Abstract
Functional imaging studies consistently report abnormal amygdala activity in major depressive disorder (MDD). Neuroanatomical correlates are less clear: imaging studies have produced mixed results on amygdala volume, and postmortem neuroanatomic studies have only examined cell densities in portions of the amygdala or its subregions in MDD. Here, we present a stereological analysis of the volume of, and the total number of neurons, glia, and neurovascular (pericyte and endothelial) cells in the basolateral amygdala in MDD. Postmortem tissues from 13 subjects with MDD and 10 controls were examined. Sections (~15/subject) taken throughout the rostral-caudal extent of the basolateral amygdala (BLA) were stained for Nissl substance and utilized for stereological estimation of volume and cell numbers. Results indicate that depressed subjects had a larger lateral nucleus than controls, and a greater number of total BLA neurovascular cells than controls. There were no differences in the number or density of neurons or glia between depressed and control subjects. These findings present a more detailed picture of BLA cellular anatomy in depression than has previously been available. Further studies are needed to determine whether the greater number of neurovascular cells in depressed subjects may be related to increased amygdala activity in depression.
Keywords: amygdala, depression, stereology, postmortem brain, cell numbers
Introduction
Neuroimaging studies have repeatedly demonstrated abnormal amygdala activity in patients with major depressive disorder (MDD). Patients respond to emotional stimuli with greater amygdala blood flow and glucose utilization than controls; subsets of patients also display abnormally high resting amygdala activity (Carlson et al. 2006; Drevets et al. 2008, 2002). Studies have also reported decreased prefrontal-amygdala correlated activity and functional connectivity, consistent with the hypothesis of impaired prefrontal regulation of the amygdala in MDD (Almeida et al. 2009; Anand et al. 2005; Matthews et al. 2008). The basolateral amygdala (BLA), which is intimately interconnected with the prefrontal cortex (and other higher-order neocortical regions, e.g., temporal; Carlsen and Heimer 1988; McDonald, 1998), is a likely source of disturbances in amygdala-prefrontal connectivity in depression, and neuroimaging data suggest that the BLA may be preferentially altered structurally and functionally in MDD (Sheline et al. 1998; Siegle et al. 2003).
Postmortem studies have reported MDD-associated abnormalities in BLA subnuclei, including changes in protein or mRNA levels for glutamatergic (Karolewicz et al. 2009) and monoaminergic (Anisman et al. 2008; Klimek et al. 2002; Xiang et al. 2008) receptors, suggesting several neurotransmitter systems may contribute to abnormal amygdala activity in depression. Less is known about basic BLA or amygdala anatomy in depression; more than a decade of neuroimaging studies have not resolved whether the volume of the adult amygdala is greater (Lange and Irle 2004; Malykhin et al. 2012; van Eijndhoven et al. 2009; Vassilopoulou et al. 2013; Weniger et al. 2006), lesser (Hastings et al. 2004; Sheline et al. 1998; Siegle et al. 2003; Tang et al. 2007; von Gunten et al. 2000; Yoshikawa et al. 2006) or unchanged (Inagaki et al. 2004; Munn et al. 2007) in MDD. The discrepant literature may be due to a number of variables, including depression duration or number of episodes (Frodl et al. 2002, 2003; Kronenberg et al. 2009; MacMaster et al. 2008), antidepressant medication (Hamilton et al. 2008), suicidality (Monkul et al. 2007; Spoletini et al. 2011), and genetic variations (Savitz and Drevets 2009; Zetzsche et al. 2008).
At the cellular level, a lower density of glia was observed in the postmortem amygdala of depressed subjects (Bowley et al. 2002; Hamidi et al. 2004). However, no MDD postmortem studies of the amygdala (Bowley et al. 2002; Hamidi et al. 2004) or BLA (Altshuler et al. 2010; Bezchlibnyk et al. 2007) have examined the amygdala or BLA in its entirety, such that the total number of cells could be estimated. The apparently discrepant neuroimaging reports regarding amygdala volume in MDD underline the necessity of whole-structure sampling for information about total cell numbers (Braendgaard and Gundersen 1986), since (for example) a decreased glia density (Bowley et al. 2002; Hamidi et al. 2004) may not indicate a lower number of glia, particularly if amygdala volume is indeed greater in depression (Lange and Irle 2004; Malykhin et al. 2012; van Eijndhoven et al. 2009; Vassilopoulou et al. 2013; Weniger et al. 2006).
Here, we undertook a stereological analysis of the volume and total number of cells in the BLA of 13 depressed and 10 control subjects. These parameters were assessed in well-preserved, celloidin-embedded tissues, containing the BLA through its rostral-caudal extent. Celloidin-embedded tissues are virtually immune to shrinkage in the z-axis following sectioning (Miguel-Hidalgo and Rajkowska 1999), which frees their analysis from some common sources of bias (i.e., overly-thin or uneven sections) in stereological studies (Dorph-Petersen et al. 2001; Gardella et al. 2003). The number of neurons, glia, and neurovascular (pericyte and endothelial) cells was estimated in the left hemisphere lateral (LAN), basal (BN), and accessory basal (ABN) nuclei. Potential associations of depression duration, antidepressant medication, and suicide with volume and cell numbers were examined.
Materials and Methods
Subjects
Basolateral amygdala tissue was examined from 13 subjects with MDD and 10 controls. The Institutional Review Boards of University Hospitals of Cleveland and University of Mississippi Medical Center approved the protocol for recruitment, tissue collection, and interviews. Retrospective, informant-based psychiatric assessments were performed using the Structured Clinical Interview for DSM-IV Psychiatric Disorders and consensus diagnosis (Stockmeier et al. 2004). Informed consent was obtained from the legally defined next-of-kin for collecting tissue and medical records and for an interview. Interviews were administered by a trained interviewer (LD), and relied on knowledgeable informants who lived with or were in frequent contact with subjects prior to the time of death. Diagnosis of Axis I disorders, and confirmation of their absence (controls), was independently assessed by a clinical psychologist (JCO) and psychiatrist (GJJ) and consensus diagnosis was reached in conference. Subject details are presented in Table 1.
Table 1.
Subject details.
| Group | Age/Sex | Cause of Death | PMI (hrs) | Toxicology (blood) | Axis I Diagnosis | Age of MDD Onset | Duration of MDD (Years) | Episodes (1 = 1; 2= >1) |
|---|---|---|---|---|---|---|---|---|
| Control | 44/F | Accidental acute intoxication from brompheniramine; CVD | 21.0 | Brompheniramine, orphenadrine | No diagnosis | |||
| Control | 50/F | CVD | 20.0 | Nothing detected | No diagnosis | |||
| Control | 60/F | CVD | 17.3 | Nothing detected | No diagnosis | |||
| Control | 65/F | CVD | 26.0 | Nothing detected | No diagnosis | |||
| Control | 18/M | Bronchial asthma | 31.3 | Midazolam | No diagnosis | |||
| Control | 28/M | Bronchial asthma | 35.5 | Nothing detected | No diagnosis | |||
| Control | 32/M | Blunt trauma to head, trunk and extremities | 25.0 | Nothing detected | No diagnosis | |||
| Control | 53/M | Acute gastric ulcer with hemorrhage | 26.5 | Meperidine, promethazine, morphinea | No diagnosis | |||
| Control | 61/M | CVD | 16.0 | Nothing detected | No diagnosis | |||
| Control | 62/M | CVD | 21.0 | Nothing detected | No diagnosis | |||
| MDD | 42/F | CVD | 12.5 | Venlafaxine | MDD | 27 | 15 | 2 |
| MDD | 44/F | Suicide, gunshot | 24.0 | Bupropion, diphenhydramine, venlafaxine, hydrocodone | MDD | 33 | 12 | 2 |
| MDD | 48/F | Suicide, hanging | 6.5 | Citalopram, trazodone | MDD | 20 | 28 | 2 |
| MDD | 50/F | CVD | 28.5 | Dextromethorphan | MDD, in partial remission; GAD; cannabis dependence | 32 | 18 | 2 |
| MDD | 56/F | Acute bronchopneumonia | 38.0 | Doxylamine | MDD; GAD | 55 | 1 | 1 |
| MDD | 61/F | Suicide, gunshot | 37.8 | Nothing detected | MDD; hypochondriasis, mild | 49 | 12 | 2 |
| MDD | 19/M | Suicide, gunshot | 24.2 | Delta 9-THC | MDD; cannabis dependence | 14 | 4.5 | 2 |
| MDD | 37/M | Suicide, gunshot | 31.5 | Ethanol 0.05 g/dL | MDD; learning disorder NOS | 37 | .08 | 1 |
| MDD | 51/M | CVD | 11.5 | Lorazepam, bupropion, citalopram | MDD | 16 | 35 | 2 |
| MDD | 54/M | CVD | 38.0 | Dextromethorphan, fluoxetine | MDD | 32 | 22 | 2 |
| MDD | 55/M | Suicide, gunshot | 10.0 | Nothing detected | MDD | 55 | .25 | 1 |
| MDD | 59/M | Suicide, carbon monoxide | 26.8 | C0, fluoxetine | MDD; GAD; specific phobia, situational type | 54 | 5 | 2 |
| MDD | 62/M | CVD; emphysema | 22.0 | Nothing detected | MDD | 49 | 13 | 2 |
| Age | Sex Distribution | PMI | Tissue pH | Weeks in Fixative | Days in EtOH | Brain Weight (gms) | Tissue Thickness (μm) | |
| Averages: | ||||||||
| Control | 47± 5.16 | 4 F/ 6 M | 24±1.94 | 6.56±.087 | 159±31.63 | 485±12 | 1372± | 39±.36 |
| MDD | 49±3.25 | 6 F/ 7 M | 24±3.06 | 6.46±.11 | 129±17.79 | 9.16 | 40.33 | 40±.43 |
| 228±61.80 | 1402±50.50 | |||||||
| p-value: | 0.77 | 0.999 | 0.997 | 0.515 | 0.398 | 0.067 | 0.663 | 0.163 |
CVD, cardiovascular disease; EtOH, ethanol; F, female; GAD, generalized anxiety disorder; M, male; MDD, major depressive disorder; NOS, not otherwise specified; PMI, postmortem interval.
These substances given on day of surgery.
Bottom: means ± standard errors are shown; p-values indicate no group differences in demographics or the given tissue qualities and storage times.
No subjects with history or neuropathological evidence of neurologic disorders were included. Tissues of six subjects (4 MDD) were collected after a postmortem interval of greater than 30 hours (but not more than 38; Table 1). These and all other tissues were histopathologically normal, with no apparent pyknosis or vacuolization. Subjects in the MDD group met DSM-IV criteria for a major depressive episode in the last two weeks of life, with the exception of one subject in partial remission. Subjects in both groups were excluded in the case of substance abuse disorders, with exceptions (cannabis) given in Table 1. Among controls, one subject met criteria for an adjustment disorder with depressed mood for three to four months that resolved nine months before death, and two other subjects had met criteria for alcohol abuse and alcohol dependence at 8–10 years prior to death. No controls met criteria for these or other Axis I disorders at the time of death. In the depressed group, three subjects had met criteria for alcohol plus cannabis abuse, alcohol abuse, or alcohol dependence, that were in remission for 9 months, 15 years and 4 months, respectively, prior to their deaths. Controls never met criteria for MDD, and no subjects ever met criteria for bipolar disorder or schizophrenia. Blood and urine samples were tested by the Cuyahoga County Coroner’s Office for psychotropic medications and psychoactive substances. Subjects with psychoactive substances other than prescribed medications or marijuana were excluded; blood alcohol levels for included subjects were ≤ 0.1%. No subjects had undergone electroconvulsive therapy or were taking lithium.
Histology
Tissues from the left temporal lobe, containing the BLA through its rostral-caudal extent, were collected at autopsy from the Cuyahoga County Coroner’s Office in Cleveland, Ohio. More dorsal tissues containing the central, medial and some cortical amygdala nuclei were unavailable for roughly half the subjects, so that these regions could not be included in a reliable analysis. Tissues were fixed in phosphate-buffered formalin, cut into 6 mm blocks, and embedded in celloidin (Rajkowska and Goldman-Rakic 1995). Codes were assigned to prevent experimenter bias. Tissue pH was determined from the cerebellum.
Tissue was sectioned coronally at 40 μm on a sliding microtome, and stored in 70% ethanol until staining. Approximately every 20th section was stained with Cresyl Violet for microscopic analysis. For one MDD subject, medial portions of the BLA were unavailable, such that only lateral nucleus volume and cells could be reliably quantified.
Quantitative Analysis
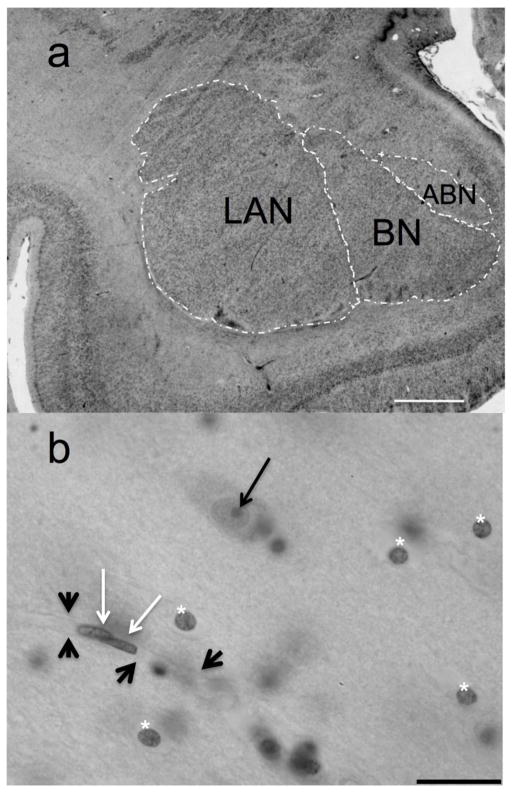
BLA subnuclei were parcellated according to cytoarchitectonic criteria (Gloor 1997; Schumann and Amaral 2005) under low power (2× objective, 0.06 NA), with a Nikon E800 light microscope attached to an Optronics video camera (Goleta, CA). StereoInvestigator software (v.8, MicroBrightField) was used to facilitate cell counts and to measure the areas of the LAN, BN, and ABN (Figure 1), across approximately 15 sections/subject (Table 2). The densely packed cells of the paralaminar nucleus were included within the borders of the basal nucleus (largely) or the lateral nucleus as appropriate (Schumann and Amaral 2005; deCampo and Fudge 2012).
Fig. 1.
The basolateral amygdala at low and high magnifications. a, Low power image of the basolateral amygdala of a male control subject at mid-level. ABN, accessory basal nucleus; BN, basal nucleus; LAN, lateral nucleus. Scale bar = 2 mm. b, LAN cells of a depressed male subject at high magnification. Neurons were identified on the bases of morphological features such as comparatively large Nissl-stained somata, clear nuclei surrounded by visible cytoplasm, and the presence of a single, clear nucleolus (black arrow). Glia (below asterisks) were distinguished from neurovascular cells (white arrows) by multiple criteria: neurovascular cells are always seen in the context of vessels (thick black arrowheads), and often are seen in close association with other neurovascular cells; peripheral chromatin in the nuclei of pericytes and endothelial cells produces the appearance of a thick membrane. These nuclei are elongated or crescent-shaped in cross-section, to conform to the vessel lumen. Glial cell nuclei tend to be rounded or slightly oval, contain conspicuous clumps of chromatin, and unlike neurons, do not display visible Nissl-stained cytoplasm and processes surrounding the nucleus. Scale bar = 20 μm
Table 2.
Stereological parameters.
| BLA subdivision | Mean CE - volume | Mean no. of sections sampled | Mean no. of neurons counted | Mean CE - neurons | Mean no. of glia counted | Mean CE - glia | Mean no. of neurovascular cells counted | Mean CE – neurovascular cells | Disector dimensions (μm) (w*l*h) | Step size between disectors (μm) |
|---|---|---|---|---|---|---|---|---|---|---|
| LAN | 0.014 | 14 | 234 | 0.066 | 1120 | 0.031 | 164 | 0.083 | 60×60×28 | 18002 |
| BN | 0.014 | 15 | 257 | 0.063 | 936 | 0.034 | 145 | 0.088 | 60×60×28 | 16002 |
| ABN | 0.016 | 15 | 233 | 0.066 | 997 | 0.032 | 189 | 0.077 | 60×60×28 | 10002 |
ABN, accessory basal nucleus; BLA, basolateral amygdala; BN, basal nucleus; CE, coefficient of error, LAN, lateral nucleus.
A minimum of 200 neurons was counted in each subnucleus. A guard zone of 3 μm was applied at the top and bottom of each sampling site. Counting frames contained the appropriate inclusion and exclusion lines.
Volume of the three subnuclei was calculated as the sum of their areas multiplied by the distance between the upper planes of the examined sections (Gundersen et al. 1988; Uylings et al. 1986), as determined using measured thickness of the tissue (West et al. 1991). In the absence of evidence for differential tissue shrinkage according to group (Table 1), these post-processing tissue dimensions were used for statistical comparisons of BLA volume, without shrinkage correction.
Cells were systematically-randomly sampled (Mouton 2002) under high magnification (100× oil objective; 1.40 NA), with a virtual three dimensional counting frame (for all subnuclei, dimensions were 60×60×28 μm (width × length × height), with a guard zone of ±3 μm applied at each sampling site; further sampling parameters and coefficients of error (Gundersen and Jensen 1987; Gundersen et al. 1999) are given in Table 2). Total cell numbers were estimated with the optical fractionator (West et al. 1991). Briefly, cell numbers were estimated by calculating the number of cells sampled throughout each subnucleus divided by the fraction of tissue sampled (sections sampled/total number of sections containing the subnucleus, area of the subnucleus sampled/section, and tissue height sampled/height of tissue; West et al. 1991). Because cell numbers are sampled from a known fraction of the tissue, cell number estimates are independent of tissue volume estimates (and any biases that may be associated with volume estimates, e.g., due to the possibility of differential shrinkage according to group). Tissue thickness was measured at every 4th sampling site (Abitz et al. 2007; Christensen et al. 2007). Neurons were sampled by counting nucleoli, and glia and neurovascular cells were sampled by counting nuclei; pericytes and endothelial cells were counted together as neurovascular cells. Preliminary data indicated no association of depression with cell size so this measure was not assessed. Further sampling parameters and coefficients of error (Gundersen and Jensen 1987; Gundersen et al. 1999) are given in Table 2. Criteria for distinguishing among neurons, glia and neurovascular cells are detailed in Figure 1.
Statistical Analysis
ANCOVA models similar to independent samples t-tests but adjusted for age, PMI, gender, and time tissue was stored in ethanol were used to compare groups. These variables were selected because they influenced volumetric or cellular measures and, in the case of time in ethanol, because there was a trend for a group difference, with MDD tissue stored in ethanol for less time than control tissue (Table 1; also see Subject Variables, below). A Kenward-Rogers adjustment was applied to account for heteroscedasticity between groups. We considered p < 0.05 evidence of statistical significance in analyses of depressed vs. control subjects; in comparisons where three groups were involved (controls versus two MDD subgroups, such as suicide/no suicide), ANCOVAs were followed by Bonferroni-adjusted (p < 0.0167) pairwise comparisons.
Volume and cell numbers were analyzed for each of the three BLA subnuclei and for the total BLA (reflecting the sum of the volumes and cell numbers respectively of the LAN, BN, and ABN). Cell densities were analyzed for each of the subnuclei; no attempt was made to average cell densities across the total BLA, because of the different parameters used to sample the three subnuclei (Table 2).
Depression duration was analyzed by comparing subjects depressed more than 5 years (LMDD) versus subjects depressed for 5 years or fewer (SMDD). The division (as opposed to correlational analysis) and the 5-year cut-off were selected because of the bimodal distribution of depression duration, with all subjects depressed for either fewer than 5 or more than 10 years (Table 1). Effects of antidepressant medication were analyzed by comparing MDD subjects with and without positive toxicology exams for antidepressants.
Results
Subject Variables
There were no group differences in age, postmortem interval (PMI), or sex distribution, either in comparisons of MDD versus control subjects or in comparisons between MDD subgroups (sorted by suicide, antidepressant medication, or depression duration), except for a skewed sex distribution by depression duration (Table 1). There was a trend for a group difference in the average amount of time that tissue was stored in ethanol, with control tissue in ethanol somewhat longer (p = 0.067; Table 1). Age positively correlated with glia density in the LAN [r=.45; p <0.033] and with glia to neuron ratio in the ABN [r=.46; p < 0.033]. PMI negatively correlated with glia density in the BN [r=−.52; p < 0.013] and with ABN glia density [r=−.50; p < 0.019] and ABN glia number [r=−.50; p < 0.019]. There were sex differences favoring males in the volume of the BN [t(20)=2.79; p < 0.012] and the BLA as a whole [t(20)=2.27; p < 0.035]. Males had significantly greater numbers of neurovascular cells than females in the LAN [t(21)=2.56; p < 0.019], the BN [t(20)=2.17; p < 0.043] and in the BLA as a whole [t(20)=3.24; p < 0.005], and higher neurovascular cell density in the LAN [t(21)=2.27; p < 0.034]. Time that tissue was stored in ethanol correlated positively with LAN neuron number [r=.45; p < 0.033], and negatively with ABN neurovascular cell number [r=−.44; p < 0.039]. Accordingly age, PMI, sex, and days in ethanol were adjusted for in the ANCOVA analyses. To compensate for skewness, the factor of time in ethanol was defined in all analyses as the base-10 logarithmic transformation of days in ethanol. There were no group differences in tissue pH, weeks in fixative, brain weight or tissue thickness (Table 1). Tissue pH correlated positively with neuron density in the BN [r=.63; p <0.004] and the ABN [r=.64; p <0.004], and negatively with volume of the ABN [r=−.59; p <0.009]; but due to unavailability of pH values for 4 subjects (2 per group), we were unable to use pH as an ANCOVA covariate.
Diagnosis
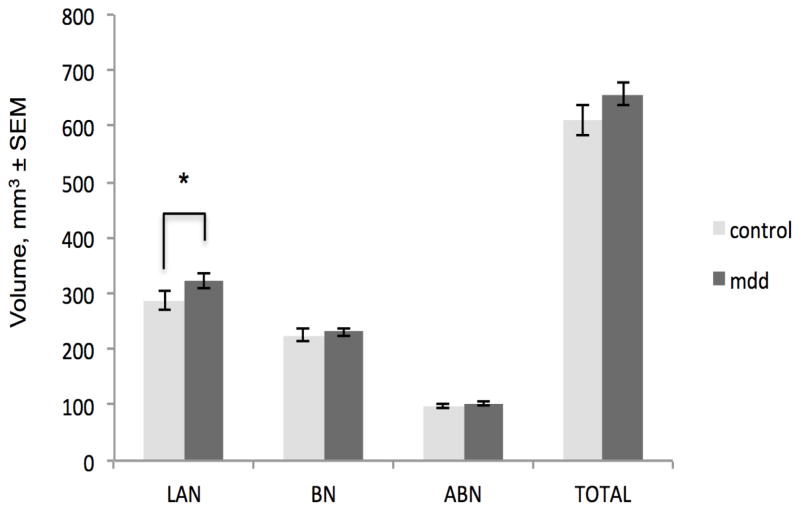
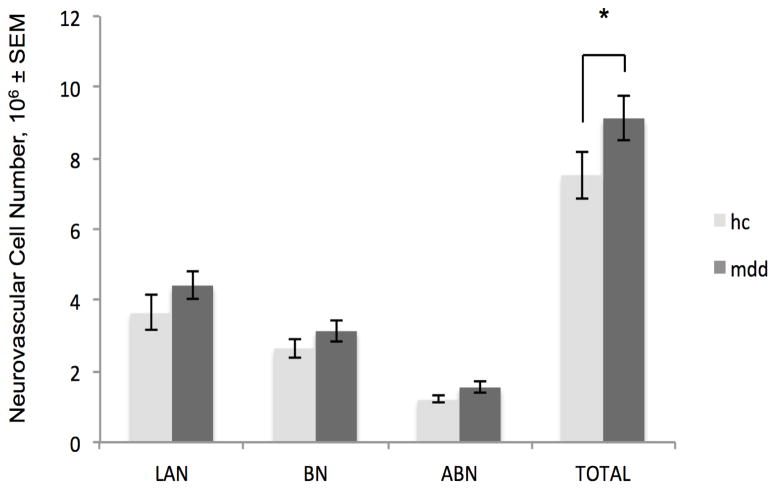
MDD subjects had a greater LAN volume than controls (p= 0.0473; Figure 2. For detailed statistical information, see Table 3). MDD subjects had a greater number of total BLA neurovascular cells than controls (p = 0.0455; Figure 3). MDD subjects did not differ from controls in neuron or glia numbers or densities in any part of the BLA. (Neurovascular cell densities did not differ between groups; for group means and standard errors on all measures, see Table 4.)
Fig. 2.
Greater volume of the lateral nucleus in major depressive disorder. Depressed subjects (mdd) had an 11% greater LAN volume than healthy control (hc) subjects. *p <0.05. Note that histograms display unadjusted means; indications of statistical significance and p-values reflect results of the adjusted statistics. LAN, lateral nucleus, BN, basal nucleus, ABN, accessory basal nucleus, TOTAL, total basolateral amygdala. SEM, standard error of the mean
Table 3.
Results of statistical analyses.
| t, df, p | F, df, p | t, df, p | F, df, p | t, df, p | F, df, p | |
|---|---|---|---|---|---|---|
|
| ||||||
| MDD vs. Control | Suicide vs. No Suicide vs. Control | Pairwise: Suicide vs. No Suicide vs. Control | sMDD vs. lMDD vs. Control | Pairwise: sMDD vs. lMDD vs. Control | Antidepressants vs. No Antidepressants vs. Control | |
|
| ||||||
| Volume | ||||||
| LAN | −2.15, (15.6), 0.047* | 2.07, (2,9.5), 0.179 | 2.47, (2,9.4), 0.138 | 2.16, (2,9.5), 0.168 | ||
| BN | −.81, (16.0), 0.428 | .25, (2,5.9), 0.784 | 1.77, (2,8.9), 0.225 | .37, (2,7.5), 0.70 | ||
| ABN | −.82, (15.3), 0.426 | .27, (2,8.4), 0.768 | .35, (2,6.1), 0.715 | .86, (2,7.0), 0.465 | ||
| BLA Total | −1.73, (15.9), 0.102 | 1.27, (2,7.4), 0.336 | 2.74, (2,7.3), 0.129 | 1.35, (2,7.6), 0.316 | ||
|
| ||||||
| Neuron Number | ||||||
| LAN | −.80, (16.6), 0.433 | .52, (2,9.1), 0.613 | .54, (2,8.6), 0.60 | .31, (2,9.0), 0.742 | ||
| BN | .64, (14.6), 0.531 | 1.03, (2,7.2), 0.405 | .51, (2,7.0), 0.620 | .71, (2,6.9), 0.523 | ||
| ABN | .63, (16.0), 0.536 | .17, (2,6.9), 0.845 | .23, (2,7.6), 0.802 | .97, (2,6.5), 0.427 | ||
| BLA Total | −.21, (15.6), 0.834 | .81, (2,8.1), 0.477 | .07, (2,7.4), 0.931 | .14, (2,7.5), 0.875 | ||
|
| ||||||
| Neuron Density | ||||||
| LAN | .47, (10.4), 0.651 | 8.68, (2,7.8), 0.011* |
LAN pairwise: Suicide vs. No Suicide: −4.31, (4.9), 0.008* Suicide vs. Control: −.51, (9.0), 0.619 No Suicide vs. Control: 1.79, (12.9), 0.097 |
.77, (2,7.5), 0.495 | .23, (2,5.7), 0.802 | |
| BN | .62, (15.9), 0.545 | .43, (2,6.4), 0.667 | .91, (2,7.2), 0.445 | .38, (2,7.6), 0.699 | ||
| ABN | 1.13, (15.9), 0.273 | .64, (2,7.4), 0.555 | .37, (2,7.9), 0.702 | .58, (2,8.0), 0.584 | ||
|
| ||||||
| Glia Number | ||||||
| LAN | −1.80, (15.6), 0.091 | 2.17, (2,6.8), 0.187 | 1.48, (2,7.2), 0.289 | 1.54, (2,7.8), 0.273 | ||
| BN | −.18, (15.5), 0.861 | 3.24, (2,7.2), 0.099a | 1.58, (2,7.6), 0.267 | .05, (2,7.4), 0.954 | ||
| ABN | −.54, (15.2), 0.597 | 2.18, (2,8.6), 0.171 | .89, (2,9.4), 0.443 | 1.33, (2,8.9), 0.313 | ||
| BLA Total | −1.31, (14.0), 0.212 | 2.05, (2,5.8), 0.213 | 1.13, (2,6.4), 0.379 | .94, (2,6.7), 0.435 | ||
|
| ||||||
| Glia Density | ||||||
| LAN | −.79, (16.0), 0.442 | 1.23, (2,8.7), 0.339 | .61, (2,7.7), 0.566 | .29, (2,9.0), 0.753 | ||
| BN | −.24, (15.6), 0.813 | 3.77, (2,8.2), 0.069a | .59, (2,7.0), 0.578 | .10, (2,7.6), 0.908 | ||
| ABN | −.37, (15.9), 0.719 | 3.22, (2,8.0), 0.094a | .16, (2,7.4), 0.858 | .09, (2,8.7), 0.918 | ||
|
| ||||||
| Glia to Neuron Ratio | ||||||
| LAN | −1.42, (14.8), 0.047 | .90, (2,2.1), 0.521 | .99, (2,10.0), 0.405 | 1.77, (2,9.5), 0.223 | ||
| BN | −.73, (13.3), 0.481 | .67, (2,7.1), 0.542 | 3.08, (2,5.7), 0.124 | 1.13, (2,6.5), 0.378 | ||
| ABN | −1.39, (15.4), 0.185 | 4.01, (2,9.1), 0.056a | 1.16, (2,7.9), 0.363 | .84, (2,8.4), 0.464 | ||
| BLA Total | −1.81, (15.7), 0.090 | 1.46, (2,8.0), 0.288 | 3.74, (2,6.2), 0.086a | 2.68, (2,7.5), 0.133 | ||
|
| ||||||
| NV Cell Number | ||||||
| LAN | −1.91, (16.2), 0.073 | 1.69, (2,9.2), 0.237 | 1.98, (2,8.7), 0.196 |
ABN pairwise: sMDD vs. lMDD: 1.17, (8.0), 0.277 sMDD vs. Control: −3.56, (9.3), 0.006* lMDD vs. Control: −.36, (8.3), 0.731 |
1.62, (2,8.5), 0.254 | |
| BN | −1.10, (15.8), 0.289 | .56, (2,7.1), 0.596 | 1.17, (2,6.8), 0.366 | 1.17, (2,7.6), 0.361 | ||
| ABN | −1.56, (14.7), 0.140 | 1.25, (2,6.3), 0.349 | 6.07, (2,9.8), 0.019* | 4.59, (2,7.0), 0.053b | ||
| BLA Total | −2.18, (14.9), 0.046* | 2.25, (2,8.2), 0.167 | 2.72, (2,7.1), 0.133 | 2.09, (2,8.9), 0.180 | ||
|
| ||||||
| NV Cell Density | ||||||
| LAN | −1.68, (17.0), 0.111 | 2.48, (2,9.3), 0.137 | 1.28, (2,7.8), 0.332 | 1.10, (2,7.8), 0.379 | ||
| BN | −1.35, (14.9), 0.197 | .60, (2,7.9), 0.574 | 2.03, (2,8.6), 0.190 | 2.23, (2,6.9), 0.179 | ||
| ABN | −1.53, (14.8), 0.148 | 1.16, (2,6.3), 0.373 | 4.02, (2,8.5), 0.059a | 2.44, (2,6.8), 0.159 | ||
Where three groups were compared (all columns except column one), significant ANCOVA results are followed by results of pairwise analyses.
Significant p-values, also given under Results, are asterisked and in boldface; trends (not in Results) are bolded. Degrees of freedom are fractional as a result of the Kenward-Rogers approach used to account for heteroscedasticity (see Materials and Methods, Statistical Analysis).
df, degrees of freedom; sMDD, subjects depressed for 5 years or fewer; lMDD, subjects depressed for longer than 5 years; LAN, lateral nucleus; BN, basal nucleus; ABN, accessory basal nucleus; NV cell, neurovascular cell.
The direction of ANCOVA trends, not elaborated in the table with pairwise comparisons, may be determined from Table 4.
Because subgroup means according to antidepressant subgrouping are not given in Table 4, the direction of any pairwise differences for the ANCOVA trend according to medication are broken down here. Pairwise comparisons of neurovascular cell numbers in the ABN indicated no difference between depressed subgroups with and without medication [t(4.7)= .93, p=0.40], and no difference between depressed subjects taking antidepressants and control subjects [t(4.5)= −.20, p=0.849]. A difference (which theoretically would have been significant, under a planned comparison) was seen between MDD subjects without antidepressants vs. controls, with MDD subjects having the greater number of neurovascular cells (as seen in MDD subjects in the BLA as a whole) [t(9.7)= −3.15, p=0.011].
Fig. 3.
Greater number of basolateral amygdala neurovascular cells in major depressive disorder. Depressed subjects (mdd) had a 19% greater total number of basolateral amygdala neurovascular cells than healthy control (hc) subjects. *p <0.05. Note that histograms display unadjusted means; indications of statistical significance and p-values reflect results of the adjusted statistics. LAN, lateral nucleus, BN, basal nucleus, ABN, accessory basal nucleus, TOTAL, total basolateral amygdala. SEM, standard error of the mean
Table 4.
Unadjusted data (means ± standard errors) are shown for all volumetric and cellular measures, sorted by gender, depression duration, and suicide.
| Control: | Volume (mm3) | Neuron number (106) | Neuron densitya (103) | Glia number (106) | Glia densitya (103) | Glia to neuron ratiob | NV cell number (106) | NV cell densitya (103) |
|---|---|---|---|---|---|---|---|---|
| LAN: | ||||||||
| Male | 310±19.3 | 5.73±.40 | 17.94±.98 | 27.05±1.85 | 85.01±5.53 | 4.75±.21 | 4.48±.59 | 13.94±1.57 |
| Female | 253±23.4 | 5.55±.65 | 22.12±.96 | 24.19±2.08 | 97.73±4.71 | 4.43±.25 | 2.43±.36 | 9.61±.62 |
| All | 288±16.9 | 5.66±.33 | 19.61±.95 | 25.91±1.39 | 90.10±4.18 | 4.62±.16 | 3.66±.49 | 12.21 ±1.17 |
| BN: | ||||||||
| Male | 245±6.2 | 5.60±.25 | 23.36±1.39 | 18.69±1.10 | 77.61±4.41 | 3.34±.13 | 2.81±.39 | 11.70±1.66 |
| Female | 192±15.1 | 4.81±.11 | 24.78±2.08 | 18.45±.86 | 94.16±4.37 | 3.84±.16 | 2.42±.36 | 12.11±1.19 |
| All | 224±10.9 | 5.28±.20 | 23.93±1.13 | 18.59±.71 | 84.23±4.04 | 3.54±.13 | 2.65±.27 | 11.86±1.05 |
| ABN: | ||||||||
| Male | 104±4.0 | 1.86±.11 | 17.63±.60 | 7.38±.63 | 69.82±4.40 | 3.97±.24 | 1.28±.15 | 12.00±1.14 |
| Female | 90±4.3 | 1.56±.11 | 18.36±.73 | 6.84±.19 | 81.06±3.11 | 4.44±.25 | 1.13±.12 | 13.34±1.18 |
| All | 98±3.5 | 1.74±.09 | 17.92±.45 | 7.16±.38 | 74.32±3.33 | 4.16±.18 | 1.22±.10 | 12.54±.82 |
| TOTAL: | ||||||||
| Male | 659±19.9 | 13.19±.50 | 53.12±2.27 | 3.97±.14 | 8.56±.80 | |||
| Female | 535±41.5 | 11.92±.57 | 49.49±2.59 | 4.20±.10 | 5.98±.62 | |||
| All | 610±27.7 | 12.68±.41 | 51.67±1.72 | 4.06±.10 | 7.53±.66 | |||
| MDD:
| ||||||||
| LAN: | ||||||||
| Male | 330±20.8 | 6.07±.51 | 19.81±.42 | 30.52±2.72 | 99.81±4.08 | 5.03±.16 | 4.85±.59 | 16.17±2.25 |
| Female | 313±12.6 | 5.59±.18 | 18.47±.75 | 26.93±1.92 | 88.19±4.02 | 4.79±.21 | 3.90±.37 | 12.77±.95 |
| ≤5 Yrs | 353±19.7 | 6.46±.58 | 19.54±.91 | 32.05±3.47 | 97.23±8.10 | 4.95±.22 | 5.41±.65 | 17.03±3.14 |
| > 5 Yrs | 302±12.0 | 5.47±.23 | 18.98±.47 | 26.87±1.58 | 92.71±2.10 | 4.91±.16 | 3.79±.30 | 13.08±.76 |
| Suicide | 330±19.6 | 6.20±.44 | 20.11±.44 | 30.39±2.69 | 98.27±4.53 | 4.89±.19 | 4.67±.65 | 15.43±2.46 |
| No suicide | 312±14.9 | 5.44±.31 | 18.11±.54 | 27.08±2.02 | 89.98±4.23 | 4.96±.17 | 4.11±.31 | 13.63±.64 |
| All | 322±12.4 | 5.85±.29 | 19.19±.44 | 28.86±1.72 | 94.45±3.22 | 4.92±.13 | 4.41±.37 | 14.60±1.33 |
| BN: | ||||||||
| Male | 238±9.5 | 5.18±.20 | 22.97±.98 | 20.20±1.56 | 88.02±3.09 | 3.89±.26 | 3.59±.42 | 15.66±1.43 |
| Female | 222±12.4 | 4.77±.53 | 22.33±1.72 | 16.97±.89 | 80.56±5.53 | 3.66±.27 | 2.48±.23 | 11.72±1.12 |
| ≤5 Yrs | 250±5.2 | 5.05±.34 | 21.29±1.42 | 20.73±2.09 | 86.56±6.17 | 4.10±.29 | 3.45±.51 | 14.29±1.56 |
| > 5 Yrs | 218±9.9 | 4.99±.36 | 23.72±1.02 | 17.51±.86 | 83.74±3.05 | 3.58±.22 | 2.90±.37 | 13.82±1.60 |
| Suicide | 236±13.3 | 5.35±.37 | 23.39±1.36 | 20.84±1.45 | 90.58±3.02 | 3.96±.31 | 3.18±.50 | 13.48±1.48 |
| No suicide | 226±8.2 | 4.67±.28 | 22.02±1.15 | 16.87±1.13 | 79.25±4.15 | 3.63±.20 | 3.08±.38 | 14.55±1.70 |
| All | 231±7.6 | 5.01±.24 | 22.71±.87 | 18.85±1.06 | 84.91±2.98 | 3.79±.18 | 3.13±.30 | 14.02±1.09 |
| ABN: | ||||||||
| Male | 106±6.7 | 1.69±.12 | 16.76±.66 | 7.80±.62 | 76.68±2.83 | 4.60±.18 | 1.71±.22 | 16.57±1.56 |
| Female | 101±7.2 | 1.67±.08 | 17.53±.99 | 6.87±.33 | 72.43±5.29 | 4.13±.21 | 1.31±.11 | 13.92±1.68 |
| ≤5 Yrs | 108±5.5 | 1.72±.13 | 16.66±1.04 | 7.77±.68 | 75.42±5.91 | 4.53±.26 | 1.58±.10 | 15.32±.95 |
| > 5 Yrs | 101±7.3 | 1.66±.09 | 17.37±.63 | 7.15±.49 | 74.54±2.42 | 4.31±.18 | 1.52±.24 | 15.57±1.96 |
| Suicide | 101±3.1 | 1.71±.12 | 17.24±.70 | 7.89±.48 | 79.81±2.73 | 4.65±.18 | 1.55±.12 | 15.82±1.34 |
| No suicide | 107±9.3 | 1.66±.10 | 16.91±.91 | 6.93±.61 | 70.00±3.81 | 4.15±.19 | 1.53±.28 | 15.11±2.03 |
| All | 104±4.7 | 1.68±.08 | 17.07±.55 | 7.41±.40 | 74.91±2.68 | 4.40±.15 | 1.54±.14 | 15.47±1.17 |
| TOTAL: | ||||||||
| Male | 674±28.5 | 12.94±.62 | 58.52±4.33 | 4.49±.17 | 10.14 ±.83 | |||
| Female | 635±29.3 | 12.03±.57 | 50.90±2.85 | 4.17±.21 | 7.69 ±.50 | |||
| ≤5 Yrs | 711±21.9 | 13.23±.89 | 60.56±5.87 | 4.52±23 | 10.44 ±1.12 | |||
| > 5 Yrs | 619±22.5 | 12.09±.37 | 51.61±2.15 | 4.24±.17 | 8.18 ±.53 | |||
| Suicide | 670±36.1 | 13.35±.70 | 59.81±4.55 | 4.48±.23 | 9.52±1.23 | |||
| No suicide | 646±22.4 | 11.78±.33 | 50.88±2.93 | 4.23±.15 | 8.72±.37 | |||
| All | 658±20.6 | 12.56±.44 | 55.34±2.91 | 4.36±.14 | 9.12 ±.63 | |||
Note that statistical analyses were performed on adjusted means; see text for statistically significant differences among groups. Volumetric calculations were performed on post-processing tissue dimensions without correction for shrinkage; cell numbers were calculated with the optical fractionator, which generates estimates that are independent of shrinkage (West et al. 1991).
Density values reflect cells/mm3.
Glia to neuron ratio was lowest in the BN, which contains a large proportion of the paralaminar nucleus, containing densely packed neurons.
ABN, accessory basal nucleus; BN, basal nucleus; LAN, lateral nucleus; NV cell, neurovascular cell.
TOTAL refers to the basolateral amygdala as a whole. ≤5 Yrs, depression duration of five years or fewer; > 5 Yrs, depression duration of more than five years.
Depression Duration
Subjects depressed for 5 years or fewer (SMDD; n=5, 1 female, 4 male) were compared to controls (n=10, 4 female, 6 male) and to subjects depressed more than 5 years (LMDD; n=8, 5 female, 3 male).
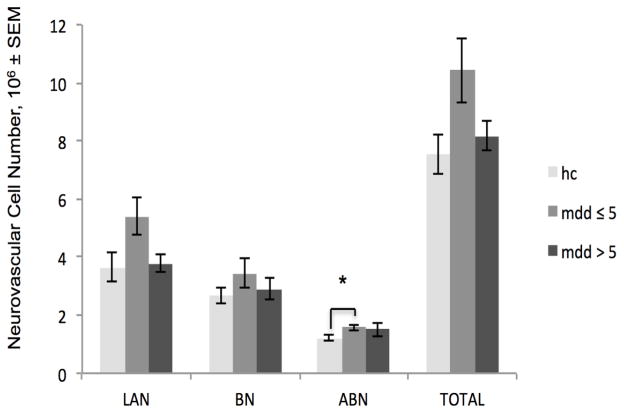
ANCOVA analysis indicated a significant association of depression duration with the number of neurovascular cells in the ABN (p= 0.0194). Pairwise comparisons indicated SMDD subjects had greater neurovascular cell numbers than controls (p= 0.0058; Figure 4). No other measures in the BLA or individual subnuclei significantly differed in the analyses of depression duration in male and female subjects combined.
Fig. 4.
Depression duration is significantly associated with the number of neurovascular cells in the accessory basal nucleus. Subjects who were depressed for 5 years or fewer (mdd≤5) had 27% more ABN neurovascular cells than control (hc) subjects. (Apparent differences in other BLA regions or between subjects depressed for more than 5 years (mdd>5) and mdd ≤5 were not significant in statistical analyses, which were adjusted for gender, age, postmortem interval and time in ethanol.) *p <0.0167. Note that histograms display unadjusted means; indications of statistical significance and p-values reflect results of the adjusted statistics. LAN, lateral nucleus, BN, basal nucleus, ABN, accessory basal nucleus, TOTAL, total basolateral amygdala. SEM, standard error of the mean
Suicide
There was a significant association of suicide with neuron density in the LAN (p= 0.0105). Pairwise comparisons indicated MDD suicide victims (n=7, 3 female, 4 male) had greater neuron density than MDD subjects who died of natural causes (n=6, 3 female, 3 male; p= 0.0079).
Antidepressant Medication
There were no significant group differences on any volumetric or cellular measures associated with antidepressant medication (present in 6 of 13 MDD subjects, 3 female, 3 male).
Discussion
The present study, to our knowledge, represents the first stereological analysis of the volume and total number of cells in the basolateral amygdala in major depressive disorder. Depressed subjects had a substantially (19%) greater number of total BLA neurovascular cells than controls (Figure 3). This difference might appear to be largely attributable to those who were depressed for five years or fewer (SMDD subjects; Figure 4), however a statistically significant difference between controls and SMDD subjects was only seen in the accessory basal nucleus, and SMDD did not differ from longer-term depressed (LMDD) subjects in neurovascular cell numbers (Table 3). The implication of a greater number of endothelial cells and/or pericytes in depression is not known, but may be regionally specific in response to depression. In animal models of antidepressant electroconvulsive therapy, there is evidence of endothelial cell proliferation in the hippocampus and hypothalamus (Jansson et al. 2006; Rotheneichner et al. 2014). In humans, antidepressants are associated with hippocampal angiogenesis (along with greater numbers of neural progenitor cells; Boldrini et al. 2012) and there is evidence that in older men, depression is associated with a greater serum concentration of the angiogenesis inhibitor endostatin (Almeida et al. 2014). It appears that brain endothelial cell proliferation and angiogenesis may therefore reflect antidepressant effects in some regions, but it is possible that in the amygdala, which responds to stress very differently from the hippocampus in animal models (e.g., Vyas et al. 2002; Lakshminarasimhan and Chattarji 2012), more neurovascular cells might signify the opposite, perhaps reflecting the abnormally high rate of amygdala activity in depression (Carlson et al. 2006; Drevets et al. 2008). Pericytes and endothelial cells respond intimately to neuronal and glial activation, integrating signals along the vessel (Bergers and Song 2005; Gerhardt and Betsholtz 2003) and regulating blood flow in activated brain regions (Girouard and Iadecola 2006; Moore and Cao 2008). Both cell types have active signaling roles in vessel formation (Bergers and Song 2005). In pericyte-deficient mice, diminished cerebral blood flow response to brain activity was observed (Bell et al. 2010); a profusion of pericyte and endothelial cells in MDD may conversely represent a response to increased amygdalar metabolic activity.
It is important to note that pericyte abnormalities have also been observed in neurological disorders including Alzheimer’s disease (Kalaria 1996; Sengillo et al. 2013) and amyotrophic lateral sclerosis (Winkler et al. 2013), and may relate to pathological changes in the blood-brain barrier (Armulik et al. 2010). Subjects in this study were apparently neurologically and neuropathologically normal, and no relation between cardiovascular disease and neurovascular cell numbers or densities were found. However, undiagnosed conditions affecting neurovascular cells cannot be completely ruled out. The present results are insufficient to ascertain whether the higher number of neurovascular cells in depression signal a difference in BLA vascularization, and further studies are underway to determine whether greater vascularization and/or changes in vessel morphology accompany the greater number of neurovascular cells.
The larger number of neurovascular cells (and potential vascular alterations) may have contributed to the group difference in lateral nucleus volume, which was modestly (11%) greater in MDD than in control subjects (Figure 2). The absence of any more striking or widespread volume difference is perhaps not surprising in light of the mixed imaging literature on whole amygdala volume in depression (e.g., Lange and Irle 2004; Malykhin et al. 2012; Hastings et al. 2004; Sheline et al. 1998). Variables not examined in the current study that may also contribute to a larger lateral nucleus in MDD include an increase in dendritic material and spines (Vyas et al. 2002; Sibille et al. 2009), as is seen in the BLA of rats under prolonged stress (Vyas et al. 2006; Vyas et al. 2002). Theoretically, increased amygdala activity in MDD (Carlson et al. 2006; Drevets et al. 2008) may result in an increase in BLA synapses, and potentially a concomitant increase in perisynaptic glia (He and Sun 2007; Ullian et al. 2001); in the LAN, we observed a slight trend for a difference in glia number favoring MDD subjects (see Tables 3 and 4).
Within MDD, suicide was associated with a modestly (10%) higher density of lateral nucleus neurons. Similar profiles have been noted in other brain regions in suicide. Elevated numbers and densities of a variety of neuronal populations have been linked to suicide, in cortical (Brüne et al. 2011) and subcortical (Baumann et al. 1999; Gos et al. 2008; Matthews and Harrison 2012; Underwood et al. 1999) regions. Elevated glia densities have also been reported greater in suicide (e.g., Hercher et al. 2009; Steiner et al. 2008). It is unclear whether these diverse observations of increased neuron and glia packing density represent a broader suicide-vulnerable phenotype, or a variety of region- and cell type-specific phenomena (Brüne et al. 2011; Steiner et al. 2008), but the growing evidence for suicide-specific abnormalities in cell density clearly warrants further research.
There are several limitations to this study. While the optical fractionator technique, applied here in thick, well-preserved tissues, affords a high degree of confidence in estimates of cell numbers, the possibility of differential shrinkage at stages prior to sectioning (e.g., fixation) influencing estimates of volume cannot be ruled out. Greater subject numbers are almost always desirable in postmortem experiments, including this one. Although subject numbers were comparable to those in similar studies (Altshuler et al. 2010; Bezchlibnyk et al. 2007; Bowley et al. 2002; Hamidi et al. 2004), observed associations of depression duration and suicide with cell numbers and densities occurred between relatively small subgroups. The analysis of depression duration, though statistically adjusted for gender, was nevertheless further limited by a highly skewed gender distribution, with all but one depressed female in the longer-duration group. More female subjects are needed in future studies, not only in light of the greater incidence of depression in females but critically because sexually dimorphic neural changes in response to stressors are very common (Galea et al. 2008; Juraska and Rubinow 2008). Other limitations of the present study include the lack of available tissues dorsal to the BLA in many subjects, precluding analysis of the entire amygdala. Immunohistochemical identification of the specific cell populations quantified may have provided important information, for example on the specific neurovascular cell phenotype that was more numerous in depression. Because pH values were unavailable for 2 depressed and 2 control subjects, statistical adjustment for pH was not possible; however, available pH values were quite similar between groups (Table 1), and the specific dependent measures with which pH was correlated (see Results, Subject Variables) did not differ between groups in any of the analyses. Two MDD subjects met DSM-IV criteria for cannabis dependence; there are conflicting reports on whether heavy cannabis use affects amygdala morphology (Ashtari et al. 2011; Cousijn et al. 2012). However, these subjects did not differ from other MDD subjects in any of the volumetric or cellular measures examined.
With the opportunity to sample extensively from the entire basolateral amygdala in depressed versus psychiatrically healthy subjects, our purpose was to provide a foundation regarding basic cellular makeup and volume in a region critically involved in the pathophysiology of depression. Unlike disorders including autism (Schumann and Amaral 2006), schizophrenia (Kreczmanski et al. 2007; Berretta et al. 2007), and bipolar disorder (Berretta et al. 2007), depression does not appear to be associated with differences in the number of neurons or glia in BLA subnuclei. The novel finding of a nearly 20% greater number of neurovascular cells in the BLA of depressed subjects warrants further research, specifically regarding ramifications for vascular morphology, the possibility of angiogenesis, and the potential relationship between pericyte and/or endothelial cell profusion and the well-documented increase in amygdala activity that accompanies MDD.
Acknowledgments
We acknowledge the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. We also thank the Cuyahoga County Coroner’s Office and staff, Cleveland, Ohio, for their willing assistance. For some of the subjects, the services of Timothy M. De Jong and Lisa Larkin in acquiring written consent and tissue collection, respectively, are gratefully acknowledged. This study was supported by Public Health Service Grant Nos. P30 GM103328 (CAS), MH67996 (CAS), MH054846 (DCS), and a postdoctoral grant from the Hearin Foundation (MJR). Funding sources had no other role in the study design or in the analysis or interpretation of data.
Footnotes
The authors have no conflicts of interest to declare.
Contributor Information
Marisa J. Rubinow, Email: mrubinow@umc.edu.
Gouri Mahajan, Email: gmahajan@umc.edu.
Warren May, Email: wmay@umc.edu.
James C. Overholser, Email: Overholser@case.edu.
George J. Jurjus, Email: George.jurjus@va.gov.
Lesa Dieter, Email: ldd2@case.edu.
Nicole Herbst, Email: Nicole.herbst@case.edu.
David C. Steffens, Email: STEFFENS@uchc.edu.
Jose J. Miguel-Hidalgo, Email: jmiguel-hidalgo@umc.edu.
Grazyna Rajkowska, Email: grajkowska@umc.edu.
Craig A. Stockmeier, Email: cstockmeier@umc.edu.
References
- Abitz M, Nielsen RD, Jones EG, Laursen H, Graem N, Pakkenberg B. Excess of neurons in the human newborn mediodorsal thalamus compared with that of the adult. Cereb Cortex. 2007;17:2573–2578. doi: 10.1093/cercor/bhl163. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Ford AH, Flicker L, Hankey GJ, Yeap BB, Clancy P, Golledge G. Angiogenesis inhibition and depression in older men. J Psychiatry Neurosci. 2014;39:200–205. doi: 10.1503/jpn.130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, Mintz J, Hellemann G, Vinters HV. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010;12:541–549. doi: 10.1111/j.1399-5618.2010.00838.x. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kainin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, Merali Z, Poulter MO. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B, Danos P, Diekmann S, Krell D, Bielau H, Geretsegger C, Wurthmann C, Bernstein HG, Bogerts B. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is reduced in depressed non-suicidal patients but normal in depressed suicide patients. Eur Arch Psychiatry Clin Neurosci. 1999;249:212–219. doi: 10.1007/s004060050089. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Bio Psychiatry. 2007;62:884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk YB, Sun X, Wang JF, MacQueen GM, McEwen BS, Young LT. Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neurosci. 2007;32:203–210. [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Braendgaard H, Gundersen HJ. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods. 1986;18:39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Brüne M, Schöbel A, Karau R, Faustmann PM, Dermietzel R, Juckel G, Petrasch-Parwez E. Neuroanatomical correlates of suicide in psychosis: the possible role of von Economo neurons. PLoS One. 2011;6:e20936. doi: 10.1371/journal.pone.0020936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targets. NeuroRx. 2006;3:22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, Dwork AJ, Pakkenberg B. Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec. 2007;290:330–340. doi: 10.1002/ar.20504. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- deCampo DM, Fudge JL. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci Biobehav Rev. 2012;36:520–535. doi: 10.1016/j.neubiorev.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Nyengaard JR, Gundersen HJ. Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc. 2001;204:232–246. doi: 10.1046/j.1365-2818.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jäger M, Groll C, Bottlender R, Leinsinger G, Möller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Bottlender R, Born C, Groll C, Jäger M, Leinsinger G, Hahn K, Möller HJ. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Galea LA, Urban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62:247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS. Differential tissue shrinkage and compression in the z-axis: implications for optical disector counting in vibratome-, plastic- and cryosections. J Neurosci Methods. 2003;124:45–59. doi: 10.1016/s0165-0270(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer’s disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. Oxford University Press; New York: 1997. [Google Scholar]
- Gos T, Krell D, Bielau H, Brisch R, Trübner K, Steiner J, Bernstein HG, Jankowski Z, Bogerts B. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is elevated in violent suicidal depressed patients. Eur Arch Psychiatry Clin Neurosci. 2008;258:513–520. doi: 10.1007/s00406-008-0825-8. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- He F, Sun YE. Glial cells more than support cells? Int J Biochem Cell Biol. 2007;39:661–665. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Hercher C, Parent M, Flores C, Canetti L, Turecki G, Mechawar N. Alcohol dependence-related increase of glial cell density in the anterior cingulate cortex of suicide completers. J Psychiatry Neurosci. 2009;34:281–288. [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Matsuoka Y, Sugahara Y, Nakano T, Akechi T, Fujimori M, Imoto S, Murakami K, Uchitomi Y. Hippocampal volume and first major depressive disorder after cancer diagnosis in breast cancer survivors. Am J Psychiatry. 2004;161:2263–2270. doi: 10.1176/appi.ajp.161.12.2263. [DOI] [PubMed] [Google Scholar]
- Jansson L, Hellsten J, Tingström A. Region specific hypothalamic neuronal activation and endothelial cell proliferation in response to electroconvulsive seizures. Biol Psychiatry. 2006;60:874–881. doi: 10.1016/j.biopsych.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Rubinow MJ. Hormones and memory. In: Byrne J, Eichenbaum H, editors. Learning and memory: a comprehensive reference, vol. 3, memory systems. Elsevier; London: 2008. pp. 503–520. [Google Scholar]
- Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol. 2009;12:143–153. doi: 10.1017/S1461145708008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry. 2002;52:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HW, Hof PR, Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130:678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Tebartz van Elst L, Regen F, Deuschle M, Heuser I, Colla M. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J Psychiatr Res. 2009;43:1112–1117. doi: 10.1016/j.jpsychires.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP, Lynch M, Rose M, Moore GJ, Rosenberg DR. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63:385–390. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Hegadoren KM, Seres P, Coupland NJ. Fronto-limbic volumetric changes in major depressive disorder. J Affect Disord. 2012;136:1104–1113. doi: 10.1016/j.jad.2011.10.038. [DOI] [PubMed] [Google Scholar]
- Matthews PR, Harrison PJ. A morphometric, immunohistochemical, and in situ hybridization study of the dorsal raphe nucleus in major depression, bipolar disorder, schizophrenia, and suicide. J Affect Disord. 2012;137:125–134. doi: 10.1016/j.jad.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar architecture in celloidin sections from the human cerebral cortex. J Neurosci Methods. 1999;93:69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2007;12:360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol. 2008;99:2035–2047. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton P. Principles and Practices of Unbiased Stereology. Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- Munn MA, Alexopoulos J, Nishino T, Babb CM, Flake LA, Singer T, Ratnanather JT, Huang H, Todd RD, Miller MI, Botteron KN. Amygdala volume analysis in female twins with major depression. Biol Psychiatry. 2007;62:415–422. doi: 10.1016/j.biopsych.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rotheneichner P, Lange S, O’Sullivan A, Marschallinger J, Zaunmair P, Geretsegger C, Aigner L, Couillard-Despres S. Hippocampal neurogenesis and antidepressive therapy: shocking relations. Neural Plast. 2014;2014 doi: 10.1155/2014/723915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164:300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol. 2005;491:320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, Tseng GC, Lewis DA. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals. Ann N Y Acad Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Spoletini I, Piras F, Fagioli S, Rubino IA, Martinotti G, Siracusano A, Caltagirone C, Spalletta G. Suicidal attempts and increased right amygdala volume in schizophrenia. Schizophrenia Res. 2011;125:30–40. doi: 10.1016/j.schres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Liu Y, Hu X, He Z, Blumberg HP. Reduced ventral anterior cingulate and amygdala volumes in medication-naïve females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156:83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG, Hofman MA. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods. 1986;18:19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, van Oijen K, Rijpkema M, Goraj B, Jan Verkes R, Oude Voshaar R, Fernández G, Buitelaar J, Tendolkar I. Amygdala volume marks the acute state in the early course of depression. Biol Psychiatry. 2009;65:812–818. doi: 10.1016/j.biopsych.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Vassilopoulou K, Papathanasiou M, Michopoulos I, Boufidou F, Oulis P, Kelekis N, Rizos E, Nikolaou C, Pantelis C, Velakoulis D, Lykouras L. A magnetic resonance imaging study of hippocampal, amygdala and subgenual prefrontal cortex volumes in major depression subtypes: melancholic versus psychotic depression. J Affect Disord. 2013;146:197–204. doi: 10.1016/j.jad.2012.09.003. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci. 2000;12:493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord. 2006;94:219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Szebeni K, Szebeni A, Klimek V, Stockmeier CA, Karolewicz B, Kalbfleisch J, Ordway GA. Dopamine receptor gene expression in human amygdaloid nuclei: elevated D4 receptor mRNA in major depression. Brain Res. 2008;1207:214–224. doi: 10.1016/j.brainres.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa E, Matsuoka Y, Yamasue H, Inagaki M, Nakano T, Akechi T, Kobayakawa M, Fujimori M, Nakaya N, Azizuki N, Imoto S, Murakami K, Kasai K, Uchitomi Y. Prefrontal cortex and amygdala volume in first minor or major depressive episode after cancer diagnosis. Biol Psychiatry. 2006;59:707–712. doi: 10.1016/j.biopsych.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Preuss UW, Bondy B, Frodl T, Zill P, Schmitt G, Koutsouleris N, Rujescu D, Born C, Reiser M, Möller HJ, Meisenzahl EM. 5-HT1A receptor gene C-1019 G polymorphism and amygdala volume in borderline personality disorder. Genes Brain Behav. 2008;7:306–313. doi: 10.1111/j.1601-183X.2007.00353.x. [DOI] [PubMed] [Google Scholar]