Abstract
Background
Animal models that allow concurrent access to drug and nondrug reinforcers provide unique insight into the etiology, maintenance, and treatment of drug use.
Objectives
We sought to develop and utilize a concurrent access procedure with nicotine and sucrose in rats.
Methods
Pressing one lever delivered intravenous nicotine, and pressing another lever delivered sucrose pellets, with both reinforcers freely available throughout daily sessions.
Results
Rats that had been pretrained with nicotine on some days and sucrose on other days responded on both levers when subsequently given concurrent access, but almost all responded at substantially higher rates on the sucrose lever. In contrast, rats pretrained exclusively with nicotine before being given concurrent access showed individual differences, with about half responding more on the nicotine lever. Treatment with the nicotinic receptor partial agonist varenicline selectively decreased nicotine self-administration. Food restriction and removal of the sucrose lever both increased nicotine self-administration.
Conclusions
The finding that rats continue to take nicotine when sucrose is concurrently available—and in many cases take it more frequently than sucrose—demonstrates that nicotine self-administration does not only occur in the absence of alternative reinforcement options. As a model of human nicotine use, concurrent access is more naturalistic and has higher face validity than procedures in which only one reinforcer is available or choosing one reinforcer precludes access to other reinforcers. As such, this procedure could be useful for evaluating therapeutic agents and improving our understanding of environmental conditions that promote or discourage nicotine use.
Keywords: Addiction, Choice, Individual differences, Concurrent schedule, Self-administration, Varenclines
Introduction
Drug self-administration has long been used as an animal model of drug use. As typically employed, the animal is essentially offered a choice between taking a drug and not taking the drug, with not taking the drug functioning as a catchall for behaviors and reinforcers that are unspecified and unmeasured. However, there can be advantages to offering a more focused alternative, such as concurrent access to a nondrug reinforcer (e.g., Nader and Woolverton 1991; Negus 2003; Thomsen et al. 2013). Such choice procedures provide a more complete model of the decision processes governing human drug-seeking behavior. For example, laboratory studies of choice between drug and nondrug reinforcers in humans have shown that drug use can be reduced by increasing the magnitude of the alternative reinforcer (Higgins et al. 1994; Bisaga et al. 2007) and that preference for drugs over nondrug reinforcers is associated with the individual’s level of drug dependence (Hogarth 2012; Moeller et al. 2009) and propensity to relapse (Moeller et al. 2013; Perkins et al. 2002).
Concurrent schedules have been used extensively to study choice between drug and nondrug reinforcers (see reviews by Bickel et al. 1995; Banks and Negus 2012). These schedules can be arranged in two general ways, discrete trials and free operant, that focus on qualitative and quantitative aspects of choice, respectively. In microeconomic terms, these correspond to asking which brand is preferred among similar commodities versus what are the preferred proportions of dissimilar commodities (Wetzstein 2013). In free-operant procedures, both choices are available continuously throughout the session, or there is only a brief timeout after each reinforcement, allowing the subject to allocate behavior between the alternatives. In discrete trials procedures, the levers are typically retracted for a period after each reinforcer is delivered, so taking one reinforcer automatically limits intake of the other. In other words, choice of one reinforcer in a discrete trials procedure carries a high opportunity cost in terms of lost access to the other reinforcer. For this reason, discrete trials procedures are well suited for determining the relative values of reinforcers. However, free-operant procedures might be more representative of environments in which opportunity cost is low and a reinforcer that is not at the top of the preference scale can still maintain substantial amounts of behavior.
Lenoir, Ahmed, and colleagues have shown that most rats allowed to choose between cocaine and sweet fluids (sucrose or saccharin solution) in discrete trials procedures have a strong preference for the nondrug reinforcer (Ahmed 2010; Lenoir et al. 2007). The percentage of rats preferring cocaine over saccharin is at most about 15 % even when they have been given extensive training with cocaine prior to choice testing (Cantin et al. 2010). This phenomenon suggests that cocaine might only exert strong control over behavior in certain individuals or when alternative forms of reinforcement are unavailable. In contrast, 51 % of rats given extensive pretraining with heroin preferred heroin over saccharin, compared to only 11 % of rats given no pretraining with heroin (Lenoir et al. 2013). Kerstetter et al. (2012) found that male rats took cocaine more frequently than food pellets under a free-operant concurrent schedule, but not under a discrete trials schedule similar to that of Lenoir et al. (2007); female rats took cocaine more often than food under both procedures. Thus, drug preference depends on the drug class, prior experience with the drug, individual differences in sensitivity to drug reinforcement, and the schedule conditions under which the choice is offered.
Nicotine is somewhat puzzling because it is highly addictive (Carter et al. 2009; Stolerman and Jarvis 1995), yet by some measures, its reinforcing effects are weak (Chaudhri et al. 2006; Le Foll and Goldberg 2006) or masked by its own aversive effects (Tan et al. 2009). Individual rats vary widely in their intake of oral nicotine solution when it is provided continuously in the home cage with plain water also available (Nesil et al. 2011), but Glick et al. (1996) found that most water-restricted rats prefer a low-concentration oral nicotine solution over plain water under a concurrent schedule. However, to our knowledge, concurrent access to nicotine and food has not been studied in rats or other laboratory animals. Therefore, it could be useful and informative to develop a rodent procedure for studying concurrent access to intravenous nicotine and sucrose pellets, a highly palatable food made available only in the testing situation.
In the present study, rats could obtain intravenous nicotine by pressing one lever and sucrose pellets by pressing another lever under a free-operant concurrent schedule with a low response requirement and a brief timeout. We examined the effects of pretraining with each of the reinforcers separately prior to concurrent access versus pretraining only with nicotine self-administration prior to concurrent access. To evaluate the potential of the concurrent schedule for modeling the effects of antismoking treatments, we tested the effects of varenicline, a partial agonist of α4β2 nicotinic receptors and full agonist of α7 nicotinic receptors (Mihalak et al. 2006), that has shown efficacy for maintaining long-term smoking cessation (Cahill et al. 2012). We also studied food-related manipulations that might affect nicotine self-administration; these included extinction of sucrose-lever responding and removal of the sucrose lever (to limit alternative sources of reinforcement), and discontinuation of food restriction (to decrease the value of sucrose as a reinforcer).
Methods
Subjects
Male hooded Lister rats (Harlan, Bicester, UK) were pair-housed in a temperature-controlled (20–22 °C) and humidity-controlled room on a 12-h light cycle (0730– 1930 hours). Rats received ad libitum access to food until they were 10 weeks old and weighed 280–300 g. Food was restricted to about 16 g/day to maintain body weights at about 85 % to facilitate acquisition of nicotine self-administration. A Micro-Renathane (Braintree Scientific Inc., Braintree, MA) catheter was implanted into the external jugular vein under surgical anesthesia, as described in detail previously (Wing and Shoaib 2008). Catheters were flushed daily with heparinized saline containing Baytril (enrofloxacin; Bayer PLC, Newbury, UK). All procedures complied with local and national ethical requirements and the Animals (Scientific Procedures) Act 1986, under license from the UK home office.
Apparatus
Plexiglas training chambers were enclosed in sound-attenuated, ventilated cubicles. Each chamber included a pellet dispenser that delivered 45 mg sucrose/dextrose pellets (5TUT Sucrose Reward Tablet, TestDiet Inc., Richmond, IN), a syringe pump and fluid swivel to deliver nicotine solution, and a cue light between two levers. Schedules were controlled by MED-PC software (Med Associates, St. Albans, VT).
Procedure
Sessions were conducted Monday through Friday. Each session started with illumination of the cue light and lasted for 60 min or until 100 sucrose pellets had been delivered (to prevent satiation). The two levers were randomly assigned across rats to deliver sucrose or nicotine. Under the concurrent schedule of reinforcement, pressing the nicotine lever produced a 1-s nicotine infusion (0.03 mg/kg) and terminated the cue light for a 20-s timeout period during which lever presses had no scheduled effect. Pressing the sucrose lever produced a sucrose pellet and also terminated the light for a 20-s timeout.
In experiment 1, rats (n=15) received single-lever training with sucrose and nicotine on separate days until reaching a stability criterion of three consecutive sessions with mean response rates varying no more than 15 % from the previous session. Only the sucrose lever was available during the first session, and only the nicotine lever was available during the second session. The next 17 sessions consisted of 11 nicotine sessions and 6 sucrose sessions in mixed order over days; more nicotine sessions than sucrose were required due to slower acquisition of nicotine responding. The response requirement was gradually increased from one response per reinforcement to a random-ratio 4 contingency (25 % chance of a response being reinforced) over the first four sucrose sessions and the first six nicotine sessions. In the second phase of experiment 1, there were three single-lever extinction sessions with each lever, in which pressing the lever had no programmed effect; this was intended to equalize response rates on the two levers prior to the concurrent access phase. In the third and final phase of experiment 1, concurrent access to nicotine and sucrose was provided for four sessions, with both levers available throughout each session. To avoid adventitious reinforcement of switching between levers, if a lever was pressed and did not produce reinforcement, switching to the other lever was never reinforced on the first response. The rats initially trained in experiment 1 were further studied in experiments 3, 4, and 5.
In experiment 2, an experimentally naive group of rats (n= 12) was initially trained with only the nicotine lever, with the response requirement gradually increased from one response per reinforcement to random-ratio 4 as in experiment 1, until the stability criterion was reached (which occurred in the 10th session). Then eight sessions of extinction were conducted, in which the nicotine lever was available but had no programmed effect; extinction was conducted for more sessions than in experiment 1 due to resistance to extinction in experiment 2. Finally, rats were given concurrent access to nicotine and sucrose for eight sessions under the same concurrent schedule used in experiment 1.
In experiment 3, the effects of varenicline were determined under the concurrent schedule. Varenicline was administered subcutaneously 10 min before test sessions conducted twice per week, interspersed with baseline sessions on non-test days. Varenicline doses of 0.1, 0.3, 1, and 1.5 mg/kg were tested in pseudo-randomized order, with 6, 7, 4, and 12 rats tested at these respective doses.
In experiments 4 and 5, 12 rats from experiment 3 were further studied to determine the effects of food-related manipulations on nicotine self-administration. In experiment 4, the nicotine dose was reduced by half, to 0.015 mg/kg; there were three baseline sessions under the concurrent schedule at the reduced dose, followed by four sessions in which both levers were still available but sucrose delivery was discontinued (sucrose extinction), and then three sessions in which the sucrose lever was removed. Experiment 5 consisted of a 2×2 factorial design measuring the effects of sucrose lever (present versus absent) and feeding condition (restricted versus nonrestricted) on nicotine self-administration. The nicotine dose of 0.03 mg/kg was reinstituted and maintained throughout this experiment. After three baseline sessions under the concurrent schedule, the sucrose lever was removed for seven sessions to determine whether a lack of reinforcement alternatives would increase nicotine self-administration. During the last four of these sessions without the sucrose lever, food restriction was lifted by providing standard rat chow ad libitum in the home cage between sessions. Finally, there were three sessions in which food restriction remained lifted but rats were returned to the concurrent schedule.
Drugs
Nicotine hydrogen tartrate salt (Sigma, Dorset) was dissolved in 0.9 % saline solution, with the pH adjusted to 7.2±0.2 with diluted NaOH. Varenicline tartrate (Tocris Bioscience, Bristol, UK) was dissolved in 0.9 % saline.
Data analysis
Response rates were analyzed using restricted maximum likelihood analysis, the most valid approach for repeated measures data (Littel et al. 2006). Reinforcement rates were proportional to response rates under the random-ratio contingency, so only analyses of response rate data are shown. In analyses involving both nicotine and sucrose responding, data were logarithmically transformed to compensate for skew. Paired comparisons were performed with Holm corrections (maintaining familywise p<0.05) to detect differences between the two levers within each session or (in experiment 3) within each dose of varenicline. Data for single-lever training sessions with sucrose in experiment 1 (not including the first session) were combined into blocks of two for paired comparisons.
Results
Experiment 1: concurrent access after pretraining with nicotine and sucrose separately
During acquisition training in experiment 1, only one lever (and its associated reinforcer) was available during each session (Fig. 1a). For these data, there was a significant interaction of session number and lever (F8,56=13.6, p<0.0001). Responding on the sucrose lever increased each day over the first four sessions, then stabilized for the remainder of acquisition training. Responding on the nicotine lever also increased over sessions before stabilizing, but the increase occurred at a more gradual rate than with sucrose. Response rates were significantly higher on the sucrose lever than the nicotine lever in adjacent-day sessions throughout acquisition training. During extinction training, responding on the sucrose lever was significantly decreased during all three extinction sessions compared to the last day of single-lever sucrose training, and responding on the nicotine lever was significantly decreased during the second and third extinction sessions compared to the last 2-day block of single-lever nicotine training (p’s<0.05). Response rates on the two levers did not differ from each other during extinction training (p’s>0.32).
Fig. 1.
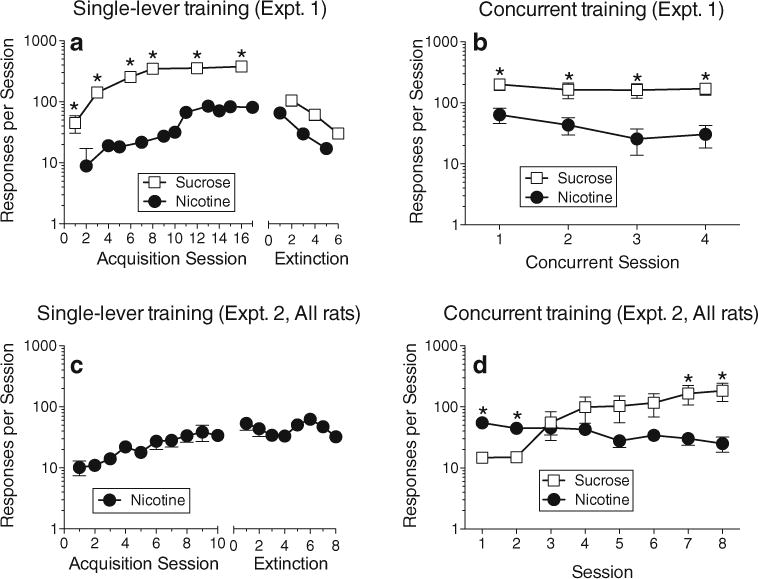
Results of experiments 1 and 2. In experiment 1, rats were first trained to self-administer sucrose and nicotine with only one of the levers available during each session (a, Acquisition Sessions). Extinction training was then conducted, in which there was still only one lever available at a time, but no sucrose or nicotine reinforcement was delivered (a, Extinction). In the final phase of experiment 1, rats were allowed to self-administer sucrose and nicotine with both levers concurrently available (b). In experiment 2, rats were initially trained to self-administer nicotine for 10 sessions, but were not given access to sucrose pellets (c, Acquisition Sessions). Then, the nicotine lever remained available but nicotine delivery was discontinued for eight sessions (c, Extinction). Finally, the rats of experiment 2 were given concurrent access to the sucrose and nicotine levers (d). All points represent mean (±sem). All sessions lasted for 1 h or 100 sucrose deliveries. Asterisks indicate significant differences (p<0.05) between sucrose and nicotine lever responding within specific sessions (or between each sucrose session and the corresponding two-session block of nicotine sessions during single-lever training in (a))
During concurrent training in experiment 1, both levers were available in each session. Throughout this phase, rats responded at a significantly higher rate on the sucrose lever than the nicotine lever (Fig. 1b). The effect of lever was significant (F1,14=51.9, p<0.0001), and response rates on each lever remained stable (i.e., did not change significantly) over all 4 days of concurrent training.
Experiment 2: concurrent access without prior sucrose training
In experiment 2, all rats acquired the nicotine self-administration response during the 10-day acquisition phase in which only the nicotine lever was available (Fig. 1c; effect of session number: F17,187=4.46, p<0.0001). However, compared to the last day of acquisition, responding on the nicotine lever did not decrease significantly during any of the 8 days of extinction training. When rats were offered both levers under the concurrent schedule, average response rates were significantly higher on the nicotine lever than on the sucrose lever during the first two sessions, but sucrose responding increased over the course of training and became significantly higher than nicotine responding during the last two sessions (Fig. 1d; interaction of session number and lever (F7,77=2.58, p<0.02). However, when the distribution of individual rats’ allocation of behavior was depicted graphically (Fig. 2a), it became clear that—unlike experiment 1 where almost all rats responded substantially more on the sucrose lever—the rats in experiment 2 exhibited a bimodal distribution (or at least a wider range of outcomes), with five rats responding more on the sucrose lever and seven responding more on the nicotine lever. Logistic regression of the data in Fig. 2a indicated that, compared to experiment 1, the training procedure used in experiment 2 produced a 19-fold increase in the odds of a rat responding predominantly on the nicotine lever (χ21=6.26, p<0.012).
Fig. 2.
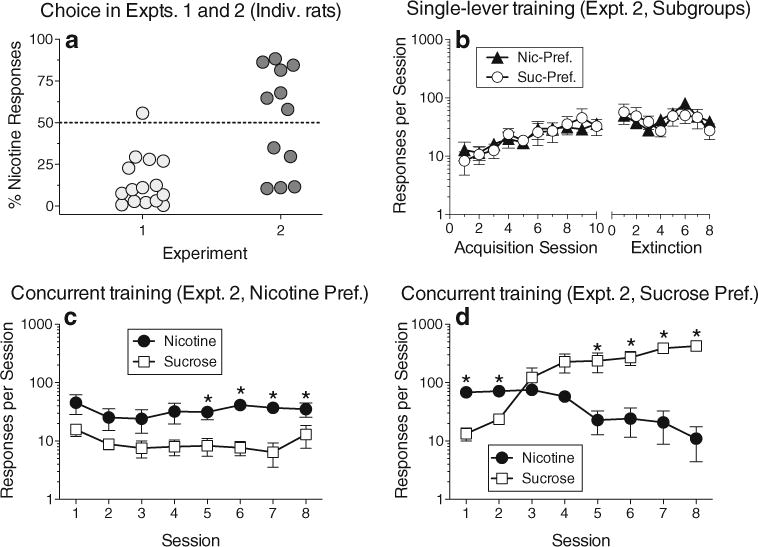
Individual-subject data from experiments 1 and 2 and subgroup data from experiment 2. a Individual rats’ choice of the nicotine lever as a percentage of all responses within the session, averaged across all concurrent access sessions. b Acquisition and extinction training from experiment 2, presented separately for subgroups of rats with a preference for nicotine that was above 50 % (Nic-Pref.) or below 50 % (Suc-Pref.) in (a); (b) corresponds to Fig. 1c, where all rats’ data were averaged. c and d Response rates from concurrent access testing in experiment 2 presented separately for the two subgroups; these panels correspond to Fig. 1d, where all rats’ data were averaged. All points except those in (a) represent mean (±sem)
To further compare the behavior of nicotine-preferring and sucrose-preferring rats in experiment 2, response rates were analyzed with nicotine preference included as a grouping variable, dichotomized for each rat as being either above or below 50 % (Fig. 2b, c, d). The nicotine-preferring and sucrose-preferring subgroups did not differ during the single-lever acquisition and extinction phases (Fig. 2b; p’s> 0.2). However, during concurrent access (Fig. 2c, d), there was a significant interaction of preference subgroup, session number, and lever (F7,70=11.18, p<0.0001). Although both subgroups sampled both levers and responded at similar rates on the sucrose lever on the first day of concurrent access, only the nicotine-preferring subgroup consistently responded more on the nicotine lever than the sucrose lever throughout the concurrent access phase (Fig. 2c), with this difference becoming significant during the last four sessions. In contrast, the sucrose-preferring subgroup responded significantly more on the nicotine lever than the sucrose lever during the first two sessions, but switched to responding significantly more on the sucrose lever by the fifth session (Fig. 2d).
Experiment 3: effects of varenicline
Treatment with the highest dose of varenicline selectively decreased nicotine self-administration under the concurrent schedule. There was a significant interaction of varenicline dose and lever (Fig. 3; F4,26=5.65, p<0.003). Paired comparisons revealed that nicotine responding was significantly decreased at 1.5 mg/kg of varenicline, but sucrose responding was not significantly affected at any dose. Although average rates of nicotine responding increased slightly at the two lowest doses of varenicline, these increases were not significant and were due to several rats showing an increase in nicotine responding at either 0.1 or 0.3 mg/kg. The percentage of responses on the nicotine lever decreased from 8.7 % (±2.6) without varenicline to 1.4 % (±0.6) at the highest dose of varenicline.
Fig. 3.
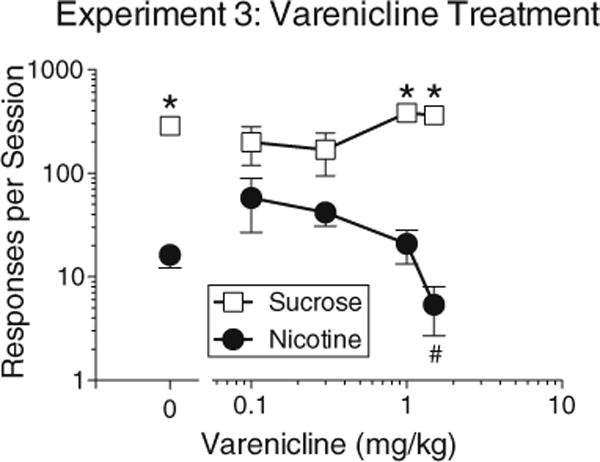
Effects of varenicline treatment on nicotine and sucrose responding under the concurrent schedule in experiment 3. Asterisks indicate significant differences between sucrose and nicotine lever responding within specific treatment levels. Pound sign indicates a significant decrease in nicotine responding at the highest dose of varenicline compared to nicotine responding under baseline conditions (the zero dose of varenicline). A significant familywise significance level of p<0.05 was maintained for all paired comparisons. All points represent mean±sem
Experiment 4: effects of sucrose extinction and sucrose-lever removal
Analysis of baseline response rates in experiments 3, 4, and 5 (main effect of experiment: F2,22=3.82, p<0.05) showed that responding on both levers decreased significantly when the nicotine dose was reduced from 0.03 to 0.15 mg/kg in experiment 4 (p’s<0.05); mean (±standard error of the mean (sem)) rates of nicotine responding dropped from 16.3±4.1 to 8.0± 5.3 responses/session, and sucrose responding dropped from 299.7±29.9 to 184.9±34.7 responses/session. During sucrose extinction in experiment 4, sucrose responding was significantly decreased (versus the last baseline session) during all four extinction sessions, and by the second extinction session, it no longer differed significantly from nicotine responding (Fig. 4a; interaction of phase and lever: F1,11=25.8, p<0.0004). With the results of experiment 4 summarized by averaging response rates on the nicotine lever within each phase (Fig. 4b), it can be seen that nicotine responding increased slightly during the sucrose extinction phase and increased further when the sucrose lever was removed; however, the effect of phase only approached significance (p=0.07).
Fig. 4.
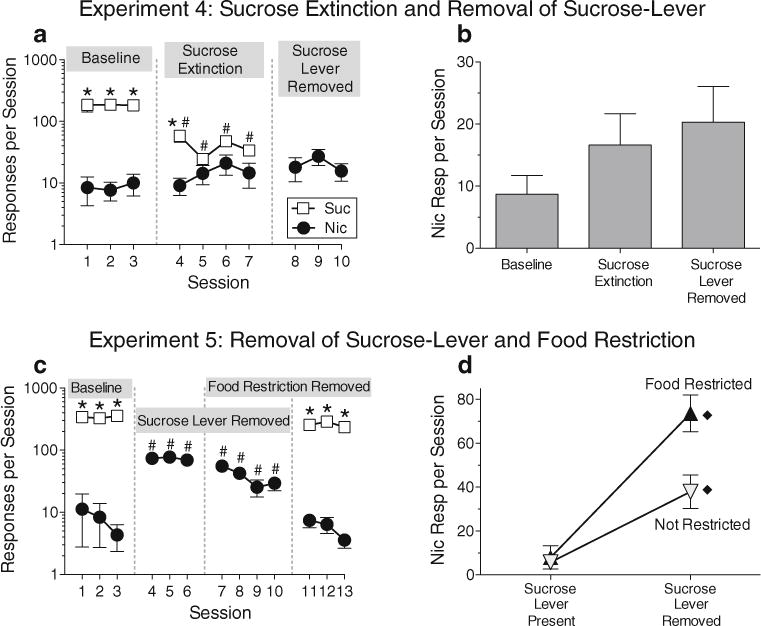
Results of experiments 4 and 5. In experiment 4, the dose of nicotine was reduced to 0.015 mg/kg (from 0.03 mg/kg, the dose used in all other experiments). After three sessions under the concurrent nicotine-sucrose schedule (a, Baseline), delivery of sucrose was discontinued (a, Sucrose Extinction), and finally, the sucrose lever was removed (a, Sucrose Lever Removed). Average levels of nicotine-lever responding within each phase of experiment 4 are shown in (b). In experiment 5, the nicotine dose was returned to 0.03 mg/kg. After three baseline sessions under the concurrent nicotine-sucrose schedule (c, Baseline), the sucrose lever was removed for seven sessions (c, Sucrose Lever Removed). During the last three sessions without the sucrose lever, food restriction was discontinued (c, overlap of Sucrose Lever Removed and Food Restriction Removed). Finally, the sucrose lever was returned but food restriction remained lifted (c, Food Restriction Removed). Average levels of nicotine-lever responding during each phase of experiment 5 were combined into the interaction profile shown in (d). Diamond symbols in (d) indicate that (1) with the sucrose lever removed, nicotine responding was significantly higher during food restriction than when food was not restricted and (2) under both the food-restricted and nonrestricted phases, nicotine responding was significantly higher with the sucrose lever removed than with the sucrose lever present. Asterisks in (a) and (c) indicate significant differences (p<0.05) between sucrose and nicotine lever responding within specific sessions. Pound signs in (a) and (c) indicate significant differences compared to the same lever on the last session of the baseline phase. All points and bars represent mean(±sem)
Experiment 5: effects of food satiation and sucrose-lever removal
At the beginning of experiment 5, with the nicotine dose restored to 0.03 mg/kg, baseline rates of nicotine responding (8.9±3.0 responses/session) were about the same as the baseline rates in experiment 4, and baseline rates of sucrose responding (341.3±40.9 responses/session) increased compared to experiment 4, but not significantly (p=0.3, paired comparison from analysis of baseline rates for Experiments 3, 4, and 5, described above). However, removing the sucrose lever had a more robust effect in experiment 5 than in experiment 4, producing an immediate, significant increase in responding on the nicotine lever compared to the final day of the baseline phase (Fig. 4c; main effect of lever: F1,11=357.6, p<0.0001; main effect of phase: F1,11=91.86, p<0.0001). When food restriction was lifted (with the sucrose lever still removed), rates of nicotine responding decreased but remained higher than those during the final baseline session. When the sucrose lever was returned and the rats were allowed to respond on the concurrent schedule without being food-restricted, nicotine self-administration immediately decreased to about the level seen during the baseline phase. Response rates were averaged within each phase of experiment 5 to produce an interaction profile based on the factorial design (Fig. 4d); this analysis revealed a significant interaction effect of food restriction and sucrose-lever availability on nicotine self-administration (F1,11=9.6, p<0.01). Nicotine responding was lowest when the sucrose lever was available, and food restriction did not affect nicotine-lever responding when the sucrose lever was present. However, when the sucrose lever was removed, rates of nicotine responding were significantly higher during food restriction than during food satiation.
Discussion
These findings clearly indicate that nicotine self-administration can be robust in rats even when alternative reinforcers are available. In experiment 1, rats that were given single-lever training with nicotine and sucrose separately continued to take both reinforcers when they were later given concurrent access; in fact, they took them at nearly the same rates that they had during single-lever training. In experiment 2, rats that were pretrained exclusively with nicotine diverged into two subgroups when they were later given concurrent access. One subgroup continued to self-administer nicotine at about the same rate they that had during single-lever training, while responding less frequently on the sucrose lever. The other subgroup doubled their nicotine responding initially, before switching to responding more on the sucrose lever than the nicotine lever.
Preference for drugs over alternative reinforcers may be an important marker of addiction. The finding that 58 % of the rats in experiment 2 responded more on the nicotine lever than the sucrose lever is surprising in light of the facts that only 7 % of the rats in experiment 1 did so, that cocaine generally has a higher reinforcing efficacy than nicotine under progressive-ratio procedures in rats (e.g., Panlilio et al. 2007, 2013), and that no more than 15 % of rats have been found to prefer cocaine over sweet reinforcers with discrete trials concurrent testing (Cantin et al. 2010). This raises the question of whether responding more on the nicotine lever than the sucrose lever under the free-operant concurrent procedure can be considered a preference for nicotine. When only one reinforcer is available at a time, intravenous doses of nicotine and other drugs are self-administered less frequently than 45-mg sucrose pellets. This is largely a function of pharmacokinetics rather than reward value (i.e., magnitude of reinforcement); drug self-administration responding temporarily ceases while drug levels are above a certain point, but the temporally intermittent response patterns that this produces are not replicated with food by simply increasing the number of food pellets per delivery (Panlilio et al. 2003, 2008). Thus, a higher rate of responding on the sucrose lever than the nicotine lever under the free-operant concurrent schedule does not necessarily indicate a higher magnitude of reinforcement for sucrose. However, the opposite—a higher response rate on the nicotine lever than the sucrose lever—clearly indicates a departure from the norm and does suggest that the reward value of nicotine might be elevated in that individual.
Under a discrete trials concurrent schedule, temporal intermittency is imposed by a long intertrial interval, and choosing one reinforcer limits access to the other. Under these conditions, saccharin is preferred over cocaine. However, the same rats still self-administer cocaine when saccharin is not available (Lenoir et al. 2007). We contend that most tobacco smokers are not faced with a qualitative, either-or choice, but allocate their behavior quantitatively between tobacco and other reinforcers (see Heyman 2013). Increasing the price of cigarettes and limiting the areas where they can be smoked can alter behavior, but in most cases the opportunity cost associated with these measures is not sufficient to induce smoking cessation (Cavazos-Rehg et al. 2014; Hahn 2010). Therefore, the free-operant concurrent schedule might provide a model of the human environment that is more naturalistic than procedures in which only one reinforcer is available (such as in a simple schedule of nicotine self-administration) or in which choosing one reinforcer precludes access to other reinforcers (such as in discrete-trials choice procedures). To the extent that nicotine self-administration in rats is controlled by variables that that also influence human tobacco use, closer correspondence between the model and the human environment not only lends face validity to the procedure but might also be found to improve its predictive validity.
Compared to rats pretrained with nicotine and sucrose (experiment 1), nicotine responding was resistant to extinction in the rats that had been pretrained exclusively with nicotine (experiment 2). Resistance to nicotine extinction has been observed before (Cohen et al. 2005; Diergaarde et al. 2008; Le Foll et al. 2007) and suggests a more robust nicotine-seeking response that might be due to conditioned reinforcing effects, such as sensory properties of the lever that were exclusively associated with nicotine in experiment 2 but not in experiment 1. It might also represent a lack of behavioral flexibility, which could indicate an addiction-like state (Galli and Wolffgramm 2011). Although resistance to extinction occurred in experiment 2 regardless of whether the individual would later develop a preference for nicotine over sucrose, a more robust nicotine-seeking response would be consistent with the overall greater propensity for the rats in experiment 2 to take nicotine more frequently than sucrose.
Kerstetter et al. (2012) found that food restriction decreased food responding and increased cocaine responding under a free-operant concurrent schedule. This finding is counterintuitive because food satiation might be expected to primarily decrease motivation to receive food. However, it is consistent with studies showing that food restriction increases drug self-administration in general (Carr 2002; Carroll and Meisch 1984; Corrigall 1991). Rats were food-restricted during most of the present study, but they were given ad libitum food in the home cage during part of experiment 5. Free feeding decreased nicotine self-administration when only the nicotine lever was available, but had little or no effect when both levers were available. It is uncertain why sucrose responding was not decreased by food satiation, but it is possible that the sweet taste of sucrose pellets had reinforcing effects that were relatively independent of the motivation to feed, unlike the grain-based pellets used by Kerstetter et al. (2012).
Removal of the sucrose lever robustly increased nicotine self-administration in experiment 5. An effect in the same direction was obtained in experiment 4 but was weaker, possibly due to the lower, presumably less reinforcing dose of nicotine used in experiment 4. These results suggest that the rate of nicotine self-administration is determined at least in part by the relative value of nicotine compared to the concurrently available reinforcers. It should be noted that the order of test conditions was not counterbalanced across subjects in experiments 4 and 5, so these findings are tentative. Also, it is possible that the behavior of the rats in Experiments 4 and 5 could have been influenced in some way by their experience in experiments 1 and 3. Still, the present results are consistent with demonstrations that the availability of alternative rein-forcers can decrease drug use in animals (Carroll et al. 2001; Lesage 2009) and humans (Higgins et al. 1994; Heishman et al. 2000) and that tobacco seeking in humans can be increased by devaluation of a concurrently available food reinforcer through specific satiety (Hogarth 2012; Hogarth and Chase 2011). Interestingly, the option of receiving an alternative reinforcer (money) instead of smoking cigarettes can decrease smoking (e.g., Bisaga et al. 2007), but in a meta-analysis, such options were found to have a weaker effect on tobacco use than on heroin or cocaine use (Prendergast et al. 2006).
Nicotine can have anorectic effects that might be specific for sweet foods (Grunberg et al. 1985). Nicotine can also support conditioned taste aversion when paired with a novel, sweet taste (Pescatore et al. 2005). These findings raise the possibility that nicotine-induced taste aversion or decreased appetite for sucrose might have been responsible for the unexpected finding that a substantial number of rats preferred nicotine over sucrose when their first exposure to sucrose pellets occurred within the concurrent nicotine-sucrose schedule in experiment 2. However, this possibility seems unlikely given that the rats that would later develop a sucrose preference (Fig. 2d) had higher and more consistent levels of nicotine intake (i.e., they received doses that would be more likely to produce anorectic or emetic effects) during the first two sessions of concurrent access than the rats that would later develop a nicotine preference (Fig. 2c). Furthermore, the potential of cocaine to produce anorectic effects (Cooper and van der Hoek 1993), to support conditioned taste aversion (D’Mello et al. 1981), and to disrupt food-maintained operant responding (Panlilio et al. 2000) does not seem to prevent the development of robust preference for sweet fluids over cocaine, even when rats are not exposed to the sweet solution prior to choice testing (Lenoir et al. 2007).
Intranasal nicotine spray (Hogarth 2012) and smoking deprivation or satiety (Perkins et al. 1994, 1997) can affect the choice between tobacco and nondrug reinforcers in humans under laboratory conditions, but studies of the effects of varenicline under such conditions have yet to be published. We examined varenicline in the present study because it is known to promote smoking cessation (Cahill et al. 2012) and to decrease nicotine self-administration in rats under a simple (non-choice) schedule of reinforcement (Costello et al. 2014; O’Connor et al. 2010). At the highest dose tested, varenicline produced a selective decrease in nicotine self-administration under the concurrent schedule. The slight increase in nicotine responding at lower doses of varenicline could be due to a priming effect like that seen with low-dose nicotine replacement therapy in highly dependent tobacco smokers (Hogarth 2012). Overall, our findings with varenicline suggest that the free-operant nicotine-sucrose procedure could be useful for testing other drugs for potentially therapeutic effects. For these purposes, sucrose responding can serve as a control condition, for example, showing that varenicline does not produce a general disruption of operant behavior or appetitive motivation. More importantly, the concurrent schedule might provide an animal model of tobacco use that models human environments where tobacco use is persistent despite the availability of nondrug reinforcers. Therefore, it would be sensible to develop a systematic program of translational research using this paradigm to evaluate the predictive validity of the method for assaying the effectiveness of treatment agents and to use it to better understand individual differences in dependence vulnerability, sensitivity to opportunity costs, and sensitivity to pharmacotherapy.
Acknowledgments
All experiments were conducted at Newcastle University and supported by internal funds from Newcastle University and funds donated by Dr. L. Hogarth (MRC G0701456). Preparation of the manuscript was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services (LVP).
Footnotes
Conflict of interest The authors declare no conflicts of interest.
Contributor Information
Leigh V. Panlilio, Email: lpanlili@mail.nih.gov, Preclinical Pharmacology Section, Behavioral Neuroscience Branch, Intramural Research Program, National Institute on Drug Abuse, Baltimore, MD 21224, USA.
Lee Hogarth, School of Psychology, University of Exeter, Washington Singer Building, Perry Road, Exeter EX4 4QG, UK.
Mohammed Shoaib, Institute of Neuroscience, Medical School, Newcastle University, Framlington Place, Newcastle upon Tyne NE2 4HH, UK.
References
- Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology. 1995;118:250–259. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Padilla M, Garawi F, Sullivan MA, Haney M. Effects of alternative reinforcer and craving on the choice to smoke cigarettes in the laboratory. Hum Psychopharmacol. 2007;22:41–47. doi: 10.1002/hup.816. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One. 2010;5(7):e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Bickel WK, Higgins ST. Nondrug incentives to treat drug abuse: laboratory and clinical developments. In: Carroll ME, Overmeir JB, editors. Animal research and human psychological health: advancing welfare through behavioral science. American Psychological Association; Washington, DC: 2001. pp. 139–154. [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. In: Thompson T, Dews PB, Barrett JE, editors. Advances in behavioral pharmacology. Vol. 4. Academic; New York: 1984. pp. 47–88. [Google Scholar]
- Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18:3241–3262. doi: 10.1158/1055-9965.EPI-09-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Chaloupka FJ, Luke DA, Waterman B, Grucza RA, Bierut LJ. Differential effects of cigarette price changes on adult smoking behaviours. Tob Control. 2014;23:113–118. doi: 10.1136/tobaccocontrol-2012-050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacol. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrié P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacol. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, van der Hoek GA. Cocaine: a microstructural analysis of its effects on feeding and associated behaviour in the rat. Brain Res. 1993;608:45–51. doi: 10.1016/0006-8993(93)90772-f. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. A rodent model for nicotine self-administration. In: Boulton A, Baker GB, Wu PH, editors. Animal models of drug addiction, neuromethods. Vol. 21. Humana; Clifton: 1991. pp. 315–344. [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39:1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello GD, Goldberg DM, Goldberg SR, Stolerman IP. Conditioned taste aversion and operant behavior in rats: effects of cocaine, apomorphine and some long-acting derivatives. J Pharmacol Exp Ther. 1981;219:60–68. [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Galli G, Wolffgramm J. Long-term development of excessive and inflexible nicotine taking by rats, effects of a novel treatment approach. Behav Brain Res. 2011;217:261–270. doi: 10.1016/j.bbr.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Glick SD, Visker KE, Maisonneuve IM. An oral self-administration model of nicotine preference in rats: effects of mecamylamine. Psychopharmacol. 1996;128:426–431. doi: 10.1007/s002130050153. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Bowen DJ, Maycock VA, Nespor SM. The importance of sweet taste and caloric content in the effects of nicotine on specific food consumption. Psychopharmacol. 1985;87:198–203. doi: 10.1007/BF00431807. [DOI] [PubMed] [Google Scholar]
- Hahn EJ. Smokefree legislation: a review of health and economic outcomes research. Am J Prev Med. 2010;39(6 Suppl 1):S66–S76. doi: 10.1016/j.amepre.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Schuh KJ, Schuster CR, Henningfield JE, Goldberg SR. Reinforcing and subjective effects of morphine in human opioid abusers: effect of dose and alternative reinforcer. Psychopharmacol. 2000;148:272–280. doi: 10.1007/s002130050051. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Addiction and choice: theory and new data. Front Psychiatry. 2013;4(31):1–5. doi: 10.3389/fpsyt.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Hogarth L. Goal-directed and transfer-cue-elicited drug-seeking are dissociated by pharmacotherapy: evidence for independent additive controllers. J Exp Psych: Animal Beh Proc. 2012;38:266–278. doi: 10.1037/a0028914. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Chase HW. Parallel goal-directed and habitual control of human drug-seeking: implications for dependence vulnerability. J Exp Psych: Animal Beh Proc. 2011;37:261–276. doi: 10.1037/a0022913. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacol. 2012;37:2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacol. 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS One. 2007;2(2):e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacol. 2013;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2(8):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage MG. Toward a nonhuman model of contingency management: effects of reinforcing abstinence from nicotine self-administration in rats with an alternative nondrug reinforcer. Psychopharmacol. 2009;203:13–22. doi: 10.1007/s00213-008-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littel RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2. SAS Institute; Cary: 2006. [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Beebe-Wang N, Woicik PA, Konova AB, Maloney T, Goldstein RZ. Choice to view cocaine images predicts concurrent and prospective drug use in cocaine addiction. Drug Alcohol Depend. 2013;130:178–185. doi: 10.1016/j.drugalcdep.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, et al. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacol. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with D-amphetamine and flupenthixol. Neuropsychopharmacol. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacol. 2011;61:189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacol. 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacol. 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacol. 2007;32:646–657. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. A stimulus-control account of regulated drug intake in rats. Psychopharmacol. 2008;196:441–1450. doi: 10.1007/s00213-007-0978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Stimulus compounding enhances conditioned suppression produced by cocaine-paired stimuli. Exp Clin Psychopharmacol. 2000;8:6–13. doi: 10.1037//1064-1297.8.1.6. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR. Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacol. 2013;38:1198–1208. doi: 10.1038/npp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Broge M, Gerlach D, Sanders M, Grobe JE, Cherry C, Wilson AS. Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychol. 2002;21:332–339. doi: 10.1037//0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe J, Fonte C. Influence of acute smoking exposure on the subsequent reinforcing value of smoking. Exp Clin Psychopharmacol. 1997;5:277–285. doi: 10.1037//1064-1297.5.3.277. [DOI] [PubMed] [Google Scholar]
- Pescatore KA, Glowa JR, Riley AL. Strain differences in the acquisition of nicotine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2005;82:751–757. doi: 10.1016/j.pbb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacol. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Tan H, Bishop SF, Lauzon NM, Sun N, Laviolette SR. Chronic nicotine exposure switches the functional role of mesolimbic dopamine transmission in the processing of nicotine’s rewarding and aversive effects. Neuropharmacology. 2009;56:741–751. doi: 10.1016/j.neuropharm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–233. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzstein M. Microeconomic theory: concepts and connections. Routledge; New York: 2013. [Google Scholar]
- Wing VC, Shoaib M. Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacol. 2008;200:357–365. doi: 10.1007/s00213-008-1211-y. [DOI] [PubMed] [Google Scholar]


