Abstract
Alcohol possesses complex sensory attributes that are first detected by the body via sensory receptors and afferent fibers that promptly transmit signals to brain areas involved in mediating ingestive motivation, reinforcement, and addictive behavior. Given that the chemosensory cues accompanying alcohol consumption are among the most intimate, consistent, and immediate predictors of alcohol’s postabsorptive effects, with experience these stimuli also gain powerful associative incentive value to elicit craving and related physiologic changes, maintenance of ongoing alcohol use, and reinstatement of drug seeking after periods of abstinence. Despite the above, preclinical research has traditionally dichotomized alcohol’s taste and postingestive influences as independent regulators of motivation to drink. The present review summarizes current evidence regarding alcohol’s ability to directly activate peripheral and central oral chemosensory circuits, relevance for intake of the drug, and provides a framework for moving beyond a dissociation between the sensory and postabsorptive effects of alcohol to understand their neurobiological integration and significance for alcohol addiction.
Keywords: Chemosensory, ethanol, reinforcement, taste, trigeminal, alcohol, addiction
1. Introduction
Historically, preclinical research investigating factors that motivate alcohol drinking has tended to dichotomize whether ethanol is ingested for its ‘taste’ or ‘postingestive’ effects, often with attempts to control for or minimize the influence of the former. This dichotomy derives in part from proposed criteria for a valid animal model of alcoholism put forth in the 1970’s, including the tenet that intake of alcohol should be “based solely on its pharmacological properties and not be related to some other characteristic, such as the calories it provides or its gustatory or olfactory properties” [1,2]. This dissociation between ethanol’s sensory and postabsorptive effects has been less prominent in the clinical research literature on alcoholism, which has frequently recognized the significance of alcohol chemosensory stimuli in eliciting craving and associated drug-seeking responses in alcohol-experienced individuals [3–11]. The sensory properties of alcohol have also been of significant research interest to the alcoholic beverage industry in order to identify and manipulate those sensory attributes that maximize intake [12].
Under conditions of natural self-administration, ethanol initially produces activation of peripheral and central taste and oral somatosensory pathways [13–18], as well as a multitude of visceral sensory effects (e.g., stimulation of the gut, etc.), temporally prior to entry of pharmacologically relevant levels of ethanol into brain. Thus, ethanol sensory signals gain immediate access to the CNS (within ms) in advance of the drug’s delayed postabsorptive effects. With chronic exposure, sensory and postingestive inputs become intimately integrated, such that these stimuli gain meaning for the addicted organism. Importantly, these sensory pathways are linked to limbic forebrain and cortical areas involved in controlling ingestive motivation and feeding [19]. In this review, we examine evidence for the role of sensory mechanisms in alcohol intake and provide a framework for understanding the convergence of chemosensory and postingestive factors in the development and maintenance of alcohol addiction.
2. Oral Sensory Processing of Ethanol
Ethanol is a highly salient and complex oral chemosensory stimulus, known to directly stimulate sensory receptor and brain gustatory circuits involved in sweet taste processing [13–16] as well as oral trigeminal pathways sensitive to noxious or irritant stimulus input [17–18]. A relationship between ingestion of alcohol and sweet-tasting solutions was first recognized several decades ago with observations that ethanol-preferring C57BL mice display a significantly greater intake of both nutritive (sucrose) and non-nutritive (saccharin) sweeteners relative to their non-ethanol-preferring DBA/2J counterparts [20–21]. Subsequently, direct positive correlations between alcohol and saccharin consumption were observed in randomly bred rats [22–23], multiple inbred strains of mice [24], and seven strains of rats known to differ in ethanol preference [25]. A robust association between the intake of alcohol and sweet substances (i.e., sucrose, saccharin) has held true across a variety of independently-selected lines of alcohol-preferring and -nonpreferring rats [26–30], the F2 progeny of crosses of these lines [25, 29, 31–33], and rats selectively bred for the reciprocal phenotype of saccharin consumption [34], strongly supporting a common genetic basis for this relationship. In humans, genetic risk for alcoholism as indexed by a positive family history of the disorder has also repeatedly been associated with heightened preference for concentrated sweet solutions [35–37], including in children with a positive family history but no prior experience with alcohol [38].
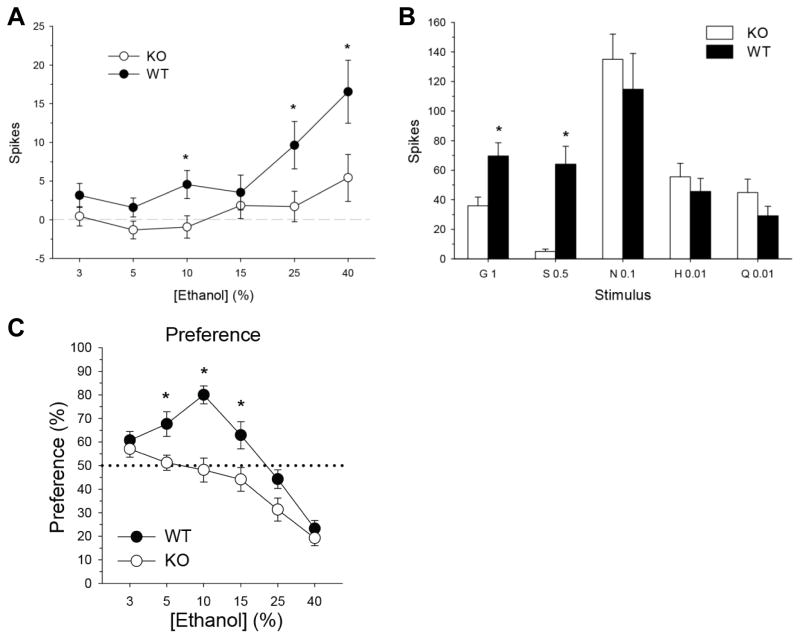
A substantive body of behavioral and neurophysiological data has now established that alcohol directly activates gustatory receptor and central neural substrates for sweet taste. Initial conditioned taste aversion generalization studies demonstrated that conditioned aversions to the taste of alcohol generalized to sucrose mixtures in randomly bred rats [39–42], with the sweet component of the mixtures being critical whenever aversion generalization was found [40]. Conditioned taste aversions also cross-generalize between ethanol and sucrose alone in C57BL/6J mice [43–44]. Neurophysiological recordings from peripheral gustatory nerves in primates have indicated that orally applied ethanol preferentially stimulates sweet-sensitive relative to other taste fibers in the chorda tympani nerve innervating the anterior tongue [14]. Studies from our laboratory have also demonstrated that oral ethanol stimulation of the tongue and palate within a clinically relevant concentration range (3–40%) selectively activates central sweet-responsive gustatory neurons in the rodent nucleus of the solitary tract (NTS), the first brain area to receive and process taste information [13,15–16]. Moreover, the response of individual central taste-sensitive neurons to sucrose is a robust predictor of their responsiveness to ethanol [15–16; Figure 1]. Ethanol-induced activity in these cells was further inhibited by peripheral pharmacological blockade of oral sweet receptors, initially implicating sweet taste receptors as candidate receptors for ethanol [15]. More recently, we specifically established that knockout of the T1r3 sweet taste receptor subunit suppresses alcohol’s ability to activate central sweet taste circuits in the NTS as well as eliminates behavioral alcohol preference in ethanol-preferring C57BL/6J mice, strongly supporting this receptor in the sensory detection and transduction of ethanol taste [13; Figure 2]. Ethanol’s ability to potently activate sweet taste pathways presumably arises from the original substrate from which it is derived and fermented (sugars in fruits, grains, etc.).
Fig. 1.
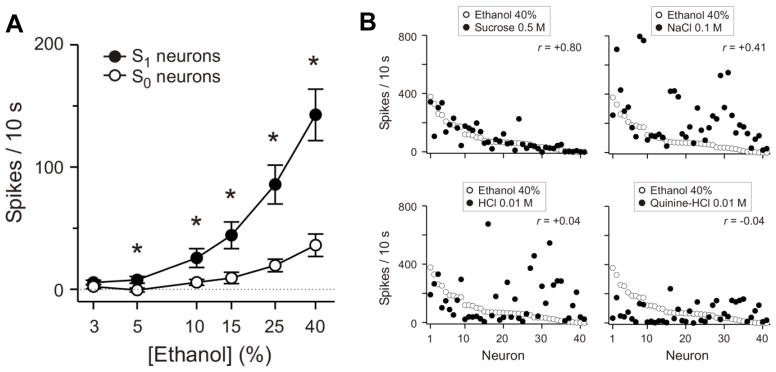
A: Mean (±SEM) responses of sucrose-responsive (S1) and sucrose-unresponsive (S0) NTS neurons to an ethanol concentration series (3–40%) recorded from anesthetized Sprague-Dawley rats. Stimuli were presented to the anterior tongue and palate in discrete 10-s trials preceded and followed by a deionized water rinse. Responses to ethanol recorded from S1 neurons were significantly greater than those observed in S0 neurons for all ethanol concentrations except 3% (*P ≤ 0.02). B: Across-neuron patterns of response produced by standard sweet, salty, acid, and bitter tastants (filled circles) relative to that evoked by 40% ethanol (open circles). Individual neurons are rank ordered along the abscissa based on their magnitude of response to 40% ethanol. Correlation coefficients (r) calculated between the across-neuron pattern evoked by ethanol and each standard tastant are shown. Responses to ethanol were highly correlated with those to 0.5 M sucrose (r=+0.80), but uncorrelated with responses to HCl (r=+0.04) or quinine (r=−0.04). Modified from [15].
Fig. 2.
A: Mean (±SEM) responses to an ascending ethanol concentration series in NTS neurons sampled from T1r3 sweet taste receptor knockout (KO) and C57BL/6J wild-type (WT) mice. B: Mean (±SEM) responses in KO and WT cells to glycine (G), sucrose (S), NaCl (N), HCl (H), and quinine (Q). Concentration follows abbreviation. Mice lacking the T1r3 receptor exhibited suppressed neural taste responses to ethanol and sweeteners, but did not differ from wild-type mice in responses to prototypic salt, acid, or bitter stimuli. C: Mean (±SEM) percent preference for ethanol in a two-bottle choice assay at each concentration in KO and WT mice. T1r3 knockouts were behaviorally indifferent to alcohol at concentrations preferred by wildtype mice. *Significant difference between KO and WT (P < 0.05). Modified from [13].
Oral ethanol stimulation of appetitive taste pathways, particularly at high concentrations, may at first glance appear counterintuitive given that heterogeneous rats often initially avoid ethanol at concentrations above 6% [45], indicating a significant aversive chemosensory component of the ethanol stimulus. This initial ethanol avoidance response to high concentrations of ethanol in randomly bred rodents has frequently been attributed to an unpalatable “bitter” gustatory component to ethanol, as has been self-reported in human studies measuring subjective taste perceptions of ethanol [46]. Conditioned taste aversions to ethanol also generalize to sweet-bitter mixtures in rats [40,42] and to quinine as well as sucrose alone in C57BL/6J mice [43]. Despite perceptual generalization of a bitter-like oral property of ethanol in rodents and humans, neurophysiological data have thus far not supported a relationship between neural taste responses elicited by ethanol and bitter stimuli in gustatory circuits of outbred Wistar, Sprague-Dawley, or selectively bred alcohol-preferring (P) rats [15–16, 47], C57BL/6J or bitter-sensitive C3HeB/FeJ and C3.SW-Soaa mice [13,48], or non-human primates [14], in contrast to robust positive associations observed between neural responses to ethanol and sweet stimuli. There is also no consistent association between behavioral alcohol preference and chemosensory responses to quinine [49] or intake of the bitter substance sucrose octaacetate [28] in alcohol-preferring and -nonpreferring rat lines. Further, in both the chorda tympani and glossopharyngeal nerves of primates (innervating the anterior and posterior tongue, respectively), ethanol in mixtures with quinine actually suppresses bitter taste responses, consistent with the properties of a sweetener [14,50].
Although additional research is needed to determine whether ethanol directly stimulates physiological substrates involved in bitter taste processing, there is perhaps a more compelling amount of data for direct ethanol-induced activation of oral somatosensory (i.e., trigeminal) mechanisms that contribute to alcohol’s aversive orosensory properties. Oral application of ethanol to the tongue activates fibers of the lingual branch of the trigeminal nerve across species [18,51–52] and produces a concentration-dependent increase in activity of central neurons in the rodent brain stem trigeminal subnucleus caudalis [17]. Ethanol also directly activates sensory nociceptors including transient receptor potential channel vanilloid receptor 1 [TRPV1; 53], the receptor for capsaicin [54–55], which shows heavy localization on sensory fibers that innervate the oral epithelium [56–57]. These trigeminal circuits process noxious chemical and thermal input from the oral cavity, and ethanol’s ability to stimulate these pathways presumably underlies the burning and irritant sensations to oral alcohol reported in human psychophysical studies, especially at high concentrations [58–62]. Human psychophysical data have further shown that noxious oral sensations processed by the trigeminal system can be confused with bitter taste [63], and thus potentially such cross-modal generalization could account for the perception of a “bitter” taste component to ethanol by humans, as well as the conditioned taste aversion generalization between ethanol and sweet-bitter mixtures in rodents [40,42]. Data from our laboratory have shown that direct manipulation of the trigeminal system, specifically knockout of the TRPV1 receptor, reduces oral ethanol avoidance in mice, although only moderately, indicating that other trigeminal or gustatory substrates must contribute to ethanol’s aversive oral component [64]. TRPV1 knockouts also display higher preference for ethanol and consume more ethanol in two-bottle choice tests than wild-type controls [65]. Overall, existing data indicate that oral ethanol consumption simultaneously activates sensory inputs that serve both appetitive and protective functions.
3. Ethanol Chemosensory Cues and Brain Reinforcement Mechanisms
Although research on oral alcohol-induced activation of central gustatory circuits has thus far largely focused on first-order brain stem systems (i.e., NTS) that process incoming taste information from the periphery, these structures subsequently transmit sensory signals downstream to limbic forebrain and cortical areas known to be important in regulating ingestive motivation and reinforcement. Following processing in brain stem gustatory areas, taste signals are transmitted via a thalamocortical projection to primary taste cortex within the insula, as well as to limbic structures, including the ventral tegmental area–nucleus accumbens pathway, amygdala, ventral pallidum, and lateral hypothalamus [19, for review]. In particular, the ability of alcohol to activate sensory receptor and associated brain circuits for sweet taste is significant, given evidence that activation of such circuitry engages central reinforcement mechanisms that motivate subsequent intake [66–70]. For example, oral sucrose stimulation via sham feeding produces an immediate concentration-dependent increase in dopamine release in the nucleus accumbens [68], which is attenuated by selective damage to limbic taste projections [69–70]. Further, both dopamine [67] and opioid [71] receptor antagonists inhibit sensory-mediated intake of sweet solutions in the latter paradigm. A coupling of sweet taste substrates to central reward mechanisms is consistent with a functional evolution of these substrates to recognize and promote ingestion of nutritive substances. Oral ethanol self-administration in rodents has also been shown to immediately elevate accumbal dopamine levels in a manner associated with the stimulus properties of ethanol, before physiologically relevant concentrations of ethanol reach the brain via absorption into the bloodstream [72]. Recently, our laboratory has demonstrated that oral ethanol stimulation (20% concentration) also induces robust c-Fos activity within the gustatory portion of the insula in outbred Wistar rats, providing direct evidence for ethanol sensory-elicited activation of cortical taste areas (Figure 3). The insula has also recently been implicated as a key area in processing cue-reinforcement associations in drug addiction, with lesions or inactivation of the insula disrupting addictive responses (e.g., craving, self-administration, reinstatement of drug-seeking) triggered by exposure to conditioned sensory cues associated with prior drug administration [73–79].
Fig. 3.
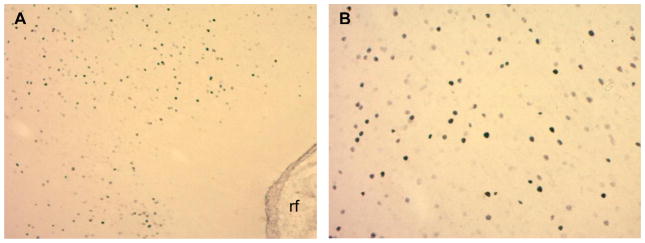
Photomicrographs of Fos-positive cells within gustatory insular cortex [+1.2 mm AP from bregma; A: 10×, B: 20×] of an outbred Wistar rat, elicited by exposure to the taste of 20% ethanol. rf, rhinal fissure.
Due to its oral route of administration, the chemosensory cues accompanying alcohol consumption are among the most intimate, consistent, and ecologically appropriate stimuli immediately predictive of the drug’s subsequent postabsorptive effects. Following experience with alcohol, significantly heightened appetitive and decreased aversive reactivity to alcohol orosensory cues are observed in animal models [80–82], responses that are maintained even after sustained periods of abstinence [82]. In alcoholics and high-risk drinkers, alcohol chemosensory stimuli elicit urges to drink and associated physiologic changes (increased salivation, skin conductance and cardiac responses [3–9]), as well as activation of mesocorticolimbic structures implicated in drug seeking and motivation [10–11]. Re-exposure to ethanol gustatory cues after extinction of ethanol self-administration also induces strong reinstatement of ethanol seeking in animal models of relapse [83] and potentiates reinstatement of ethanol responding by more distal ethanol-paired environmental stimuli [84–85]. Despite a significant literature supporting ethanol chemosensory cues as robust appetitive signals for promoting and maintaining ethanol-seeking behavior, the underlying neural substrates and functional brain alterations mediating conditioned drug-seeking responses elicited by these stimuli are not well established. Understanding the nature of experience-induced plasticity occurring in circuits that process ethanol sensory cues following chronic exposure to the drug is an important area for further investigation, given that exposure to such drug-predictive cues is believed to be a primary factor mediating craving responses, subsequent drug-seeking and approach behavior, and persistent vulnerability to relapse even long after discontinuation of drug use [9,86–89].
4. Conclusion
Sensory-mediated contributions to alcohol intake have traditionally received less attention and research focus than the postabsorptive effects of the drug on the CNS, with ethanol’s taste and postingestive influences often being treated as independent entities. It is becoming increasingly apparent that the ability of ethanol to directly and immediately stimulate complex chemosensory circuits linked to motivationally-relevant limbic and cortical areas involved in controlling intake, as well as “direct” interaction of ethanol with neural substrates following entry into brain, play critical and coordinated roles in the development and maintenance of alcohol addiction. A more thorough understanding of the central nervous system mechanisms that integrate ethanol sensory signals with postingestive reinforcement following chronic exposure, and mediate the ability of those sensory signals to acquire control over subsequent alcohol seeking behavior, are important areas for future study.
Highlights.
Alcohol directly activates peripheral and central taste and trigeminal pathways
These circuits are linked to motivationally-relevant limbic and cortical areas
Ethanol chemosensory signals can acquire control over subsequent alcohol seeking
Integration of alcohol sensory-postingestive inputs important area for future study
Acknowledgments
The research presented in this review was supported in part by NIH Grants AA023291 and AA015741 (S. M. Brasser), DC005270 and DC008194 (C. H. Lemon), DC00353 (D. V. Smith), and AA015512 (Indiana Alcohol Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cicero TJ. A critique of animal analogues of alcoholism. In: Majchrowicz E, Noble EP, editors. Biochemistry and pharmacology of ethanol. II. New York: Plenum Press; 1979. pp. 533–60. [Google Scholar]
- 2.Lester D, Freed EX. Criteria for an animal model of alcoholism. Pharmacol Biochem Behav. 1973;1:103–7. doi: 10.1016/0091-3057(73)90062-2. [DOI] [PubMed] [Google Scholar]
- 3.Grüsser SM, Heinz A, Flor H. Standardized stimuli to assess drug craving and drug memory in addicts. J Neural Transm. 2000;107:715–20. doi: 10.1007/s007020070072. [DOI] [PubMed] [Google Scholar]
- 4.Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–6. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- 5.Pomerleau OF, Fertig J, Baker L, Cooney N. Reactivity to alcohol cues in alcoholics and non-alcoholics: implications for a stimulus control analysis of drinking. Addict Behav. 1983;8:1–10. doi: 10.1016/0306-4603(83)90048-5. [DOI] [PubMed] [Google Scholar]
- 6.Rohsenow DJ, Monti PM, Colby SM, Gulliver SB, Sirota AD, Niaura RS, et al. Effects of alcohol cues on smoking urges and topography among alcoholic men. Alcohol Clin Exp Res. 1997;21:101–7. [PubMed] [Google Scholar]
- 7.Stormark KM, Laberg JC, Bjerland T, Nordby H, Hugdahl K. Autonomic cued reactivity in alcoholics: the effect of olfactory stimuli. Addict Behav. 1995;20:571–84. doi: 10.1016/0306-4603(95)00017-7. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein A, Lingford-Hughes A, Martinez-Raga J, Marshall J. What makes alcohol-dependent individuals early in abstinence crave for alcohol: exposure to the drink, images of drinking, or remembrance of drinks past? Alcohol Clin Exp Res. 1998;22:1376–81. [PubMed] [Google Scholar]
- 9.Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–52. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- 10.Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, et al. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004;28:550–7. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- 12.Babor TF. Alcohol research and the alcoholic beverage industry: issues, concerns and conflicts of interest. Addiction. 2009;104:34–47. doi: 10.1111/j.1360-0443.2008.02433.x. [DOI] [PubMed] [Google Scholar]
- 13.Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics. 2010;41:232–43. doi: 10.1152/physiolgenomics.00113.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellekant G, Danilova V, Roberts T, Ninomiya Y. The taste of ethanol in a primate model: I. chorda tympani nerve response in Macaca mulatta. Alcohol. 1997;14:473–84. doi: 10.1016/s0741-8329(96)00215-7. [DOI] [PubMed] [Google Scholar]
- 15.Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol. 2004;92:536–44. doi: 10.1152/jn.00097.2004. [DOI] [PubMed] [Google Scholar]
- 16.Lemon CH, Wilson DM, Brasser SM. Differential neural representation of oral ethanol by central taste-sensitive neurons in ethanol-preferring and genetically heterogeneous rats. J Neurophysiol. 2011;106:3145–56. doi: 10.1152/jn.00580.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80:465–92. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 18.Danilova V, Hellekant G. Oral sensation of ethanol in a primate model III: responses in the lingual branch of the trigeminal nerve of Macaca mulatta. Alcohol. 2002;26:3–16. doi: 10.1016/s0741-8329(01)00178-1. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69:243–55. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- 20.Fuller JL. Single-locus control of saccharin preference in mice. J Hered. 1974;65:33–6. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- 21.Lush IE. The genetics of tasting in mice VI. saccharin, acesulfame, dulcin and sucrose. Genet Res. 1989;53:95–9. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- 22.Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol. 1990;7:83–5. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- 23.Kampov-Polevoy AB, Overstreet DH, Crosby RD, Rezvani AH, Janowsky DS, Halikas JA. Saccharin-induced polydipsia as a predictor of voluntary alcohol intake in Wistar rats. In: Seredenin SB, Longo V, Gaviraghi G, editors. Biological basis of individual sensitivity to psychotropic drugs. Edinburgh: Graffham Press; 1994. pp. 293–8. [Google Scholar]
- 24.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–10. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 25.Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res. 1993;17:366–9. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 26.Dyr W, Kostowski W. Animal model of ethanol abuse: rats selectively bred for high and low voluntary alcohol intake. Acta Pol Pharm. 2000;57 (Suppl):90–2. [PubMed] [Google Scholar]
- 27.Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9:155–60. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- 28.Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–81. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 29.Stewart RB, Murphy JM, Lumeng L, Li TK. Intake of saccharin solution in selectively-bred high and low alcohol drinking (HAD and LAD) lines of rats and in the F2 progeny of HAD and LAD rat crosses. Alcohol Clin Exp Res. 1998;22 (Suppl):55A. [Google Scholar]
- 30.Woods JE, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, et al. Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:926–36. doi: 10.1097/01.ALC.0000071920.53470.C1. [DOI] [PubMed] [Google Scholar]
- 31.Foroud T, Bice P, Castelluccio P, Bo R, Ritchotte A, Stewart R, et al. Mapping of QTL influencing saccharin consumption in the selectively bred alcohol-preferring and -nonpreferring rat lines. Behav Genet. 2002;32:57–67. doi: 10.1023/a:1014459912935. [DOI] [PubMed] [Google Scholar]
- 32.Stewart RB, Murphy JM, Lumeng L, Li TK. Sweet preference and spontaneous motor activity are correlated with alcohol intake in the F2 progeny of an alcohol-preferring (P) and -nonpreferring (NP) rat cross. Alcohol Clin Exp Res. 1997;21 (Suppl):16A. [Google Scholar]
- 33.Stewart RB, Bice P, Foroud T, Lumeng L, Li TK, Carr LG. Correlation of saccharin and ethanol intake in the F2 progeny of HAD2 and LAD2 crosses. Alcohol Clin Exp Res. 2003;27 (Suppl):49A. [Google Scholar]
- 34.Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–8. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 35.Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res. 2003;27:1743–9. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- 36.Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–9. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wronski M, Skrok-Wolska D, Samochowiec J, Ziolkowski M, Swiecicki L, Bienkowski P, et al. Perceived intensity and pleasantness of sucrose taste in male alcoholics. Alcohol Alcohol. 2007;42:75–9. doi: 10.1093/alcalc/agl097. [DOI] [PubMed] [Google Scholar]
- 38.Mennella JA, Pepino MY, Lehmann-Castor SM, Yourshaw LM. Sweet preferences and analgesia during childhood: effects of family history of alcoholism and depression. Addiction. 2010;105:666–75. doi: 10.1111/j.1360-0443.2009.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol. 1986;3:55–61. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- 40.Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Senses. 1993;18:509–22. [Google Scholar]
- 41.Kiefer SW, Bice PJ, Orr MR, Dopp JM. Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol. 1990;7:115–20. doi: 10.1016/0741-8329(90)90071-j. [DOI] [PubMed] [Google Scholar]
- 42.Kiefer SW, Lawrence GJ. The sweet-bitter taste of alcohol: aversion generalization to various sweet-quinine mixtures in the rat. Chem Senses. 1988;13:633–41. [Google Scholar]
- 43.Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet. 2007;37:146–59. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- 44.Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res. 2000;24:253–8. [PubMed] [Google Scholar]
- 45.Richter CP, Campbell KH. Alcohol taste thresholds and concentrations of solution preferred by rats. Science. 1940;91:507–8. doi: 10.1126/science.91.2369.507. [DOI] [PubMed] [Google Scholar]
- 46.Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend. 2000;60:199–206. doi: 10.1016/s0376-8716(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 47.Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol. 2005;94:3719–29. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- 48.Wilson DM, Boughter JD, Jr, Lemon CH. Bitter taste stimuli induce differential neural codes in mouse brain. PLoS One. 2012;7:e41597. doi: 10.1371/journal.pone.0041597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brasser SM, Silbaugh BC, Ketchum MJ, Olney JJ, Lemon CH. Chemosensory responsiveness to ethanol and its individual sensory components in alcohol-preferring, alcohol-nonpreferring and genetically heterogeneous rats. Addict Biol. 2012;17:423–36. doi: 10.1111/j.1369-1600.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danilova V, Hellekant G. The taste of ethanol in a primate model. II. glossopharyngeal nerve response in Macaca mulatta. Alcohol. 2000;21:259–69. doi: 10.1016/s0741-8329(00)00094-x. [DOI] [PubMed] [Google Scholar]
- 51.Hellekant G. The effect of ethyl alcohol on non-gustatory receptors of the tongue of the cat. Acta Physiol Scand. 1965;65:243–50. doi: 10.1111/j.1748-1716.1965.tb04267.x. [DOI] [PubMed] [Google Scholar]
- 52.Simon SA, Sostman AL. Electrophysiological responses to nonelectrolytes in lingual nerve of rat and in lingual epithelia of dog. Arch Oral Biol. 1991;36:805–13. doi: 10.1016/0003-9969(91)90030-x. [DOI] [PubMed] [Google Scholar]
- 53.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–51. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 54.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 55.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–72. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 56.Ishida Y, Ugawa S, Ueda T, Murakami S, Shimada S. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Brain Res Mol Brain Res. 2002;107:17–22. doi: 10.1016/s0169-328x(02)00441-2. [DOI] [PubMed] [Google Scholar]
- 57.Kido MA, Muroya H, Yamaza T, Terada Y, Tanaka T. Vanilloid receptor expression in the rat tongue and palate. J Dent Res. 2003;82:393–7. doi: 10.1177/154405910308200513. [DOI] [PubMed] [Google Scholar]
- 58.Diamant H, Funakoshi M, Strom L, Zotterman Y. Electrophysiological studies on human taste nerves. In: Zotterman Y, editor. Olfaction and taste I. New York: Pergamon; 1963. pp. 193–203. [Google Scholar]
- 59.Green BG. The sensitivity of the tongue to ethanol. Ann NY Acad Sci. 1987;510:315–7. [Google Scholar]
- 60.Green BG. Spatial and temporal factors in the perception of ethanol irritation on the tongue. Percept Psychophys. 1988;44:108–16. doi: 10.3758/bf03208702. [DOI] [PubMed] [Google Scholar]
- 61.Green BG. Effects of thermal, mechanical, and chemical stimulation on the perception of oral irritation. In: Green BG, Mason JR, Kare MR, editors. Chemical senses, vol 2, irritation. New York: Marcel Dekker; 1990. pp. 171–5. [Google Scholar]
- 62.Wilson CW, Brien O, Mac C, Airt JG. The effect of metronidazole on the human taste threshold to alcohol. Br J Addict Alcohol Other Drugs. 1973;68:99–110. doi: 10.1111/j.1360-0443.1973.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 63.Lim J, Green BG. The psychophysical relationship between bitter taste and burning sensation: evidence of qualitative similarity. Chem Senses. 2007;32:31–9. doi: 10.1093/chemse/bjl033. [DOI] [PubMed] [Google Scholar]
- 64.Ellingson JM, Silbaugh BC, Brasser SM. Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet. 2009;39:62–72. doi: 10.1007/s10519-008-9232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blednov YA, Harris RA. Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacology. 2009;56:814–20. doi: 10.1016/j.neuropharm.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 67.Schneider LH. Orosensory self-stimulation by sucrose involves brain dopaminergic mechanisms. Ann NY Acad Sci. 1989;575:307–19. doi: 10.1111/j.1749-6632.1989.tb53252.x. [DOI] [PubMed] [Google Scholar]
- 68.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regulatory Integrative Comp Physiol. 2004;286:R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 69.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89:531–5. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84:363–9. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Kirkham TC, Cooper SJ. Attenuation of sham feeding by naloxone is stereospecific: evidence for opioid mediation of orosensory reward. Physiol Behav. 1988;43:845–7. doi: 10.1016/0031-9384(88)90386-1. [DOI] [PubMed] [Google Scholar]
- 72.Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–82. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- 73.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann NY Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–8. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 77.Scott D, Hiroi N. Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry. 2011;69:1052–9. doi: 10.1016/j.biopsych.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–71. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 79.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behavior in rats. Eur J Neurosci. 2006;24:3285–98. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 80.Badia-Elder NE, Kiefer SW. Taste reactivity in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol. 1999;18:159–63. doi: 10.1016/s0741-8329(98)00079-2. [DOI] [PubMed] [Google Scholar]
- 81.Bice PJ, Kiefer SW. Taste reactivity in alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 1990;14:721–7. doi: 10.1111/j.1530-0277.1990.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 82.Kiefer SW, Badia-Elder N, Bice PJ. Taste reactivity in high alcohol drinking and low alcohol drinking rats. Alcohol Clin Exp Res. 1995;19:279–84. doi: 10.1111/j.1530-0277.1995.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 83.Chiamulera C, Valerio E, Tessari M. Resumption of ethanol-seeking behavior in rats. Behav Pharmacol. 1995;6:32–9. [PubMed] [Google Scholar]
- 84.Bäckström P, Hyytiä P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–65. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- 85.Barak S, Liu F, Hamida SB, Yowell QV, Neasta J, Kharazia V, et al. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci. 2013;16:1111–7. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1988;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 87.Kalivas PW, Volkow ND. The neural basis of addicition: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 88.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 89.O’Brien CP. Neuroplasticity in addictive disorders. Dialogues Clin Neurosci. 2009;11:350–3. doi: 10.31887/DCNS.2009.11.3/cpobrien. [DOI] [PMC free article] [PubMed] [Google Scholar]