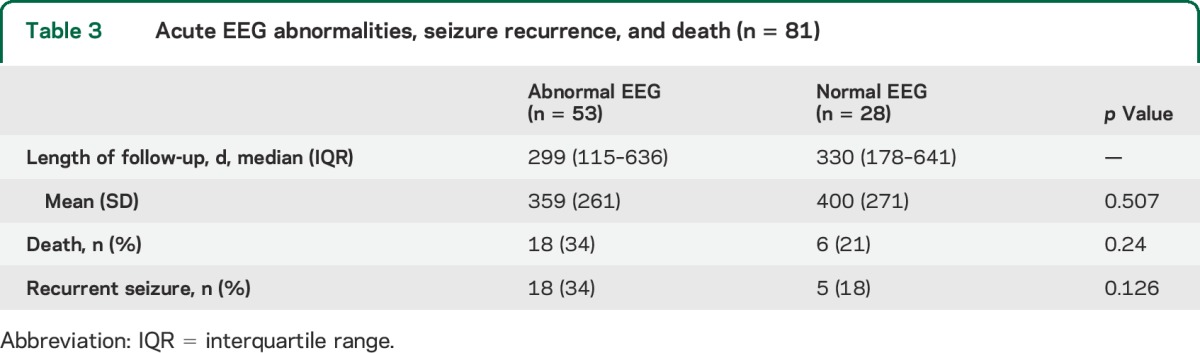
Outcomes.
Patients were followed for a median of 303 days (interquartile range 103–560) after EEG examination. There was no difference in length of follow-up based on an abnormal EEG (p = 0.507). During follow-up, 24 (30%) died and 23 (28%) had at least one recurrent seizure.
EEG abnormalities: Risk factors.
Baseline characteristics and EEG abnormalities.
Patients with advanced HIV infection were more likely to have an abnormal EEG than patients at WHO stage I or II (48/52 [92%] vs 20/27 [74%]; odds ratio [OR]: 4.2 [95% CI: 1.11–15.95], p = 0.039). Abnormal imaging findings were associated with an abnormal EEG (20/23 [87%] vs 8/16 [50%]; OR: 6.67 [95% CI: 1.4–31.72], p = 0.027), specifically cortical (p = 0.014) and white matter (p = 0.008) abnormalities. Cortical (p = 0.008) and white matter (p = 0.004) abnormalities were also associated with slow posterior dominant rhythm. Twenty-five of 33 patients (78%) with an abnormal zMMSE score, compared with 14 of 25 (58%) with a normal score, had generalized EEG slowing (OR: 2.81 [95% CI: 1.01–8.06], p = 0.048).
EEG abnormalities and outcome.
An abnormal EEG was not associated with recurrent seizures (p = 0.13) or death during follow-up (p = 0.24). Controlling for advanced HIV infection at enrollment yielded an adjusted OR of 4.74 (95% CI: 0.98–22.9, p = 0.053). Four of 6 patients who had a seizure recorded during EEG died during follow-up, compared with 20 of 75 (27%) without a recorded seizure (OR: 5.5 [95% CI: 0.93–32.38], p = 0.051).
DISCUSSION
In this prospective study of HIV-infected adults with new-onset seizure, patients with more advanced HIV disease and those with imaging abnormalities were more likely to have abnormal EEGs during admission. Recurrent seizure and mortality rates during follow-up were both high and EEG abnormalities at the time of admission for the index seizure were not associated with recurrent seizures.
Several limitations of this work deserve mention. During the course of the study, inclusion criteria to allow patients refusing LP to enroll were made. However, EEG abnormality, seizure recurrence, and death rates did not change before and after enrollment criteria adjustment (all p values >0.05). Because bedside EEG was unavailable in our setting, only patients stable enough for transport to the EEG laboratory were included in this study. Screening during the admission for index seizure for neuropsychiatric problems would have been inappropriate, but some delays in outpatient assessments occurred and it is possible the neuropsychiatric symptoms identified developed after the admission. Imaging was not available for everyone who had an EEG and not all images were acquired during the acute admission. We may have underestimated the rate of seizure recurrence among our patients. Individuals with abnormal EEGs were more likely to be lost to follow-up, either due to death or other reasons, in the first 2 months than individuals with a normal EEG. Because the average time to seizure recurrence among participants was 218 days, those lost during the first 2 months may have been more ill and experiencing more recurrent seizures than participants who remained in the study. There was no difference in length of follow-up based on EEG findings. Seizures around the time of death were likely not captured because patient deaths generally occurred in the community. Acute EEG findings informed acute clinical care and this may have affected outcome, but clinicians making long-term decisions about chronic antiepileptic medication use in these subjects were likely not aware of the EEG findings (inpatient and outpatients records are segregated) and many of the study patients were reluctant to continue chronic seizure medications unless seizures were a frequent problem for them, possibly because of the substantial medication burden associated with HIV ± OI in this population.
Despite these limitations, this study provides insights into the EEG findings in HIV+ patients presenting with new-onset seizure in Zambia. EEG abnormality prevalence is similar to that shown in South Africa,14 Cameroon,8 India,2 and Germany.5 Our patient population showed a similar rate of background slowing as in German patients, but a higher prevalence of epileptiform discharges and electrographic seizures.5 As in the German study, EEG abnormalities in this Zambian population were associated with more advanced HIV infection. Patients were followed for a median of 303 days after EEG examination, and seizure recurrence and mortality both occurred in almost a third of the study population during this period.
What value can EEG bring to the diagnostics evaluation of HIV+ patients with new-onset seizure in resource-limited settings? A substantial minority of patients had ongoing seizures requiring acute treatment. EEG may also assist in prioritizing patients for imaging. Neuroimaging in sub-Saharan Africa continues to be extremely unequally distributed.25 Where a limited number of subsidized or free imaging studies are available to public sector patients, EEG may be a viable screening tool for identifying HIV+ patients most likely to benefit from neuroimaging.
To our knowledge, this study offers the longest follow-up among HIV+ patients with new-onset seizure in sub-Saharan Africa. Among survivors in this cohort study, at least 42% had recurrent seizures during this relatively brief follow-up period. Most patients prescribed antiepileptic medications were given enzyme-inducing agents that should be avoided in conjunction with cART,26 but these are generally the only antiepileptic drugs available in the Zambian public health sector.27 Given the availability of cART, mortality rates were also alarmingly high. Mbonda et al.8 conducted a chart review to examine the etiology of recent onset seizures among HIV+ Cameroonian adults and reported in-hospital deaths among study participants but not the prevalence of recurrent seizures. Chadha et al.2 documented recurrent seizures among 15 of 23 HIV+ Indian adults with new-onset seizure; however, follow-up appeared to be limited to the index hospitalization. Unfortunately, the small sample size and substantial mortality rates limited our capacity to assess the prognostic value of acute EEG for seizure recurrence risk in this HIV+ population.
GLOSSARY
- cART
combined antiretroviral therapy
- CHASE
Cohort Study of HIV-Associated Seizure and Epilepsy
- IHDS
International HIV Dementia Scale
- LP
lumbar puncture
- OI
opportunistic infection
- OR
odds ratio
- SSQ
Shona Symptom Questionnaire
- WHO
World Health Organization
- zMMSE
Zambian Mini-Mental State Examination
AUTHOR CONTRIBUTIONS
Dr. Siddiqi contributed to the drafting of the manuscript, study concept, and analysis of data. Dr. Elafros contributed to the drafting of the manuscript, study concept, and analysis of data. Dr. Sikazwe contributed to the drafting of the manuscript, study concept, and analysis of data. Prof. Birbeck contributed to the drafting of the manuscript, study concept, and analysis of data. Ms. Kalungwana contributed to the drafting of the manuscript, study concept, and analysis of data. Dr. Potchen contributed to the drafting of the manuscript, study concept, and analysis of data. Dr. Bositis contributed to the drafting of the manuscript, study concept, and analysis of data. Dr. Koralnik contributed to the drafting of the manuscript and study concept. Dr. Theodore contributed to the drafting of the manuscript, study concept, and analysis of data.
STUDY FUNDING
Supported by NIH (R21NS073509) and an American Academy of Neurology Clinical Research Fellowship (O.K.S.).
DISCLOSURE
O. Siddiqi received grant funding from the NIH and American Academy of Neurology. M. Elafros and I. Sikazwe report no disclosures relevant to the manuscript. G. Birbeck received grant funding through the NIH and Dana Foundation. L. Kalungwana reports no disclosures relevant to the manuscript. M. Potchen received grant funding through the NIH and Dana Foundation. C. Bositis reports no disclosures relevant to the manuscript. I. Koralnik received grant funding through the NIH. W. Theodore reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Siddiqi O, Birbeck GL. Safe treatment of seizures in the setting of HIV/AIDS. Curr Treat Options Neurol 2013;15:529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadha DS, Handa A, Sharma SK, Varadarajulu P, Singh AP. Seizures in patients with human immunodeficiency virus infection. J Assoc Physicians India 2000;48:573–576. [PubMed] [Google Scholar]
- 3.Modi G, Hari K, Modi M, Mochan A. The frequency and profile of neurology in black South African HIV infected (clade C) patients: a hospital-based prospective audit. J Neurol Sci 2007;254:60–64. [DOI] [PubMed] [Google Scholar]
- 4.Isezuo SA, Sani AZ, Ezunu E, Maiyaki S, Njoku CH, Obembe A. Clinical neuropathy in HIV/AIDS: an eight-year review of hospitalized patients in Sokoto, northwestern Nigeria. Trop Doct 2009;39:133–135. [DOI] [PubMed] [Google Scholar]
- 5.Kellinghaus C, Engbring C, Kovac S, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure 2008;17:27–33. [DOI] [PubMed] [Google Scholar]
- 6.Babiloni C, Vecchio F, Buffo P, et al. Cortical sources of resting-state EEG rhythms are abnormal in naive HIV subjects. Clin Neurophysiol 2012;123:2163–2171. [DOI] [PubMed] [Google Scholar]
- 7.Wieser H, Wang J, Panayiotou P, et al. Seizure occurrence, EEG, and magnetic resonance imaging findings in 61 patients with acquired immunodeficiency syndrome. J Epilepsy 1994;7:70–75. [Google Scholar]
- 8.Mbonda P, Kuate C, Njamnshi A, Fongang Y, Fonsah J, Muna W. Etiologic aspects of epileptic seizures of recent onset in HIV-AIDS infected subjects at the Yaounde Central Hospital (Cameroon). World J AIDS 2013;3:160–167. [Google Scholar]
- 9.Newton TF, Leuchter AF, Miller EN, Weiner H. Quantitative EEG in patients with AIDS and asymptomatic HIV infection. Clin Electroencephalogr 1994;25:18–25. [DOI] [PubMed] [Google Scholar]
- 10.Harrison MJ, Newman SP, Hall-Craggs MA, et al. Evidence of CNS impairment in HIV infection: clinical, neuropsychological, EEG, and MRI/MRS study. J Neurol Neurosurg Psychiatry 1998;65:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel P, Schutte CM, Becker P, van der Meyden C. Discrimination between viral and nonviral meningitis by visually analyzed and quantitative electroencephalography. Clin Electroencephalogr 1999;30:35–38. [DOI] [PubMed] [Google Scholar]
- 12.Baldeweg T, Gruzelier J. Alpha EEG activity and subcortical pathology in HIV infection. Int J Psychophysiol 1997;26:431–442. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan PW, Rossetti AO. EEG patterns and imaging correlations in encephalopathy: encephalopathy part II. J Clin Neurophysiol 2011;28:233–251. [DOI] [PubMed] [Google Scholar]
- 14.Modi M, Mochan A, Modi G. New onset seizures in HIV: seizure semiology, CD4 counts, and viral loads. Epilepsia 2009;50:1266–1269. [DOI] [PubMed] [Google Scholar]
- 15.Satishchandra P, Sinha S. Seizures in HIV-seropositive individuals: NIMHANS experience and review. Epilepsia 2008;49(suppl 6):33–41. [DOI] [PubMed] [Google Scholar]
- 16.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky Performance Status. Cancer 1980;45:2220–2224. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva: WHO; 2007. [Google Scholar]
- 18.NINDS. NINDS Common Data Elements [online]. Available at: http://www.commondataelements.ninds.nih.gov/epilepsy.aspx#tab=Data_Standards. Accessed January 7, 2014.
- 19.Siddiqi OK, Ghebremichael M, Dang X, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis 2014;58:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birbeck G, Kvalsund M, Byers P, et al. Neuropsychiatric and socioeconomic status impact antiretroviral adherence and mortality in rural Zambia. Am J Trop Med Hyg 2011;85:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kvalsund MP, Haworth A, Murman DL, Velie E, Birbeck GL. Closing gaps in antiretroviral therapy access: human immunodeficiency virus–associated dementia screening instruments for non-physician healthcare workers. Am J Trop Med Hyg 2009;80:1054–1059. [PubMed] [Google Scholar]
- 22.Holguin A, Banda M, Willen EJ, et al. HIV-1 effects on neuropsychological performance in a resource-limited country, Zambia. AIDS Behav 2011;15:1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Available at: http://www.who.int/hiv/pub/guidelines/zambia_art.pdf. Accessed February 24, 2015.
- 24.World Health Organization. mhGAP Intervention Guide for Mental, Neurological and Substance Use Disorders in Non-specialized Settings. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 25.Birbeck GL. Epilepsy care in developing countries: part I of II. Epilepsy Curr 2010;10:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birbeck GL, French JA, Perucca E, et al. Evidence-based guideline: antiepileptic drug selection for people with HIV/AIDS: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Ad Hoc Task Force of the Commission on Therapeutic Strategies of the International League Against Epilepsy. Neurology 2012;78:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birbeck G, Chomba E, Ddumba E, Kauye F, Mielke J. Lack of appropriate treatment for people with comorbid HIV/AIDS and epilepsy in sub-Saharan Africa. Epilepsia 2007;48:1424–1425. [DOI] [PubMed] [Google Scholar]


