Abstract
Rationale
Alterations in brainstem circuits have been proposed as a possible mechanism underlying the etiology of mood disorders. Projections from the median raphe nucleus (MnR) modulate dopaminergic activity in the forebrain and are also part of a behavioral disinhibition/inhibition system that produces phenotypes resembling behavioral variations manifested during manic and depressive phases of bipolar disorder.
Objective
Assess the effect of chronic lithium treatment on behavioral disinhibition induced by MnR lesions.
Methods
MnR electrolytic lesions were performed in C57BL/6J mice, with sham operated and intact animals as control groups. Following recovery, mice were chronically treated with lithium (LiCl, added in chow) followed by behavioral testing.
Results
MnR lesion induced manic-like behavioral alterations including hyperactivity in the open field (OF), stereotyped circling, anxiolytic/risk taking in the elevated plus maze (EPM) and light/dark box (LDB) tests, and increased basal body temperature. Lithium was specifically effective in reducing OF hyperactivity and stereotypy but did not reverse (EPM) or had a nonspecific effect (LDB) on anxiety/risk taking measures. Additionally, lithium decreased saccharin preference and prevented weight loss during single housing.
Conclusions
Our data support electrolytic lesions of the MnR as an experimental model of a hyper-excitable/disinhibited phenotype consistent with some aspects of mania that are attenuated by the mood stabilizer lithium. Given lithium’s relatively specific efficacy in treating mania, these data support the hypothesis that manic symptoms derive not only from the stimulation of excitatory systems but also from inactivation or decreased activity of inhibitory mechanisms.
Keywords: bipolar disorder, mania, animal model, lithium, median raphe nucleus
INTRODUCTION
Bipolar illness is a severe disorder, and progress is still needed in understanding the underlying neurobiology as well as for the development of safe and effective treatments. Such progress will depend, partly, on the establishment of animal models that adequately reflect both depressive and manic aspects of bipolar disorder (Gould and Einat 2007). The central characteristic of bipolar disorder that distinguishes it from unipolar depression is the presence of manic or hypomanic episodes, which manifest themselves as decreased need for sleep, increased impulsiveness, risk taking, and excessive engagement in pleasurable activities among other symptoms (Bowden 2005). However, only a few well-validated animal models of bipolar mania currently exist (Gould and Einat 2007).
Alterations in serotonergic regulation of catecholaminergic circuits have been proposed as a possible mechanism related to the etiology of mood disorders (Di Giovanni et al. 2008; Prange et al. 1974). Alterations in neuronal size, as well as changes in components of the serotonin system within neurons of the raphe nucleus (Cannon et al. 2007; Matthews and Harrison 2012; Sullivan et al. 2009) have been identified postmortem from humans who were diagnosed with bipolar disorder. The median raphe nucleus (MnR) contains mostly cell bodies of serotonergic and GABAergic neurons (Beck et al. 2004; Kohler and Steinbusch 1982). Projections from the MnR modulate dopaminergic activity in the forebrain (Herve et al. 1981; Nishikawa et al. 1986), and are involved in behavioral changes related to coping responses to stress (Beck et al. 2004; Gray 1982; 1987; Paul and Lowry 2013). Bimodal modulation of behaviors (disinhibition/inhibition) regulated by the MnR resembles the variations in activity levels shown during the poles of bipolar disorder (mania/depression), which can also be triggered by exposure to stress (Beck et al. 2004; Gray 1982; 1987; Paul and Lowry 2013). Previous research in rats has shown that electrical stimulation of the MnR elicits emotional responses (i.e. crouching, defecation, micturition, piloerection) similar to those triggered in the presence of stress (Andrade et al. 2013; Graeff and Silveira Filho 1978). Inactivation of the MnR, on the other hand, produces physiological over reactivity to stress as assessed by number and size of gastric ulcers, weight loss, depressed immune responses, increased corticosterone levels, and constant theta waves in hippocampus, along with induction of general behavioral disinhibition (Andrade and Graeff 2001; Andrade et al. 2013; Graeff et al. 1996; Vertes et al. 1994).
Collectively the set of behavioral alterations induced by electrolytic or neurotoxic lesions on MnR as previously shown in rats or cats resembling manic symptoms includes hyperactivity (Asin and Fibiger 1983; Wirtshafter et al. 1986), increased sexual behavior (Albinsson et al. 1996), aggressiveness (File et al. 1979), impulsivity and risk taking (Andrade and Graeff 2001; Wogar et al. 1993), increased reinforcement value (Wogar et al. 1991), and insomnia (Arpa et al. 1998). Despite different approaches for MnR inactivation (i.e. serotonin or GABA receptor agonists, iboenic acid, NMDA) that produce some of these alterations, the inactivation by electrolytic lesion is the only one reproducibly able to produce the entire set of symptoms (Andrade and Graeff 2001; Asin et al. 1985; Jacobs et al. 1974; Kusljic et al. 2003; Martin and van den Buuse 2008; Wirtshafter et al. 1987). Additionally, antipsychotics which are effective in reducing manic symptoms including haloperidol, clozapine and chlorpromazine counteract some of the behavioral effects of MnR lesions such as hyperactivity and stereotypic rotations (Giambalvo and Snodgrass 1978; Yamamoto and Ueki 1978). It has also been shown that amphetamine, which in humans can lead to manic behavior, enhances hyperactivity induced by lesions of the MnR (Asin and Fibiger 1983; Geyer et al. 1976). Rodent studies indicate that lithium increases the brain tissue levels and release of serotonin, as well as changes the activity of dopaminergic and noradrenergic systems where imbalances are induced following lesions of the MnR (Baptista et al. 1990; Herve et al. 1981; Kostowski et al. 1974; Scheuch et al. 2010; Treiser et al. 1981). However, no studies to date have assessed the effect of lithium on behavioral changes resulting from inactivation of the MnR.
The aim of this study was to assess the effect of chronic lithium treatment on behavioral changes induced by the MnR lesions in mice. We hypothesized that chronic lithium treatment would attenuate manic-like behaviors caused by MnR lesions.
MATERIALS and METHODS
Animals
Eleven week old male C57BL/6J mice were obtained from The Jackson Laboratories (Bar Harbor, Maine). This strain was chosen because robust behavioral responses to lithium have been noted in a number of behavioral tests (Can et al. 2011; Can et al. 2013; Gould et al. 2007; O’Brien et al. 2004). Mice were housed four or five individuals per cage in an animal room with constant temperature (22±1°C) and a 12-h light/dark cycle (lights on/off at 07.00/19.00). Mice were given free access to food and water. Experiments were performed between 08:00 and 12:00. All experimental procedures were approved by the University of Maryland, Baltimore Animal Care and Use Committee, and were conducted in full compliance with the NIH Guide for the Care and Use of Laboratory Animals. Mice were randomly assigned to three experimental conditions: electrolytic lesion of median raphe nucleus (Group L, n=22), sham lesioned (Group S, n=21), or intact (Group I, n=20). The sequence, repetitions, and intervals between procedures and behavioral tests are detailed in Figure 1 timeline.
Figure 1.
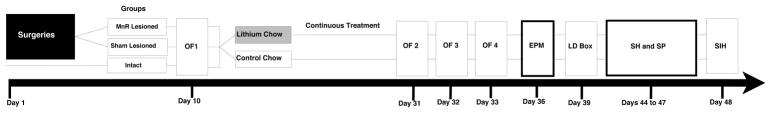
Experimental timeline for surgical procedures, lithium treatment, and behavioral testing. Prior to surgery, mice were randomly assigned to three experimental groups (MnR Lesioned, Sham Lesioned and Intact). Ten days following surgery, mice were tested in the open field test (OF1) and then half of the mice were provided chow containing 4 g/kg LiCl (lithium treated group), while the other half were provided with control chow (control group) throughout the remainder of the experiment. The OF was conducted three times on successive days (OF2, OF3 and OF4, days 31, 32 and 33 respectively), followed by the elevated plus maze test (EPM), and the light dark box test (LDB). After the LDB mice were single housed for 96h for habituation (48h, days 44 to 46) and then tested in saccharin preference (SP) and stress-induced hyperthermia (SIH)
Surgical procedure
After anesthesia induction and stabilization using 2–3% isoflurane, surgical preparation and placement of the animals were made with a stereotaxic apparatus (David-Kopf, Tujunga, CA/Model 962). A single incision was made along the midline to the back of the skull. Connective tissue was removed down to the periosteum, and a drill (David-Kopf/Model 1471) fitted to the stereotaxic frame was used for trepanation. A stainless steel unipolar electrode (insulated except the tip with dimensions of 2.5mm length × 0.20mm in diameter/AM Systems, Sequim, WA) was lowered into the burr hole at an angle of 20° from the lateral plane to the following MnR coordinates: AP= −4.48 posterior, LR= 1.64 from the bregma and DV= −4.79 ventral to skull surface (Franklin and Paxinos 2007). A 0.7 mA direct current was applied for 10 seconds, resulting in electrocoagulation in the lesion group animals. Only a single lesion was made in each animal. As the MnR is a midline structure a bilateral lesion was made (see Figure 6). In the sham-lesioned mice, the electrode was inserted and removed without the application of the electrical current. The incision was closed with sutures prior to removal of the animal from the stereotaxic frame. Following surgery, animals were housed individually for 48h for recovery, kept under analgesic treatment (Carprofen 0.5mg/kg, once a day for two days) and closely monitored for signs of pain and distress. Intact animals were also kept under the same housing conditions, but received no surgery.
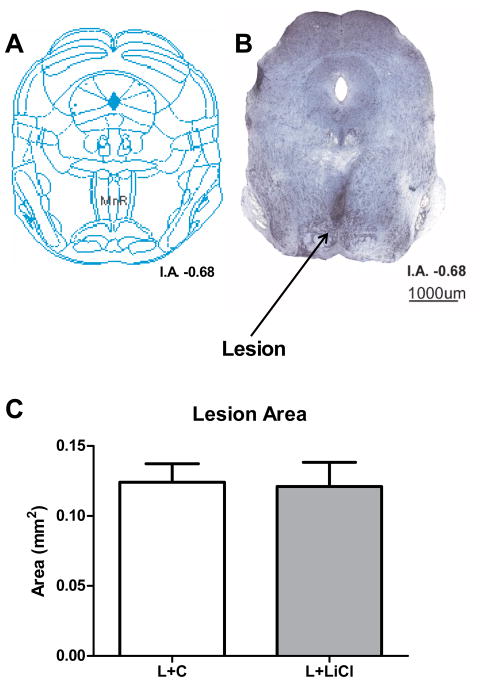
Figure 6.
MnR lesion analysis. (A) Coronal representation at I.A. −0.68 (Franklin and Paxinos 2007). (B) Representative photo of a Nissl method stained coronal slice of the brainstem of a MnR lesioned mouse; arrow points to gliosis at the site of the lesion (in darker blue). (C) Size of the lesioned area (in mm2) obtained from histological analysis of mice after electrolytic lesion of MnR treated with lithium (L+LiCl) or control (L+C) chow (mean±SEM). I.A.: interaural level
Lithium treatment
Regular food was removed from all cages and lithium chow containing 4 g/kg LiCl (Bioserv, Frenchtown, NJ) was provided in half of the cages as previously described (Can et al. 2011; Kovacsics and Gould 2010; O’Brien et al. 2004), while the other half were provided with control chow (Bioserv, Frenchtown, NJ). In both groups, mice received a regular water bottle, and a 0.9% saline bottle to reduce ion imbalances caused by chronic lithium.
Open-Field Test (OF)
Locomotion of animals was assessed individually over a period of 30 minutes in 50 × 50 × 38 cm arenas under 30–35 lux illumination. All sessions were videotaped. The distance moved by each mouse was calculated using Top Scan software (CleverSys Inc, Reston, VA). OF was conducted once prior to, and then three additional times on successive days following chronic lithium treatment (Figure 1).
Elevated Plus Maze Test (EPM)
The plus shaped apparatus used was comprised of two open arms (39.5 × 5 cm) across from each other and perpendicular to two closed arms (35 × 5 cm × 16 cm wall height) with a center platform (5 × 5 cm). Mice moved freely in the maze for 10 min under 30–35 lux illumination. During all trials, the experimenter was present in the room. Each session was videotaped. The number of entries into each arm and the percent of entries into the open arms were calculated with Top Scan software from CleverSys Inc (Reston, VA). The assessment of ethological parameters was performed manually using the Etholog software (Ottoni 2000). The set of parameters analyzed included: circling (turning around a body center point more than 360°), head dips (nose below edge of an open arm), grooming (licking of the fur, beginning by the nose and moving to the ears and then the rest of the body), freezing (absence of any movement, except for breathing), stretching, and fecal boli.
Light/Dark Box Test (LDB)
The natural aversion of mice to brightly illuminated areas as well as their exploratory behaviors were assessed in an arena (35 × 35 × 35 cm) divided into two sections by a partition with a door. One chamber was larger (23 × 35 ×35 cm) and brightly illuminated (approximately 400 lux), whereas the adjacent chamber was smaller (12 × 35 × 35 cm) and darker (less than 5 lux). Mice were placed into the dark chamber first and then allowed to move freely between the two chambers through a small door located between the chambers for 10 min. Each session was videotaped. The number of transitions between light and dark sides was scored manually using Anno Star software (CleverSys Inc, Reston, VA).
Saccharin Preference Test (SP) and Social Isolation (SI)
Each mouse was single housed. In each cage, two bottles, one containing tap water and a second containing saccharin solution (1%) were randomized to each side within a dual liquid dispenser. The bottles had their positions reversed and then were refilled and weighed every 24h. After a 48h habituation period the amount of water and saccharin solution consumed during the third day was recorded and calculated in percentage of preference for the solutions. Mice were weighed before and after the social isolation and changes in body weight were calculated.
Stress-Induced Hyperthermia Test (SIH)
After 96 hours of social isolation mice were tested for the body temperature increase induced by acute restraint stress as previously described (Dao et al. 2010; Vinkers et al. 2009). Each animal was handled and immobilized for 20 seconds, and the baseline body temperature measured by a rectal thermometer (Omega, Stamford, CT, # RET-3). After 10 minutes, a second body temperature measurement was taken from the same animal in the same manner as the first measurement.
Lithium Concentration Assay
Mice were anesthetized with 4% isoflurane, and then quickly decapitated. The brain was removed and sectioned in two coronal halves (section approximately at the intraaural level 1.34mm). The anterior portion was frozen at −80° C and used for lithium concentration assays. For that procedure, the brain tissue was homogenized with a polytron homogenizer (Kinematica AG, Model PT-MR 2100, Littau, Switzerland) in 3 volumes of 0.5 N trichloroacetic acid, followed by centrifugation as previously described (Can et al. 2011; Hamburger-Bar et al. 1986; Kovacsics and Gould 2010). Lithium levels of brain (mmol/kg, wet weight) samples were measured with a flame photometer (Cole-Palmer Model 2655-00, Chicago, IL).
Histology
The posterior portion of the brain was immersion fixed in paraformoldehyde (4%) for 48h, followed by immersion in a sucrose solution (30%) for another 48h and then was sectioned (30 μm) with a cryostat (Leica, Model 1850). After placement on a glass micro slide, the sections were stained with thionine using the Nissl method. The slides were photographed with an optical microscope (Scope.A1 and AxioCam Mrc, Carl Zeiss, LLC) and images analyzed with a computer. The lesion placement (start and end section points, dorso-ventral position and laterality) was determined by comparison with the Mouse Brain Atlas (Franklin and Paxinos 2007). For the morphometric analysis, the gliosis produced by the lesion was measured with the image processing program ImageJ (NIH), at the point where the area of cross section of lesion was maximal. Data analyses were only performed cases in which the correct site of the lesion was confirmed (restricted to the MnR coronal and dorso/ventral positions) as well as the gliosis not extended laterally beyond the region of the MnR (MnR and PMnR).
Statistics
Data were analyzed with Statistica 7 (StatSoft, Tulsa, OK). Each experimental outcome was evaluated with a 2×3 factorial ANOVA with two treatments (control or lithium chow) x three groups (L, S, or I) or with a repeated measures factorial ANOVA (3×3) when applicable. Significant interactions where followed by Fisher LSD post hoc tests. Results are reported as mean±SEM. Statistical significance level was set as p<0.05, two-tailed analysis.
RESULTS
OF
A repeated measures ANOVA for distance travelled showed a main effect of group (F(2,55)=163.98; p<0.001), a main effect of time (F(3,165)=26.13; p<0.001), as well as an interaction between group and time (F(6,165)=2.83; p<0.05), an interaction between time and treatment (F(3,165)=4.08; p<0.01), and a three-way interaction between time, group and treatment (F(6,165)=2.72; p<0.05). Post hoc analysis with Fisher LSD for distance moved before treatments (Day 10) revealed significant differences between MnR lesioned (L+C and L+LiCl) and control groups (S and I + C and LiCl) (p<0.001) and no differences between the lesioned groups. These results indicated that MnR lesion was effective in inducing hyperactivity in mice.
Following 21 days of treatment (Day 31) the activity of MnR lesioned groups remained significantly higher than control groups (Fisher LSD; p<0.001 for all). However, we observed significant differences between L+C and L+LiCl groups along the repeated days of testing (L+C x L+LiCl/Fisher LSD, p<0.05; Days 32 and 33) indicating reduced distance travelled only in lesioned animals that were treated with lithium chow (L+LiCl). There were no differences between control groups regardless of LiCl treatment (Figure 2).
Figure 2.
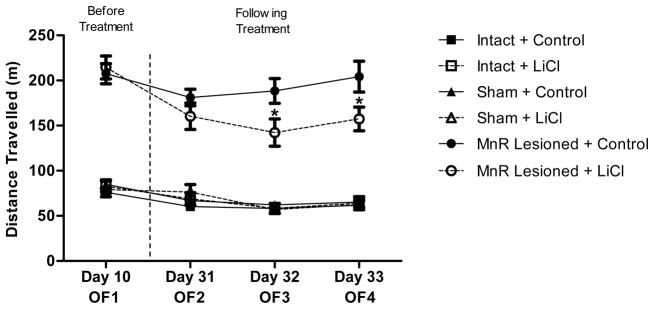
Effect of chronic lithium treatment on the locomotor activity of mice after lesion of the MnR. Values represent the mean (± SEM) of locomotor activity (distance in meters) of groups treated with lithium (I+LiCl, S+LiCl and L+LiCl) or control chow (I+C, S+C and I+C). Mice were tested before the start of treatment (OF1-Day 10) and later three additional times on successive days following chronic lithium treatment (OF2, OF3, and OF4 on days 31, 32 and 33 respectively). *p<0.05 (Fisher LSD test) for differences in the activity of L+C and L+LiCl groups
EPM
A factorial ANOVA for percentage of entries in open arms and number of total entries revealed an effect of group (F(2,55)=5.63; p<0.01 and F(2,55)=35.95; p<0.001, respectively). However, we observed no significant main effect of treatment or an interaction between these factors for either outcome (Figure 3A and B). These results indicate that MnR lesions increased the number of crossings between arms and exploration of open arms but lithium had no effect on these responses.
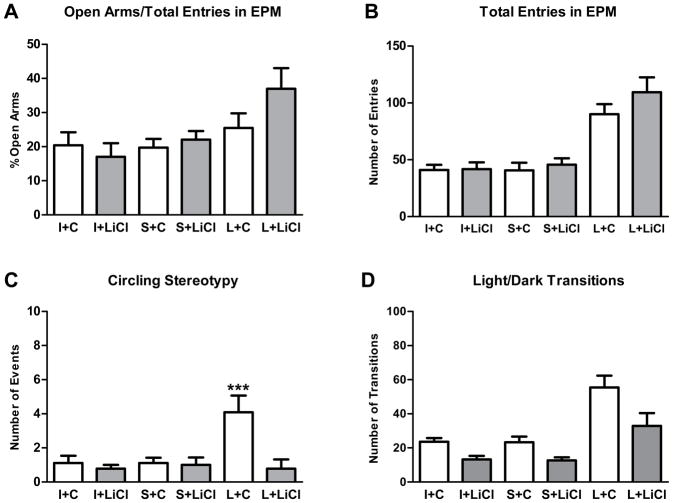
Figure 3.
Effects of MnR lesion and chronic lithium treatment in the elevated plus maze and light/dark box tests. (A) Percentage of entries into open arms relative to the total number of entries (open arms+closed arms) and (B) the total number of crossings between arms in EPM test. (C) Number of stereotypical circling behavior episodes during the elevated plus maze test (EPM). (D) Total number of transitions between light and dark chambers in the LDB test. ***p<0.001 (Fisher LSD test) for differences between L+C and all other groups. All data are presented as mean±SEM. MnR lesioned (L), sham lesioned (S) and intact (I) mice treated with lithium (LiCl) or control (C) chow
During our observations of the EPM trials, we noticed a particular stereotypy that appeared to occur more often in lesioned than control mice in which animals rotate on their axes in a circular motion while exploring the closed arms and center areas of the EPM. An ANOVA for the number of instances of circling stereotypy behavior indicated a significant main effect of group (F(2,55)=4.44; p<0.05) and treatment (F(1,55)=8.16; p<0.01), and an interaction between group and treatment (F(2,55)=5.30; p<0.01). MnR lesions increased the number of occurrences of this behavior approximately four fold, and this increase was prevented with lithium treatment (Figure 3C; post hoc Fisher LSD/L+C x L+LiCl and control groups, p<0.001). To address the specificity of this stereotypy, we additionally assessed other behaviors where there was a significant effect of group (head dip, grooming, freezing) and treatment (head dip, number of fecal boli) on some outcomes, but no significant interactions (Table 1).
Table 1.
Ethological analysis of the elevated plus maze. Mean frequency (±SEM) of behaviors observed in the EMP. MnR lesioned (L), sham lesioned (S) and intact (I) mice treated with lithium (LiCl) or control (C) chow.
| Parameters | I+C | I+LiCl | S+C | S+LiCl | L+C | L+LiCl |
|---|---|---|---|---|---|---|
| Circling | 1.20±0.55 | 0.80±0.55 | 1.09±0.52 | 1.00±0.55 | 4.09±0.52*** | 0.78±0.58 |
| Head Dip | 10.10±2.92 | 18.30±2.92 | 8.36±2.78 | 16.30±2.92 | 22.00±2.78 | 36.56±3.07 |
| Grooming | 5.70±0.87 | 6.00±0.87 | 5.55±0.83 | 5.00±0.87 | 3.73±0.83 | 2.67±0.91 |
| Freezing | 6.70±1.57 | 7.40±1.57 | 8.36±1.49 | 8.60±1.57 | 1.36±1.49 | 4.22±1.65 |
| Stretching | 7.20±1.51 | 10.20±1.51 | 9.36±1.44 | 10.70±1.51 | 8.36±1.44 | 6.00±1.59 |
| Fecal Boli | 2.00±0.70 | 2.40±0.70 | 1.55±0.67 | 3.30±0.70 | 0.55±0.67 | 1.89±0.74 |
p<0.001 (Fisher LSD test) for differences between L+C group and all other groups
LDB
A factorial ANOVA for number of transitions between light and dark compartments revealed a main effect of group (F(2,55)=21.31; p<0.001) and treatment (F(1,55)=15.16; p<0.001), but no significant interaction between these factors (Figure 3D). Thus, according to these results, lesioning the MnR increased the number of transitions between chambers and lithium overall reduced transitions.
SP
We observed a significant effect of lithium treatment to reduce the preference for saccharin (F(1,54)=39.39; p<0.001), but there was no main effect of group or an interaction between these factors (Figure 4A). Lithium also had a significant effect on the weight of animals during the social isolation. A repeated measures ANOVA revealed a significant main effect of treatment (F(1,55)=18.21; p<0.001) and an interaction between time and treatment (F(2,110)=40.57; p<0.001). Overall, lithium treatment prevented the weight loss observed in the animals treated with control chow during the social isolation phase (Figure 4B).
Figure 4.
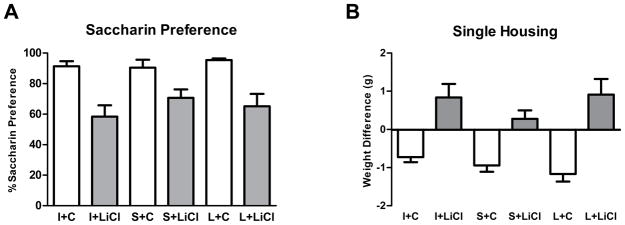
Effects of MnR lesion and chronic lithium treatment on saccharin preference and body weight following single housing. (A) Saccharin preference in relation to the total liquid consumption (water + saccharin consumption) over a period of 24h in the third day of the saccharin preference test. (B) Changes in body weight (in grams) after 96h of single housing. All data are presented as mean±SEM. MnR lesioned (L), sham lesioned (S) and intact (I) mice treated with lithium (LiCl) or control (C) chow
SIH
A repeated measures ANOVA for body temperature revealed a main effect of group (F(2,50)=14.00; p<0.001) and a main effect of acute restraint stress (F(1,50)=313.80; p<0.001). MnR lesions increased the baseline and post stress body temperatures and the restraint stress induced hyperthermia in all the groups (Figure 5). However, there was no effect of treatment or interactions between factors.
Figure 5.
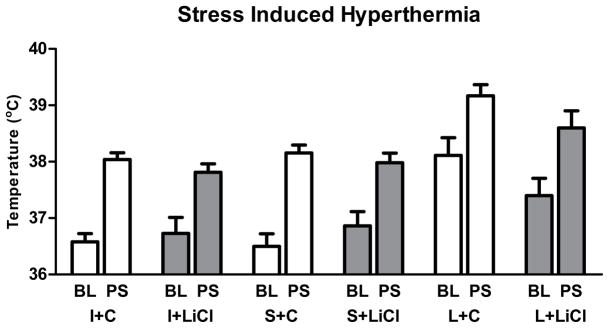
Effect of MnR lesion and chronic lithium treatment on baseline and stress induced temperature change. Baseline (BL) and post-stress (PS) body temperatures (in Celsius; mean±SEM) in the stress induced hyperthermia test (SIH) (mean±SEM) in mice from MnR lesioned (L), sham lesioned (S) and intact (I) mice treated with lithium (LiCl) or control (C) chow groups
Lithium Concentration Assay
A one-way ANOVA indicated no significant difference between brain lithium levels (mmol/kg, wet weight) for all treated groups (F(2,27)=1.35; p=0.28; I+LiCl=0.91±0.04; S+LiCl=0.83±0.04 and L+LiCl=0.93±0.05). Lithium levels were below detectable limits in all mice from the groups treated with control chow. These brain lithium concentrations in lithium treated mice were within the human therapeutic range (0.8 to 1.2 mmol/L (Gelenberg et al. 1989). We have previously shown that following chronic treatment brain and blood levels of lithium are generally very similar in the C57Bl6J mouse strain (Can et al. 2011; Gould et al. 2007).
Histology
Histological analysis of the brains of lesioned mice (L+C and L+LiCl) allowed for confirmation of the lesion location and quantification of gliosis. An example of a MnR lesion can be seen in Figure 6A. Manual assessment in a blinded manner of the location of lesions revealed that the target area was correctly lesioned in all but two mice, which were excluded from the experimental analyses described above. Analysis of the size of lesioned areas (independent samples t-test) indicated no significant differences between L+C and L+LiCl groups (t(18)=0.14; p=0.89) (Figure 6B). Similarly, we did not observe any differences in lesion position between groups, and there was not any significant effect of side of lesion (medial versus lateral) on the frequency of circling (Factorial ANOVA F(1,16)=0.42; p=0.53).
DISCUSSION
These results demonstrate that electrolytic lesions of the MnR in C57BL/6J mice produce locomotor hyperactivity, stereotyped circling, and increases in the frequency of both open arms and light side exploration in EPM and LDB tests. Many of the effects of electrolytic lesions of MnR in mice that we observed are similar to those previously demonstrated in rats (Andrade and Graeff 2001; Asin and Fibiger 1983; Giambalvo and Snodgrass 1978; Wirtshafter et al. 1986). Chronic lithium treatment attenuated or reversed some of the MnR lesion induced behavioral changes. Given lithium’s relatively specific efficacy in treating mania, these data support the hypothesis that manic symptoms may derive not only from the stimulation of excitatory systems (e.g. dopaminergic and noradrenergic) but also from inactivation or lowering activity of serotonergic inhibitory mechanisms. However, we also note that electrolytic lesion of the MnR not only destroys serotonergic neurons, but also fibers that pass in proximity to this structure. While some of the behavioral effects of electrolytic lesions have been reproduced by selective destruction of serotonergic cell bodies, others have not (Andrade and Graeff 2001; Asin et al. 1985; Jacobs et al. 1974; Kusljic et al. 2003; Martin and van den Buuse 2008; Wirtshafter et al. 1987). As such, further validation for the role of specific neuronal populations and their sensitivity to lithium treatment will require administration of cell-type selective neurotoxins or other approaches.
We utilized a single concentration of lithium chloride in the food of mice. This concentration of lithium chloride was chosen on the basis of previous experiments that had demonstrated that the resulting brain lithium levels were within human therapeutic levels, and which resulted in behavioral effects (Can et al. 2011; Flaisher-Grinberg and Einat 2010; Gould et al. 2007; Kovacsics and Gould 2010; O’Brien et al. 2004; Shaltiel et al. 2008) in C57Bl6J mice. As expected, our treatment paradigm resulted in brain lithium levels ~ 0.9 mmol/kg within the human dose range efficacious for the treatment of mania (typically 0.8 to 1.2mM) (Gelenberg et al. 1989). Similar to humans, above this level in rodents results in deleterious side effects and potentially death. The OF test revealed that lesions of the MnR led to a robust increase in locomotor activity. Lithium attenuated the locomotor hyperactivity induced by the MnR lesions on days 32 and 33 (Figure 2). It is possible that this effect may be in part due to lack of habituation occurring in the non-lithium treated MnR lesioned animals. Lithium also reduced MnR lesion-induced stereotypic circling as measured on the EPM. This hyperactivity, as well as the finding of an induction of stereotypic circling in lesioned animals on the EPM, may result from downstream effects of the MnR lesion on the dopaminergic system (Giambalvo and Snodgrass 1978; Yamamoto and Ueki 1978). Our data also revealed that lesions of the MnR produced another behavioral alteration observed in animal models of mania: increased exploration of the open arms of the EPM that may be related to decreased anxiety or an increased exposure to risk (Dao et al. 2010; Einat 2006; Yen et al. 2013). Increased open arms exploration in the EPM may be related to the reduced availability of serotonin from MnR projections to the forebrain (Graeff et al. 1996). However, there was an absence of effectiveness of lithium on the increased exposure to the open arms induced by the MnR lesion.
An anxiolytic or risk taking phenotype induced by the MnR lesion was also observed in the LDB test. Lesions of the MnR increased the number of transitions, whereas chronic lithium treatment significantly decreased them. However, there was no interaction between lesion and chronic lithium treatment, indicating that lithium did not specifically reduce the behavioral effects of MnR lesions. Nevertheless, the effect of lithium to reduce the number of transitions is likely not a non-specific action of lithium on the locomotor activity, since lithium treatment had no effect on locomotor activity in non-lesioned animals as demonstrated in the OF test.
The saccharin preference test indicated a high basal preference for saccharin solution over water suggesting that the absence of an effect of lesion may have resulted from a ceiling effect. Our results revealed that chronic lithium treatment significantly reduced the preference for saccharin regardless of lesion status. These findings are consistent with antihedonic effects of lithium in this test as reported previously (Flaisher-Grinberg and Einat 2010; Flaisher-Grinberg et al. 2009), though polydipsia induced by lithium administration cannot be excluded as a possible confounding variable. We also found that administration of lithium prevented weight loss during social isolation, confirming its protective effect on attenuating the chronic consequences of stress (Ihne et al. 2012; Silva et al. 2008; Vasconcellos et al. 2003) including weight loss (O’Leary et al. 2012). The absence of significant differences in the weight of the lesioned animals suggests that there is not an clear effect of the MnR lesion to modify sensitivity to at least one outcome of chronic stress as previously described in rats (Andrade et al. 1998; Andrade and Graeff 2001).
The results of the stress-induced hyperthermia test similarly revealed that the MnR lesions did not affect acute stress induced changes in body temperature. It is possible that a ceiling effect in the L+C group prevented the assessment of the real stress effect, since post stress temperatures were higher than 39°C in the mice of this group.
It is relevant to note that chronic lithium treatment was specifically effective in reducing MnR lesion effects primarily attributed to imbalances in catecholaminergic activity, namely open field hyperactivity and stereotypic circling. This could be due to effects on dopaminergic signaling through direct molecular targets of lithium including the inhibition of glycogen synthase kinase-3 (GSK-3) (Can et al. 2014). On the other hand, there was an absence of effectiveness of lithium treatment on the anxiolytic/risk taking profile induced by MnR lesion in EPM. The EPM data, taken together with the general behavioral effect of lithium in the LDB, suggests that lithium does not reverse increases in risk-taking or anxiety decreases induced by MnR hypofunction.
Commonly used animal models of mania are based on the assessment of the effects of mood stabilizers on the spontaneous increases in activity as well as other behaviors induced by psychostimulants, sleep deprivation, intracerebroventricular administration of oubain, genetic mutations, or evaluation of strain differences (Einat 2006; Gessa et al. 1995; Gould and Einat 2007; Gould et al. 2007; Herman et al. 2007; Roybal et al. 2007). Overall, our data support electrolytic lesions of the MnR as an additional experimental model of some aspects of mania. Compared to most approaches the MnR lesion has the advantage of specifically implicating a specific brain region. Most genetic mutations that model mania are in the germ line, rather than conditional in nature, which may have profound effects on developmental processes. Additionally, the chronicity of the manic-like behavioral effects of MnR lesion is an additional advantage over some approaches such as administration of stimulants. This allows the evaluation of the effectiveness of ongoing treatments, potentially at multiple time points. Overall, our results are consistent with a model of hyper-excitable/disinhibited phenotype that is attenuated by the mood stabilizer lithium. This finding is especially relevant because it allows the possibility of reinterpretation of the behavioral effects of MnR lesions in a transnationally relevant context. Additional studies will be necessary to explore the interaction between lithium treatment and other aspects of behavioral disinhibition induced by the lesions of MnR, such as those observed in models of cognitive and motor impulsivity and sexual behaviors, as well as other drugs clinically effective in treatment of mania (antipsychotics, valproate acid, carbamazepine).
Acknowledgments
Funded by MH091816 to TDG. FAP received CNPq (Proc. 140649/2010-7) and CAPES (Proc. 13751/12-3) research fellowships from Brazilian government. The experiments fully comply with the current United States of America laws of Care and Use of Laboratory Animals.
Contributor Information
Fernanda A. Pezzato, Department of Psychiatry, University of Maryland School of Medicine, Rm. 934D MSTF, 685 W. Baltimore St., Baltimore, MD 21201, USA.
Adem Can, Department of Psychiatry, University of Maryland School of Medicine, Rm. 934D MSTF, 685 W. Baltimore St., Baltimore, MD 21201, USA.
Katsumasa Hoshino, Universidade Estadual Paulista Júlio de Mesquita Filho, Instituto de Biociências, Departamento de Fisiologia, 18618-000 - Botucatu, SP – Brazil.
José de Anchieta C. Horta, Junior, Universidade Estadual Paulista Júlio de Mesquita Filho, Instituto de Biociências, Departamento de Anatomia, 18618970 - Botucatu, SP – Brazil.
Miriam G. Mijares, Universidade de São Paulo, Instituto de Psicologia, Av. Prof. Mello Moraes, 1721, Sala A-6, Cidade Universitaria, 05508-900 - Sao Paulo, SP – Brazil.
Todd D. Gould, Email: gouldlab@me.com, Department of Psychiatry, University of Maryland School of Medicine, Rm. 934D MSTF, 685 W. Baltimore St., Baltimore, MD 21201, USA, Phone: (410) 706-5585, Fax: (410) 706-4002.
References
- Albinsson A, Andersson G, Andersson K, Vega-Matuszczyk J, Larsson K. The effects of lesions in the mesencephalic raphe systems on male rat sexual behavior and locomotor activity. Behav Brain Res. 1996;80:57–63. doi: 10.1016/0166-4328(96)00020-4. [DOI] [PubMed] [Google Scholar]
- Andrade T, Silva A, Silva C, Graeff F. Effect of electrolytic lesion of the median raphe nucleus on behavioral and physiological measures of stress. Acta physiologica, pharmacologica et therapeutica latinoamericana. 1998;49:279–289. [PubMed] [Google Scholar]
- Andrade TG, Graeff FG. Effect of electrolytic and neurotoxic lesions of the median raphe nucleus on anxiety and stress. Pharmacol Biochem Behav. 2001;70:1–14. doi: 10.1016/s0091-3057(01)00512-3. [DOI] [PubMed] [Google Scholar]
- Andrade TG, Zangrossi H, Jr, Graeff FG. The median raphe nucleus in anxiety revisited. J Psychopharmacol. 2013;27:1107–15. doi: 10.1177/0269881113499208. [DOI] [PubMed] [Google Scholar]
- Arpa J, Padrino C, Rodriguez-Albarino A, de Andres I. Centralis superior raphe, reticularis pontis nuclei, and sleep-wakefulness cycle in cats. Journal of sleep research. 1998;7:263–75. doi: 10.1046/j.1365-2869.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Asin KE, Fibiger HC. An analysis of neuronal elements within the median nucleus of the raphe that mediate lesion-induced increases in locomotor activity. Brain Res. 1983;268:211–23. doi: 10.1016/0006-8993(83)90487-0. [DOI] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D, Fibiger HC. Electrolytic, but not 5,7-dihydroxytryptamine, lesions of the nucleus medianus raphe impair acquisition of a radial maze task. Behavioral and neural biology. 1985;44:415–24. doi: 10.1016/s0163-1047(85)90784-8. [DOI] [PubMed] [Google Scholar]
- Baptista T, Hernandez L, Burguera J, Burguera M, Hoebel B. Chronic lithium administration enhances serotonin release in the lateral hypothalamus but not in the hippocampus in rats. A microdialysis study. Journal of neural transmission. 1990;82:31–41. doi: 10.1007/BF01244832. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. Journal of Affective Disorders. 2005;84:117–125. doi: 10.1016/S0165-0327(03)00194-0. [DOI] [PubMed] [Google Scholar]
- Can A, Blackwell RA, Piantadosi SC, Dao DT, O’Donnell KC, Gould TD. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes, brain, and behavior. 2011;10:434–43. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Piantadosi SC, Gould TD. Differential antidepressant-like response to lithium treatment between mouse strains: effects of sex, maternal care, and mixed genetic background. Psychopharmacology (Berl) 2013;228:411–8. doi: 10.1007/s00213-013-3045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy. Pharmacol Biochem Behav. 2014 doi: 10.1016/j.pbb.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–7. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O’Donnell P, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Levinson DF, Thompson SM, Potash JB, Gould TD. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2010;68:801–10. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Esposito E. Serotonin-dopamine interaction: electrophysiological evidence. Prog Brain Res. 2008;172:45–71. doi: 10.1016/S0079-6123(08)00903-5. [DOI] [PubMed] [Google Scholar]
- Einat H. Modelling facets of mania--new directions related to the notion of endophenotypes. J Psychopharmacol. 2006;20:714–22. doi: 10.1177/0269881106060241. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR, MacLeod NK. 5,7-dihydroxytryptamine lesions of dorsal and median raphe nuclei and performance in the social interaction test of anxiety and in a home-cage aggression test. J Affect Disord. 1979;1:115–22. doi: 10.1016/0165-0327(79)90030-2. [DOI] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Einat H. Strain-specific battery of tests for domains of mania: effects of valproate, lithium and imipramine. Front Psychiatry. 2010;1:10. doi: 10.3389/fpsyt.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Overgaard S, Einat H. Attenuation of high sweet solution preference by mood stabilizers: a possible mouse model for the increased reward-seeking domain of mania. J Neurosci Methods. 2009;177:44–50. doi: 10.1016/j.jneumeth.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3. Academic Press; New York: 2007. [Google Scholar]
- Gelenberg AJ, Kane JM, Keller MB, Lavori P, Rosenbaum JF, Cole K, Lavelle J. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. N Engl J Med. 1989;321:1489–93. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5(Suppl):89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Puerto A, Menkes DB, Segal DS, Mandell AJ. Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic pathways. Brain Res. 1976;106:257–69. doi: 10.1016/0006-8993(76)91024-6. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT, Snodgrass SR. Effect of p-chloroamphetamine and 5,7-dihydroxytryptamine on rotation and dopamine turnover. Brain Res. 1978;149:453–67. doi: 10.1016/0006-8993(78)90487-0. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev. 2007;31:825–31. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–33. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–41. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Silveira Filho NG. Behavioral inhibition induced by electrical stimulation of the median raphe nucleus of the rat. Physiol Behav. 1978;21:477–84. doi: 10.1016/0031-9384(78)90116-6. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsycology of anxiety. Oxford University Press; New York: 1982. [Google Scholar]
- Gray JA. The psychology of fear and stress. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- Hamburger-Bar R, Robert M, Newman M, Belmaker RH. Interstrain correlation between behavioural effects of lithium and effects on cortical cyclic AMP. Pharmacol Biochem Behav. 1986;24:9–13. doi: 10.1016/0091-3057(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Herman L, Hougland T, El-Mallakh RS. Mimicking human bipolar ion dysregulation models mania in rats. Neurosci Biobehav Rev. 2007;31:874–81. doi: 10.1016/j.neubiorev.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Herve D, Simon H, Blanc G, Lemoal M, Glowinski J, Tassin JP. Opposite changes in dopamine utilization in the nucleus accumbens and the frontal cortex after electrolytic lesion of the median raphe in the rat. Brain Res. 1981;216:422–8. doi: 10.1016/0006-8993(81)90144-x. [DOI] [PubMed] [Google Scholar]
- Ihne JL, Fitzgerald PJ, Hefner KR, Holmes A. Pharmacological modulation of stress-induced behavioral changes in the light/dark exploration test in male C57BL/6J mice. Neuropharmacology. 2012;62:464–73. doi: 10.1016/j.neuropharm.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Wise WD, Taylor KM. Differential behavioral and neurochemical effects following lesions of the dorsal or median raphe nuclei in rats. Brain Res. 1974;79:353–61. doi: 10.1016/0006-8993(74)90433-8. [DOI] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–75. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Samanin R, Bareggi SR, Marc V, Garattini S, Valzelli L. Biochemical aspects of the interaction between midbrain raphe and locus coeruleus in the rat. Brain Res. 1974;82:178–82. doi: 10.1016/0006-8993(74)90904-4. [DOI] [PubMed] [Google Scholar]
- Kovacsics CE, Gould TD. Shock-induced aggression in mice is modified by lithium. Pharmacol Biochem Behav. 2010;94:380–6. doi: 10.1016/j.pbb.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Kusljic S, Copolov DL, van den Buuse M. Differential Role of Serotonergic Projections Arising from the Dorsal and Median Raphe Nuclei in Locomotor Hyperactivity and Prepulse Inhibition. Neuropsychopharmacology. 2003;28:2138–2147. doi: 10.1038/sj.npp.1300277. [DOI] [PubMed] [Google Scholar]
- Martin S, van den Buuse M. Phencyclidine-induced locomotor hyperactivity is enhanced in mice after stereotaxic brain serotonin depletion. Behav Brain Res. 2008;191:289–93. doi: 10.1016/j.bbr.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Matthews PR, Harrison PJ. A morphometric, immunohistochemical, and in situ hybridization study of the dorsal raphe nucleus in major depression, bipolar disorder, schizophrenia, and suicide. J Affect Disord. 2012;137:125–34. doi: 10.1016/j.jad.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–36. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–8. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, O’Connor RM, Cryan JF. Lithium-induced effects on adult hippocampal neurogenesis are topographically segregated along the dorso-ventral axis of stressed mice. Neuropharmacology. 2012;62:247–55. doi: 10.1016/j.neuropharm.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Ottoni EB. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behavior research methods, instruments, & computers: a journal of the Psychonomic Society, Inc. 2000;32:446–9. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- Paul ED, Lowry CA. Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol. 2013;27:1090–106. doi: 10.1177/0269881113490328. [DOI] [PubMed] [Google Scholar]
- Prange AJ, Jr, Wilson IC, Lynn CW, Alltop LB, Stikeleather RA. L-tryptophan in mania. Contribution to a permissive hypothesis of affective disorders. Archives of general psychiatry. 1974;30:56–62. doi: 10.1001/archpsyc.1974.01760070040006. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuch K, Höltje M, Budde H, Lautenschlager M, Heinz A, Ahnert-Hilger G, Priller J. Lithium modulates tryptophan hydroxylase 2 gene expression and serotonin release in primary cultures of serotonergic raphe neurons. Brain Research. 2010;1307:14–21. doi: 10.1016/j.brainres.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, Rogawski M, Gasior M, Luckenbaugh D, Chen G, Manji HK. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, Almeida OF, Sousa N. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience. 2008;152:656–69. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–30. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiser SL, Cascio CS, O’Donahue TL, Thoa NB, Jacobowitz DM, Kellar KJ. Lithium increases serotonin release and decreases serotonin receptors in the hippocampus. Science. 1981;213:1529–31. doi: 10.1126/science.6269180. [DOI] [PubMed] [Google Scholar]
- Vasconcellos AP, Tabajara AS, Ferrari C, Rocha E, Dalmaz C. Effect of chronic stress on spatial memory in rats is attenuated by lithium treatment. Physiol Behav. 2003;79:143–9. doi: 10.1016/s0031-9384(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kinney GG, Kocsis B, Fortin WJ. Pharmacological suppression of the median raphe nucleus with serotonin1A agonists, 8-OH-DPAT and buspirone, produces hippocampal theta rhythm in the rat. Neuroscience. 1994;60:441–51. doi: 10.1016/0306-4522(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Oorschot RV, Olivier B, Groenink L. Stress-induced hyperthermia in the mouse. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Humana Press; New York: 2009. pp. 139–152. [Google Scholar]
- Wirtshafter D, Klitenick MA, Asin KE. Evidence against serotonin involvement in the hyperactivity produced by injections of muscimol into the median raphe nucleus. Pharmacol Biochem Behav. 1987;27:45–52. doi: 10.1016/0091-3057(87)90475-8. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Montana W, Asin KE. Behavioral and biochemical studies of the substrates of median raphe lesion induced hyperactivity. Physiol Behav. 1986;38:751–9. doi: 10.1016/0031-9384(86)90039-9. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Evidence for an involvement of 5-hydroxytryptaminergic neurones in the maintenance of operant behaviour by positive reinforcement. Psychopharmacology (Berl) 1991;105:119–24. doi: 10.1007/BF02316873. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology (Berl) 1993;111:239–43. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Effects of drugs on hyperactivity and aggression induced by raphe lesions in rats. Pharmacol Biochem Behav. 1978;9:821–6. doi: 10.1016/0091-3057(78)90362-3. [DOI] [PubMed] [Google Scholar]
- Yen YC, Anderzhanova E, Bunck M, Schuller J, Landgraf R, Wotjak CT. Co-segregation of hyperactivity, active coping styles, and cognitive dysfunction in mice selectively bred for low levels of anxiety. Frontiers in behavioral neuroscience. 2013;7:103. doi: 10.3389/fnbeh.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]