Abstract
Background
Mast cells (MCs) are hemopoietic cells that mature in tissues and are involved in allergy, immunity and inflammation by secreting multiple mediators. The natural flavone luteolin (lut) has anti-inflammatory actions and inhibits human MCs.
Objective
To investigate the ability of lut, and its novel structural analog 3’,4’,5,7-tetramethoxyluteolin (methlut), to inhibit human MCs mediator expression and release in vitro and in vivo.
Methods
Human LAD2 cells and primary human umbilical cord-blood derived cultured MC (hCBMCs) were stimulated by substance P (SP) or IgE/anti-IgE with or without pre-incubation with lut, methlut or cromolyn (1–100 μM) for 2 or 24 hr following which a mediator secretion was measured. The effect of the compound on MC intracellular calcium levels and NF-κB activation was also investigated. Pretreatment with methlut was also studied in mice passively sensitized with dinotrophenol-human serum albumin (DNP-HSA) and challenged intradermally.
Results
Methlut is a more potent inhibitor than lut or cromolyn for beta-hexosaminidase (β-hex) and histamine secretion from LAD2 cells stimulated by either SP or IgE/anti-IgE, but only methlut and lut significantly inhibit preformed tumor necrosis factor (TNF) secretion. Methlut is also a more potent inhibitor than lut of de novo synthesized TNF from LAD2, and of chemokine (C-C motif) ligand 2 (CCL2) from hCBMCs. The mechanism of action from methlut may be due to its ability to inhibit intracellular calcium increase, as well as NF-κB induction at both the transcriptional and translational levels in LAD2 cells stimulated by SP without affecting cell viability. Treatment (ip) with methlut significantly decreases skin vascular permeability of Evans blue in mice passively sensitized to DNP-HAS and challenged intradermaly.
Conclusion
Methlut is a promising MC inhibitor for the treatment of allergic and inflammatory conditions.
Keywords: Allergy, inflammation, mast cells, luteolin, tetramethoxyluteolin, calcium, NF-κB
Introduction
Mast cells (MC) are immune cells derived from hematopoietic precursors that mature in tissue microenvironments.1–3 In addition to allergic triggers, MC can be stimulated by neuropeptides, such as substance P (SP).3;4 Upon stimulation, MC release preformed mediators stored in their numerous secretory granules; these include beta-hexosaminidase (β-hex), histamine, TNF, and tryptase through rapid degranulation, as well as newly-synthesized PGD2, TNF and chemokine (C-C motif) ligand 2 (CCL2, MCP1).{Theoharides, 1982 3114 /id;Theoharides, 2004 12642 /id;Wedemeyer, 2000 10873 /id}
MC-derived histamine induce bronchoconstriction and mucus secretion, contributing to asthma. 8;9 MC is probably the only cell type that stores preformed TNF, 10 which is rapidly released and influences T cell recruitment and activation.11;12 MC-derived CCL2 13 and CXCL-8 ( IL-8) enhance recruitment of immune cells to the site of inflammation.6;7;14 The ability to release multiple mediators allows MC to actively interact with other cell types in their surrounding environment, and participate in the induction and/or propagation of various immune and inflammatory responses including mastocytosis, 15 asthma16, atopic dermatitis,17 and psoriasis.3;16;18;19 Therefore, inhibition of MC activation has clear therapeutic potential.
Disodium cromoglycate (cromolyn, Fig. 1A) is the only clinically available “MC stabilizer” because it was reported to reduce gastrointestinal effects in mastocytosis patients. 20 Even though cromolyn inhibits rat peritoneal MC histamine secretion,21 it does not inhibit rat mucosal MC22;23 or mouse MC.24 A recent study concluded that the beneficial effect of cromolyn in reducing pruritus in humans may be mediated through inhibition of sensory nerve endings instead of MC. 25–28 In addition, poor intestinal absorption of cromolyn severely limits its clinical efficacy. Consequently, there is urgent need for developing effective inhibitors of human MC.
Figure 1. Methlut inhibits degranulation of LAD2 cells stimulated by SP.
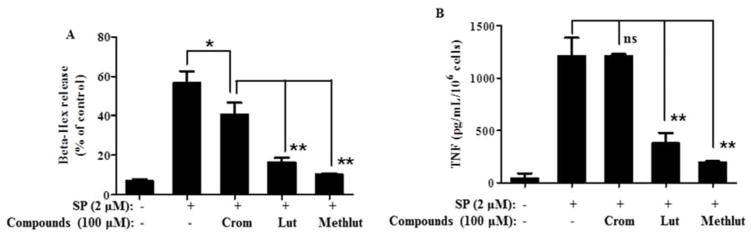
MC were pre-incubated with either cromolyn, lut or methlut (10–100 μM, 30 min) before stimulation with SP (2 μM, 30 min). (A) β-hex and (B) preformed TNF release. *p<0.05, **p<0.01; ns=not significant.
In search for potential MC inhibitors, we noticed that the part of the structure of cromolyn is similar to the backbone of flavones (Supplemental Fig1 highlighted areas), which are naturally occurring compounds with potent antioxidant, anti-inflammatory and MC blocking activities.29 The flavone luteolin (lut, Supplemental Fig.1) inhibits the release of histamine and PGD2 from human cultured MC.30 Lut also inhibits mercury-induced VEGF release from human MC31 and activated T cells. 32 The structural analogue of lut, 3’, 4’, 5, 7-tetramethoxyluteolin (methlut, Supplemental Fig.1), is more lipid soluble than lut, more likely to penetrate cells and less metabolized. 33 However, the action of methlut on MC activation has not been investigated.
In this study, we examined whether methlut could inhibit mediator release from human cultured MC stimulated by an allergic and a neuropeptide triggers, and compared it to lut and cromolyn. We also studied the effect of methlut in mice passively sensitized to dinitrophenol-human serum albumin (DNP-HAS) and challenged intradermally.
Methods
Reagents
Recombinant human stem cell factor (rhSCF) was kindly donated by Orphan Biovitrum AB (Stockholm, Sweden). Cromolyn, lut and SP were purchased from Sigma-Aldrich (St. Louis, MO). Methlut was obtained from Pharmascience Nutrients (Clear Water, FL) and was 100% pure by HPLC-Mass spectroscopy ( Supplemental Fig. 2). Cromolyn, lut and methlut were dissolved in DMSO. SP was prepared in distilled water. Working dilutions for all compounds were prepared in culture media immediately before use. The final concentration of DMSO was less than 0.1% and had no effect on cell viability
Human MC culture
The limited number of MC obtained from normal human tissues has led to the increased use of human LAD2 cells derived from a human MC leukemia34 or primary human cord blood-derived MC (hCBMCs)35 Human LAD2 cells (kindly supplied by Dr. A Kirshenbaum, NIH, Bethesda, MD) were cultured in StemPro-34 medium (Life Technologies, Carlsbad, CA) supplemented with 2 mM L-glutamine, 1% penicillin/streptomycin and 100 ng/mL rhSCF (Orphan Biovitrum AB).
To culture primary hCBMCs, human umbilical cord blood was obtained from normal deliveries in accordance with established Institutional guidelines36 Mononuclear cells were isolated by layering heparin-treated cord blood onto Lymphocyte Separation Medium (INC Biomedical, Aurora, OH). CD34+ progenitor cells were isolated by positive selection of AC133 (CD133+/CD34+) cells by magnetic cell sorting (CD133 Microbead Kit, Miltenyi Biotech, Auburn, CA). For the first six weeks, CD34+ progenitor cells were cultured in IMDM medium (Life Technologies) supplemented with 0.1% BSA, 1% insulin-transferin-selenium, 50 ng/mL IL-6, 0.1% β-mercaptoethanol, 1% penicillin/streptomycin and 100 ng/mL rhSCF. After six weeks the cells were cultured in IMDM supplemented with 10% FBS, 50 ng/mL IL-6, 0.1% β-mercaptoethanol, 1% penicillin/streptomycin and 100 ng/mL rhSCF. hCBMCs cultured for at least 15 weeks were used for experiments and cell purity was above 98%. Cell viability was determined by trypan blue (0.4%) exclusion.
Degranulation assays
β-Hex release was assayed as an index of MC degranulation. LAD2 cells (0.5×105) were pre-incubated with cromolyn, lut, or methlut (10–100 μM, 30 min) before stimulation with SP (2 μM, 30 min). To test the effect of cromolyn, LAD2 cells were treated with cromolyn (100 μM) and SP (2 μM) at the same time. Control cells were treated with 0.1% DMSO. Supernatant fluids were collected and cell pellets were lysed with 1% Triton X-100. Supernatants and cell lysates were incubated in the reaction buffer (p-nitrophenyl-N-acetyl-β-D-glucosaminide from Sigma) for 1.5 hour (h) and then 0.2 M glycine was added to stop the reaction. Absorbance was measured at 405 nm. The results are expressed as the percentage of β-hex released over the total.
MC degranulation was also assessed by measuring histamine release. After the same treatment of LAD2 cells were pretreated with cromolyn, lut or methlut and subsequently stimulated with SP. Supernatant fluids were collected and histamine release was measured using a Histamine EIA Kit (Cayman Chemical Co., Ann Arbor, MI).
To assay degranulation in primary MC, hCBMCs (0.5×105) were first primed with human IgE (1 μg/mL, Millipore, Billerica, MA) overnight and pre-incubated with cromolyn, lut or methlut (10–100 μM) for 30 min before stimulation with anti-IgE (10 μg/mL, 30 min, Life technologies). Supernatant fluids were collected and PGD2 was measured using a PGD2-MOX EIA Kit (Cayman Chemical Co.).
TNF and CCL2 ELISA
For TNF release, LAD2 cells (1×105) were pre-incubated with cromolyn, lut or methlut (10–100 μM) and subsequently stimulated with SP (2 μM) for 30 min to measure preformed TNF release. LAD2 cells were also stimulated for 24 h to measure de novo synthesized TNF release, which occurs in addition to the rapid preformed TNF release . TNF was measured in the supernatant fluids using a TNF ELISA assay kit (R&D Systems, Minneapolis, MN).
For CCL2 release, primary hCBMCs (1×105) were primed with human IgE (1 μg/mL, Millipore) overnight and pre-incubated with lut or methlut (50 μM) for 30 min before stimulation with anti-IgE (10 μg/mL, 2 hr, Life technologies). CCL2 was measured using a CCL2 ELISA assay kit (R&D Systems).
RNA isolation and quantitative real time PCR (qRT-PCR)
LAD2 cells and hCBMCs (5×105) were treated with lut or methlut (10–100 μ, 30 min) before stimulation with either SP (2 μ) or anti-IgE (10 μg/mL) for 6 h. Total RNA was extracted with an RNeasy Mini kit (Qiagen Inc., Valencia, CA). An iScript cDNA synthesis kit (BioRad, Hercules, CA) was used for reverse-transcription of each sample. qRT-PCR was performed using Taqman gene expression assays (Applied Biosystems, Foster City, CA) for TNF (Hs99999043_m1) and CCL2 (Hs00234140_m1), and the two genes encoding different subunits of the NF-κB protein complex, NFKB1 (NF-κB p50 subunit, Hs00765730_m1) and RELA (NF-κB p65 subunit, Hs00153294_m1). Samples were run at 45 cycles using a real-time PCR system (7300, Applied Biosystems). The mRNA gene expressions were normalized to human GAPDH endogenous control (4310884E, Applied Biosystems).
Intracellular calcium measurements
LAD2 cells were incubated in Tyrode’s buffer with the calcium indicator Fura-2AM (30 nM, 20 min, Life Technologies). Cells were washed and resuspended in plain Tyrode’s buffer and incubated for another 20 min. Cells were then transferred to 96-well plates (1 × 105 cells per well), and pretreated with lut or methlut (10, 50 μM, 30 min) before stimulation with SP (2 μM). Chanegs in Fura-2 fluorescence was immediately read by MDC FlexStation II (Molecular Devices, Sunnyvale, CA) at an excitation wavelength of 340 nm/380 nm and emission wavelength of 510 nm. Results were processed according to the Life Technologies Fura-2AM protocol and reported as relative ratio.
NF-κB inhibitor alpha (Iκ Bα) phosphorylation assay
The nuclear transcription factor NF-κB plays a pivotal role in the regulation of inflammatory mediator expression37 Upon stimulation, the NF-κB inhibitor alpha (IκBα) is rapidly phosphorylated and degraded, allowing NF-κB to translocate into the nucleus, where it binds to the promoter region of a number of target genes37 We investigated effect of lut and methlut on IκBα phosphorylation and NF-κB DNA-binding activity, as described later. After pre-incubation with either lut or methlut (10, 50 μM, 30 min h), LAD2 cells (4 × 106) were stimulated with SP (2 μM, 15 min). Phosphorylation of IκBα (serine 32) was detected by the PathScan Inflammation Sandwich ELISA kit (#7276, Cell Signaling). Whole cell lysates were assayed at a protein concentration of 5 mg/mL. Absorbance was read at 450 nm. Relative phospho-IκBα levels were normalized to control cells treated with 0.1% DMSO.
NF-κB DNA-binding activity
After lut and methlut pre-incubation (10, 50 μM,30 min), LAD2 cells (4 × 106) were stimulated with SP (2 μM, 15 min). Cells were harvested and cytosolic and nuclear extracts were isolated using a NE-PER nuclear extraction kit (Thermo Scientific, Rockford, IL). DNA-binding activity of NF-κB p65 in the extracts was detected by the NF-κB (p65) Transcription Factor Assay Kit (#10007889, Cayman Chemical Co.). Cytosolic and nuclear extracts (each containing 10 μg of protein) were added to a 96-well plate coated with a specific double stranded DNA sequence containing the NF-κB response element. NF-κB was detected by addition of specific primary antibody directed against NF-κB followed by HRP-conjugated secondary antibody to provide a colorimetric readout at 450 nm. Relative NF-κB p65 DNA-binding activities in the cytosolic and nuclear extracts were normalized to control cells treated with 0.1% DMSO.
Intracellular ATP measurement
In order to determine if lut and methlut have any effect on cellular energy production, intracellular ATP content was measured. After incubation with lut or methlut (10–100 μM, 24 h), 1 × 106 LAD2 cells were lysed and intracellular ATP contents were determined using an ATP assay kit (Abcam, Cambridge, MA).
Cell viability
Cell viability was assayed by Trypan blue exclusion 38 The effect of lut and methlut (100 μM) was tested on LAD2, hCBMC mast cells, as well as on cultured keratinocytes and microglial cells and was >98% viable after 24 hr of incubation. Lut was recently reported not to affect viability of HaCaT keratinocytes and primary cultured keratinocytes. 39
Evans blue extravasation
Evan’s blue (EB) extravasation was performed as previously described 40 Passive cutaneous anaphylaxis was produces as follows: normal saline (NS) or anti-DNP IgE (20 μg/50 μl, Sigma) was administered intradermally at two skin sites on each mouse (n=5). Two days later, DNP-HSA (200 μg) along with 1% Evans blue in sterile NS (100 μl) was injected in the tail vein. Male (BALB/c, 4–6 wk old, Charles River, MA) were then anesthetized by an intraperitoneal injection (0.1 ml) of ketamine (100 mg/kg)/xylazine (10 mg/kg), following which the dorsal subscapular skin was shaved and either 10 μg SP or DNP-HSA (200μg) were injected intradermally in a total volume of 50 μl in the subscapular region with a tuberculin syringe. Mice were pretreated with an ip injection of NS or methlut (100 mg/kg) 30 min before the intradermal injections. Mice were sacrificed, the skin removed, turned over and photographed. Circular pieces of skin at each injection site (8 mm diameter) were removed with a puncher, weighted and placed in Eppendorf tubes. EB was extracted in 1 ml of N,N-dimethylformamide overnight at 55 ºC and the optical density was measured at 620 nm using a PerkinElmer Luminescence Spectrophotometer (Perkin Elmer, Norwalk, CT). EB concentration was calculated using a standard curve and values were normalized to the tissue weight. Intravascular EB and non-specific effects of injection were accounted for by subtracting the EB extracted after injection of PNS.
Statistical analysis
All experimetns were performed in triplicate and were repeated for at least three times (n=3). Results are presented as mean ± SD. Data between different treatment groups were analyzed using the unpaired, 2-tailed, Student’s t-test. Significance of comparisons between treatment groups is denoted by * p<0.05 and ** p<0.01.
Results
Methlut inhibits SP-stimulated LAD2 cells degranulation
SP (2 μ, 30 min) triggers significant release of β-hex (Fig. 1A) and preformed TNF (Fig. 1B from LAD2 cells Lut and methlut inhibit β-hex release by 74% and 85%, respectively (Fig. 1A). Cromolyn reduces β-hex release (Fig. 1A), but does not reduce TNF release (Fig. 1B), while lut and methlut significantly inhibit both β-hex and TNF release by more than 70% (Fig. 1). The inhibitory effect of methlut is greater than that of lut.
Cromolyn reduces SP-stimulated β-hex release by 30% (Fig. 1A) but it must be added together with the trigger as pre-incubation for 30 min eliminates its inhibitory activity (Supplemental Fig. 3). Instead, lut and methlut inhibit β-hex release whether added together or 30 min prior to the trigger (Fig.1A).
The inhibitory effect of the flavonoids is dose-dependent (10–100 μ, 30 min) with almost complete inhibition of SP-stimulated β-hex, histamine and preformed TNF release at 100 μ (Fig. 2B).
Figure 2. Dose-response of the inhibitory effect of methlut on degranulation of LAD2 cells stimulated by SP.
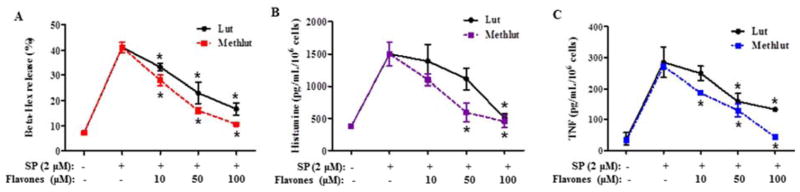
MC were pre-incubated with either lut or methlut (10–100 μM, 30 min) before stimulation with SP (2 μM, 30 min). (A) β-hex, (B) histamine and (C) preformed TNF release. *p<0.05.
Methlut inhibits IgE/anti-IgE stimulated β-hex and TNF release from LAD2 cells
We also stimulated LAD2cells by IgE/anti-IgE. Pre-incubation with lut or methlut (100 μ, 30 min) significantly inhibits IgE/anti-IgE-stimulated β-hex release by 70% and 99%, respectively (Fig. 3A). Similar inhibition is seen for the release of preformed TNF (Fig. 3B).
Figure 3. Methlut inhibits degranulation of LAD2 cells stimulated by IgE/anti-IgE.
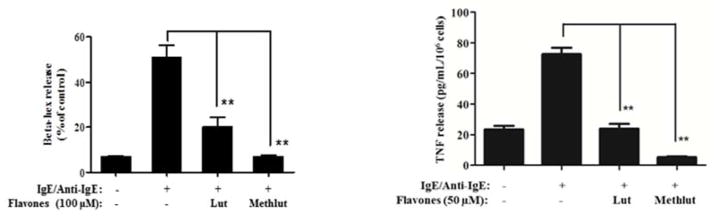
After overnight priming with IgE (1 μg/mL), LAD2 cells were incubated with lut or methlut (50 or 100 μM 30 min) before anti-IgE stimulation (10 μg/mL) for 2 hr. (A) β-hex and (B) preformed TNF.**p<0.001.
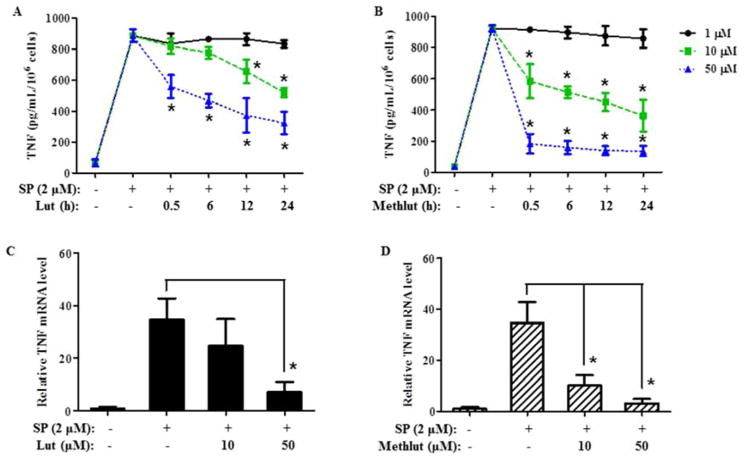
Methlut inhibits SP-stimulated de novo TNF synthesis and release from LAD2 cells
In addition to preformed TNF, MCs also release newly synthesized TNF 12–24 h later. SP (2 μM, 24 h) triggers significant TNF release (Fig. 4A, B), which is dose-dependently inhibited by pre-incubation with lut or methlut (1–100 μM, 30 min). Prolonged pre-incubation times do not increase the extent of inhibition, except for lut where inhibition at 10 μM becomes more prominent after 12 and 24 h as compared to 30 min (Fig. 4A). Methlut is more effective than lut at 10 μM. Moreover, lut at 50 μM reduces TNF release by about 50%, while methlut achieves 95% inhibition at the same concentration (Fig. 4B). Cromolyn has no inhibitory effect (results not shown).
Figure4. Methlut inhibits TNF mRNA expression in LAD2 cells stimulated by SP.
LAD2 cells were incubated with lut (A) or methlut (B) (1–50 μM, up to 24 h) before SP stimulation (2 μM, 24 h), TNF release was assayed. In another set of experiments, LAD2 cells were incubated with lut (C) or methlut (D) (10, 50 μM, 30 min) before SP stimulation (2 μM, 6 h), mRNA expression was examined. *p<0.05.
Pre-incubation with lut or methlut for 30 min also significantly decreases TNF mRNA expression (Fig. 4C, D). Methlut even at 10 μM is more effective than lut and reduces SP-stimulated TNF expression by 73% (Fig. 4D). Cromolyn has no effect (results not shown) as before.
Methlut inhibits IgE/anti-IgE stimulated CCL2 synthesis and release from IgE/anti-IgE-stimulated hCBMCs
We investigated the effect of the flavonoids on the release of CCL2 because it is known to be synthesized by MC13 and it stimules recruitment of immune cells to the site of inflammation.
Stimulation of hCBMCs with IgE/anti-IgE (10 μg/mL, 24 h)-triggers CCL2 release from primary hCBMCs. This effect is blocked by pre-incubation with lut or methlut (10 or 50 μM, 30 min, Fig. 5A). Anti-IgE stimulation (10 μg/mL, 6 h) also triggers 2-fold increase in CCL2 mRNA expression compared to control cells primed with IgE only (Fig. 5B); this effect is also completely inhibited by pre-incubation with lut or methlut (50 μM, 30 min) and not by cromolyn (results not shown).
Figure 5. Methlut inhibits CCL2 synthesis and release from hCBMCs stimulated by IgE/anti-IgE-stimulated.
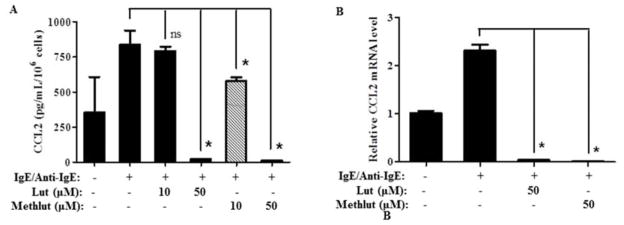
After overnight priming with IgE (1 μg/mL), hCBMCs were incubated with lut or methlut (10, 50 μM, 30 min) before anti-IgE stimulation (10 μg/mL). (A) CCL2 release was assayed at 24 h after stimulation. (B) CCL2 mRNA expression. hCBMCs were incubated with lut or methlut (50 μM, 30 min) before stimulation with anti-IgE (10 μg/mL, 6 h) and mRNA expression of CCL2 was examined. *p<0.05.
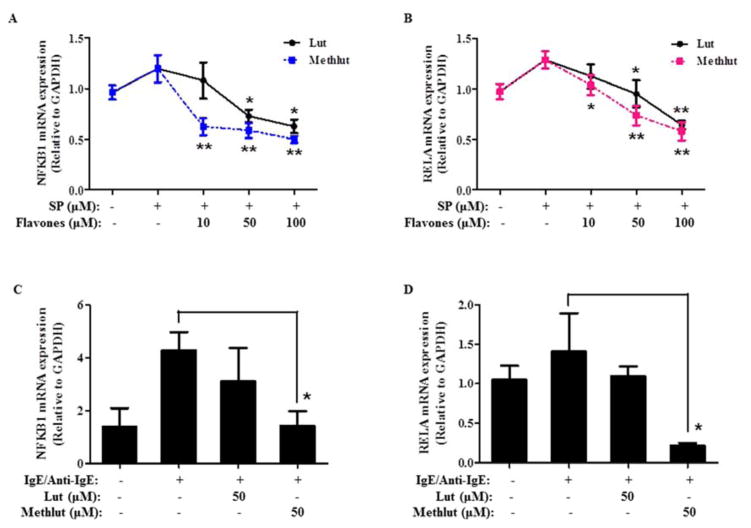
Methlut inhibits NF-κB activation
In LAD2 cells, SP (2 μM, 15 min) rapidly causes IκBα phosphorylation on serine 32 (Fig. 6A), which allows subsequent activation of NF-κB. Pre-incubation with lut or methlut (10 or 50 μM, 6 h) dose-dependently decreases IκBα phosphorylation compared to control cells (Fig. 6A). In addition, pre-incubation with lut or methlut (10 or 50 μM, 6 h) significantly decreases the DNA-binding activity of NF-κB p65 in the nuclear extract (Fig. 6B). The DNA-binding activity of NF-κB p65 in the cytosolic extract was not affected (data not shown). At 10 μM, methlut is more effective than lut in reducing both IκBα phosphorylation and DNA-binding activity of NF-κB p65 in the nucleus.
Figure 6. Methlut inhibits NF-κ B activation.
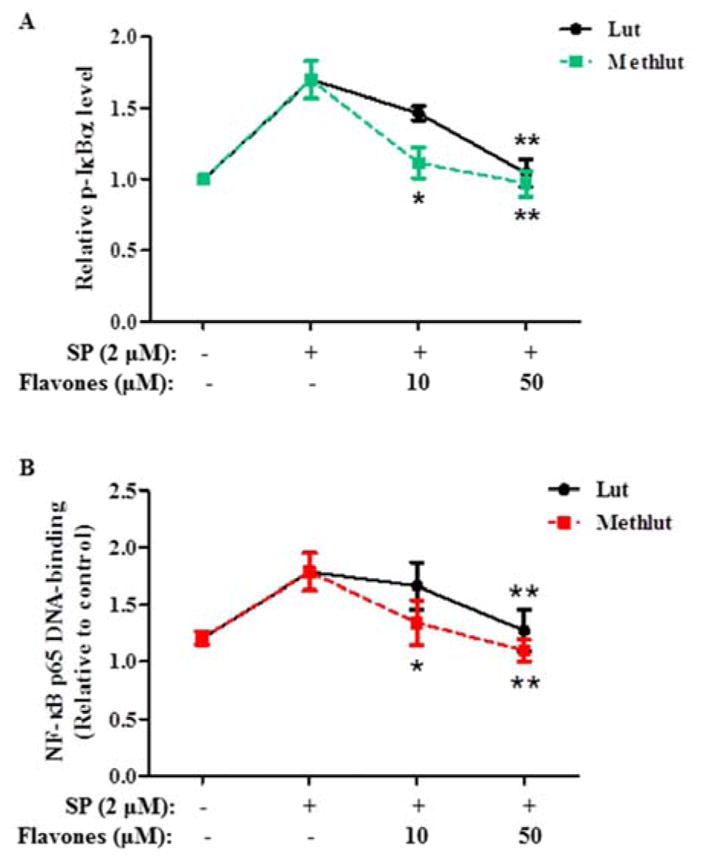
LAD2 cells were incubated with lut or methlut (10, 50 μM, 6 h) before SP stimulation (2 μM, 15 min). (A) IκBα phosphorylation was detected and presented as fold relative to control. (B) NF-κB p65 DNA-binding activity in the nuclear fraction was examined and expressed as fold relative to control. * p<0.05, **p<0.01.
Methlut inhibits mRNA expression of NF-κ B-related genes
NFKB1 encodes for the NF-κB p50 subunit and RELA encodes for the NF-κB p65 subunit. In LAD2 cells, SP (2 μM, 6 h) slightly induces mRNA expression levels of NFKB1 (Fig. 7A) and RELA (Fig. 7B), which are decreased by pre-incubation (10–100 μM, 30 min) with lut or methlut Methlut is more effective than lut. Similar results are obtained in primary hCBMCs, where pre-incubation with methlut (50 μM, 30 min) significantly decreases mRNA expression levels of both NFKB1 (Fig. 7C) and RELA (Fig. 7D). Pre-incubation with lut (50 μM, 30 min) only slightly reduces the mRNA expression of NFKB1 and RELA, but is not statistically significant.
Figure 7. Methlut inhibits NF-κ B mRNA expression.
LAD2 cells (A, B) were incubated with lut or methlut (10–100 μM, 30 min) before SP stimulation (2 μM, 6 h). hCBMCs (C, D) were primed overnight with IgE (1 μg/mL), and then incubated with lut or methlut (50 μM, 30 min) before anti-IgE stimulation (10 μg/mL, 6 h). mRNA expression levels of NFKB1 and RELA were examined. *p<0.05.
Methlut inhibits intracellular calcium increase
SP (2 μM) triggers a rapid intracellular calcium increase in LAD2 cells (Supplemental Fig. 5). Pre-incubation with lut (50 μM, 30 min) inhibits calcium increase by 50%. Pre-incubation with methlut (50 μM, 30 min) completely blocks calcium elevation, which is even lower than control cells treated with DMSO (Supplemental Fig. 4).
Methlut does not alter intracellular ATP production
Neither lut or methlut (100 μ) reduce cell viability (results not shown). In order to further document the lack of any detrimental effect on cellular function, we examined the effect of lut and methlut on intracellular ATP production. Pre-incubation with lut or methlut (100 μM, 24 h) decreases intracellular ATP content by 10% and 15%, respectively (Supplemental Fig. 5), which is not statistically significant.
Methlut prevents increased vascular permeability due to either SP or DNP-HSA
Intradermal injection of SP (10 μ) or DNP-HAS in mice passively sensitized to HSA significantly increases Evans Blue skin extravasation (Fig. 8A). Mice injected intraperitonealy with methlut (100 mg/kg, 30 min) prior to intradermal injection have significantly reduced vascular permeability to either SP or DNP-HSA (Fig. 8B).
Figure 8. Methlut inhibits mouse skin Evans blue extravasation.
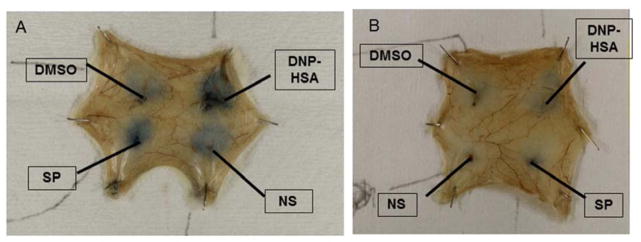
Mice were passively sensitized to DBP-HSA and injected with Evans blue via the tail vain. MC were pretreated with methlut (100 μ) 10 min prior to intradermal challenge with. Photograph of mouse skin showing (A) Extravasated Evans blue. (B) Extent of extravasated Evans blue after extravasation and assay thorometrically.
Discussion
Our results indicate that the novel flavone methlut is more potent than lut and signifiantly inhibits human LAD2 cells release of histamine, β-hex, and preformed TNF. In contrast, equimolar concentration of cromolyn, the only compound marketed as a MC “stabilizer”, reduces only β-hex release by about 30%, but not preformed TNF, when LAD2 cells are stimulated by SP. In addition, both lut and methlut are effective when administered before the trigger while cromolyn has to be added together with the trigger for any inhibition to be evident due to rapid tachyphylaxis.38;41 We had also previously shown that the structurally related to lut flavonol quercetin is more effective than cromolyn in blocking hCBMC cytokine release. 38 Nevertheless, cromolyn (100 μ) inhibited histamine release from hCBMC by about 60%, PGD2 release by 80% and LTC4 by 100%. 38 A recent publication also reported that cromolyn inhibited PGD2 release from hCBMC stimulated by IgE/Anti-IgE by 100%. 42 It is, therefore apparent that cromolyn can inhibit the release of histamine and arachidonic acid products, but not cytokines, whether due to IgE/anti-IgE or SP stimulation. In contrast, lut and methlut are more effective MC inhibitors than cromolyn regardless of the trigger and the mediator measured. Moreover, lut and methlut were effective when MC were pre-incubated, while cromolyn had to be added together with the trigger for its inhibition to manifest. Further incubation with lut up to 48 hr does not reduce TNF gene expression any more than what we observe at 24 hr (results not shown). There is no evidence the flavonoids are metabolized inside the cells. The inhibitory effect of methlut also remains the same up to 48 hr (results not shown).
We also report that both lut and methlut inhibit the release of CCL2 from hCBMCs. While luteolin had been reported to inhibit histamine release from hCBMCs 30 this is the first time that lut and methlut are reported to inhibit CCL2 release. CCL2 is known to be synthesized by MC13 and stimulates recruitment of immune cells to the site of inflammation.
We also examined several key signaling processes involved in MC activation, including intracellular calcium levels43 intracellular ATP production,44 as well as activation of the nuclear transcriptional factor NF-κB.45;46 Here, we show that methlut inhibits the inducible transcription factor NF-κB, which is a protein complex that translocates from the cytoplasm into the nucleus upon activation and regulates gene expression of various inflammatory mediators.47 Methlut prevents phosphorylation of IκBα, which is an upstream inhibitor of NF-κB and is degraded upon phosphorylation.37 We also show that methlut decreases NF-κB p65 DNA-binding activity in the nuclear extract. Apart from inhibiting NF-κB activation at the protein level, we also report for the first time to our knowledge methlut decreases mRNA expression of two genes encoding different subunits in the NF-κB protein complex, NFKB1 (encoding NF-κB p50 subunit) and RELA (encoding NF-κB p65 subunit). By blocking NF-κB activation at both the gene and protein levels, methlut and lut can effectively regulate pro-inflammatory mediator production. Lut had been reported to inhibit cytokine production by blocking NF-κB in leukemic HMC-1 cells.46 However, HMC-1 cells do not express the high affinity FcεRI receptor for IgE48 and they proliferate independent of SCF, which is absolutely required for proliferation of primary human MC. In our studies, we used LAD2cells, which is a more more mature human MC line than HMC-1 with functional FcεRI and dependence on SCF.34
Lut and methlut effectively block stimulated intracellular calcium increase, known to be required for MC degranulation.35;49 Because methlut has four methoxyl groups substituted for the four hydroxyl groups on luteolin (Fig. 1C, D), the calcium-blocking action of luteolin is unlikely to be due any polyphenolic structure, where calcium-chelation may occur. These flavones may also inhibit or activate the regulatory components of calcium signaling pathways in MC. For insance, luteolin-7-O-glucoside inhibited phospholipase C phosphorylation in mouse MC50 and the structurally related flavonol quercetin blocked calcium-dependant PKCθ. 51 This effect on stimulated calcium increase should NOT be of concern for cardiac cells and neurons as the calcium spikes in these cell types are voltage-gate regulated, while the calcium increase in MC is not 52. In fact, lut did not have any effect on calcium ion levels in neonatal cultured cardiomyocytes (results not shown). 53
We were obviously interested in knowing if the flavones tested affect cell viability and whether any such effect may be mediated via reduction in cellular ATP levels. Neither flavone significantly reduced viability of human MC. Moreover, lut does not affect viability of either T cells,32 keratinocytes, 39 or microglia 54 for up to 24 hr. Both lut and methlut had not significant effect on intracellular ATP content in MC.
Here we also show that methlut also inhibits skin vascular permeability stimulated by intradermal SP and DNP-HAS in passively sensitized mice. This method was previously used to document the effect of other inhibitors on MC activation 40 as well as of resveratrol on passive cutaneous anaphylaxis in mice 55 Moreover, the flavonoid concentrations used here in this mouse “model” (100 mg/kg) have already been shown to have a statistically significant benefit at least for lut in children with autism 56 many of whom have been reported to have “allergic-like” symptoms 57 implicating MC activation. 58
Suprisingly, we show that methlut is more effective than lut in decreasing MC activation. The substitution of the four hydroxy groups in lut by methoxyl groups (Fig. 1D) apparently enhances the inhibitory activity (Fig. 1C). This finding seems to contradict previous studies that showed flavones containing more hydroxyl groups have greater anti-asthmatic effects.59 However, additional publications support our present findings. For instance, 6-methoxyluteolin, significantly inhibits histamine release and intracellular calcium increase in human cultured basophilic KU812F cells.60 Two polymethoxy flavones [nobiletin (3’,4’,5,6,7,8-hexamethoxy flavone) and tangeretin (4’,5,6,7,8-pentamethoxy flavone)] prevent LPS-induced bone loss in a mouse model of periodontitis and inhibit PGE2 production in co-cultures of bone marrow cells and osteoblasts.61
Another possible explanation may be that the presence of the methyl groups in methlut permits greater penetration in MC and higher intracellular concentration from lut. In addition, methoxy flavones are less metabolized thereby increasing their biological activities 33 an important attribute since flavonoids are extensively metabolized. 62
Lut apparently does not have any cell selectivity for MC, as it had previously been shown to also inhibit stimulation of human T cells 32 and keratinocytes, 39 as well as microglia. 63 However, our preliminary evidence (unpublished) indicates that both methlut and lut are preferentially taken up by immune cells, especially those with phagocytic activity such as MC, macrophages and microglia. A potential mechanism of selectivity of flavonoids, where luteolin was the best inhibitor of histamine and β-hex release, may be disruption of distinct MC vesicle secretion by interacting with granule-dependent SNARE complexes. 64 Interestingly, the beneficial action of cromolyn may also not to be selective for MC as it could be mediated through inhibition of sensory nerve endings, recently shown for a cromolyn containing skin cream. 27 Other studies have shown that cromolyn may stimulate afferent nerves in humans.26
Our present results indicate that the natural flavones methlut is a potent inhibitor of human MC inflammatory mediator release. Its likely mechanism(s) of action involve decreasing intracellular calcium levels and decreasing NF-κB activation at both the transcriptional and translational levels. Methlut has the potential to be developed into a promising MC blocker for the treatment of allergic and inflammatory conditions where MC activation is involved, particularly when made into formulations that increase its solubility and absorption.
Supplementary Material
Key Messages.
Methlut inhibits release of inflammatory mediators from human mast Cells stimulated by allergic and neuropeptide triggers.
Methlut is more effective than lut and cromolyn, for inhibition of MC (NO PGD2 data)Methlut inhibits skin vascular permeability in a passively sensitized mouse model.
Acknowledgments
Declaration of funding sources
This work was supported in part by National Institute of Health Grant AR47652, a Translational Grant from the National Psoriasis Foundation and by Theta Biomedical Consulting and Development Co., Inc. (Brookline, MA) to TCT.
We thank Dr. A.S. Kirshenbaum (NIH) for providing the LAD2 human MC and Swedish Orphan Biovitrum AB for their generous gift of rhSCF. We also thank Dr. Errol (Dept. of Obstetrics and Gynecology, Tufts Medical Center) for providing the umbilical cord blood. This study was supported in part by NIH grant R01 NS071361-05 and a National Psoriasis Foundation Translational Award to TCT.
Abbreviations
- β-Hex
Beta-hexosaminidase
- CCL2
Chemokine (C-C motif) ligand 2
- hCBMC
Human cord-blood derived MC
- h
Hours
- IκBα
Nuclear factor-kappa B inhibitor, alpha
- IL
Interleukin
- Lut
Luteolin
- Methlut
3’,4’,5,7-tetramethoxyluteolin
- NF-κB
Nuclear factor-kappa B
- PGD2
Prostaglandin D2
- rhSCF
Recombinant human stem cell factor
- SP
Substance P
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–22. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005;102(32):11408–13. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, et al. Mast cells and inflammation. Biochim Biophys Acta. 2010;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 5.Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW. Differential release of serotonin and histamine from mast cells. Nature. 1982;297:229–31. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- 6.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146(1–2):1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–31. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 8.Deckers J, Branco MF, Hammad H. Innate immune cells in asthma. Trends Immunol. 2013;34(11):540–7. doi: 10.1016/j.it.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Holgate ST. The role of mast cells and basophils in inflammation. Clin Exp Allergy. 2000;30 (Suppl 1):28–32. doi: 10.1046/j.1365-2222.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Weng Z, Sismanopoulos N, Asadi S, Therianou A, Alysandratos KD, et al. Mitochondria distinguish granule-stored from de novo synthesized tumor necrosis factor secretion in human mast cells. Int Arch Allergy Immunol. 2012;159(1):23–32. doi: 10.1159/000335178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Askenase PW. Mast cells and the mediation of T-cell recruitment in arthritis. N Engl J Med. 2005;349(13):1294. doi: 10.1056/NEJM200309253491319. [DOI] [PubMed] [Google Scholar]
- 12.Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, et al. Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol. 1993;151(9):4637–41. [PubMed] [Google Scholar]
- 13.Eglite S, Morin JM, Metzger H. Synthesis and secretion of monocyte chemotactic protein-1 stimulated by the high affinity receptor for IgE. J Immunol. 2003;170(5):2680–7. doi: 10.4049/jimmunol.170.5.2680. [DOI] [PubMed] [Google Scholar]
- 14.Salamon P, Shoham NG, Gavrieli R, Wolach B, Mekori YA. Human mast cells release interleukin-8 and induce neutrophil chemotaxis on contact with activated T cells. Allergy. 2005;60(10):1316–9. doi: 10.1111/j.1398-9995.2005.00886.x. [DOI] [PubMed] [Google Scholar]
- 15.Petra AI, Panagiotidou S, Stewart JM, Conti P, Theoharides TC. Spectrum of mast cell activation disorders. Expert Rev Clin Immunol. 2014;10(6):729–39. doi: 10.1586/1744666X.2014.906302. [DOI] [PubMed] [Google Scholar]
- 16.Sismanopoulos N, Delivanis DA, Mavrommati D, Hatziagelaki E, Conti P, Theoharides TC. Do mast cells link obesity and asthma? Allergy. 2013;68(1):8–15. doi: 10.1111/all.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasiadi M, Therianou A, Sideri K, Smyrnioti M, Delivani D, Sismanopoulos N, et al. Increased serum CRH levels with decreased skin CRH-R1 gene expression in psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2012;129(5):1410–3. doi: 10.1016/j.jaci.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer M, Theoharides TC, Granstein RD, Bischoff SC, Bienenstock J, Henz B, et al. What is the physiological function of mast cells? Exp Dermatol. 2003;12:886–910. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- 19.Castells M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol Allergy Clin North Am. 2006;26(3):465–85. doi: 10.1016/j.iac.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Horan RF, Sheffer AL, Austen KF. Cromolyn sodium in the management of systemic mastocytosis. J Allergy Clin Immunol. 1990;85(5):852–5. doi: 10.1016/0091-6749(90)90067-e. [DOI] [PubMed] [Google Scholar]
- 21.Theoharides TC, Sieghart W, Greengard P, Douglas WW. Antiallergic drug cromolyn may inhibit histamine secretion by regulating phosphorylation of a mast cell protein. Science. 1980;207(4426):80–2. doi: 10.1126/science.6153130. [DOI] [PubMed] [Google Scholar]
- 22.Barrett KE, Metcalfe DD. The histologic and functional characterization of enzymatically dispersed intestinal mast cells of nonhuman primates: effects of secretagogues and anti-allergic drugs on histamine secretion. J Immunol. 1985;135:2020–6. [PubMed] [Google Scholar]
- 23.Pearce FL, Befus AD, Gauldie J, Bienenstock J. Mucosal mast cells. II: Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982;128:2481–6. [PubMed] [Google Scholar]
- 24.Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn's effectiveness and selectivity as a 'mast cell stabilizer' in mice. Lab Invest. 2012;92(10):1472–82. doi: 10.1038/labinvest.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holgate ST. Reflections on the mechanism(s) of action of sodium cromoglycate (Intal) and the role of mast cells in asthma. Respir Med. 1989;83 (Suppl A):25–31. doi: 10.1016/s0954-6111(89)80247-1. [DOI] [PubMed] [Google Scholar]
- 26.Collier JG, Fuller RW. Evidence for an effect of sodium cromoglycate on sensory nerves in man. Br J Clin Pharmacol. 1983;16(6):639–43. doi: 10.1111/j.1365-2125.1983.tb02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieira Dos SR, Magerl M, Martus P, Zuberbier T, Church MK, Escribano L, et al. Topical sodium cromoglicate relieves allergen- and histamine-induced dermal pruritus. Br J Dermatol. 2010;162(3):674–6. doi: 10.1111/j.1365-2133.2009.09516.x. [DOI] [PubMed] [Google Scholar]
- 28.Kudo H, Tanaka T, Miyachi Y, Imamura S. Contact dermatitis from sodium cromoglycate eyedrops. Contact Dermatitis. 1988;19(4):312. doi: 10.1111/j.1600-0536.1988.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 29.Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- 30.Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000;30:501–8. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 31.Asadi S, Zhang B, Weng Z, Angelidou A, Kempuraj D, Alysandratos KD, et al. Luteolin and thiosalicylate inhibit HgCl(2) and thimerosal-induced VEGF release from human mast cells. Int J Immunopathol Pharmacol. 2010;23(4):1015–20. doi: 10.1177/039463201002300406. [DOI] [PubMed] [Google Scholar]
- 32.Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, et al. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008;155(7):1076–84. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walle T. Methylation of dietary flavones greatly improves their hepatic metabolic stability and intestinal absorption. Mol Pharm. 2007;4(6):826–32. doi: 10.1021/mp700071d. [DOI] [PubMed] [Google Scholar]
- 34.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–82. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 35.Kempuraj D, Huang M, Kandere-Grzybowska K, Basu S, Boucher W, Letourneau R, et al. Azelastine inhibits secretion of IL-6, TNF-α and IL-8 as well as NF-κB activation and intracellular calcium ion levels in normal human mast cells. Int Arch Allergy Immunol. 2003;132:231–9. doi: 10.1159/000074304. [DOI] [PubMed] [Google Scholar]
- 36.Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–8. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 37.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 38.Weng Z, Zhang B, Asadi S, Sismanopoulos N, Butcher A, Fu X, et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PloS One. 2012;7(3):e33805-k. doi: 10.1371/journal.pone.0033805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weng Z, Theoharides TC. Luteolin inhibits inflammatory cytokine release and proliferation of human cultured keratinocytes: application in psoriasis. Exp Biology. 2013 Ref Type: Journal (Full) [Google Scholar]
- 40.Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–64. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells E, Mann J. Phosphorylation of a mast cell protein in response to treatment with anti-allergic compounds; implications for the mode of action of sodium cromoglycate. Biochem Pharmacol. 1983;32:837–42. doi: 10.1016/0006-2952(83)90585-3. [DOI] [PubMed] [Google Scholar]
- 42.Yazid S, Sinniah A, Solito E, Calder V, Flower RJ. Anti-allergic cromones inhibit histamine and eicosanoid release from activated human and murine mast cells by releasing Annexin A1. PloS One. 2013;8(3):e58963. doi: 10.1371/journal.pone.0058963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douglas WW. Exocytosis and the exocytosis-vesiculation sequence: with special reference to neurohypophysis, chromaffin and mast cells, calcium and calcium ionophores. In: Thorn NA, Petersen AH, editors. Secretory Mechanisms of Exocrine Glands. Copenhagen: Munksgaard; 1974. pp. 116–36. [Google Scholar]
- 44.Douglas WW. Stimulus-secretion coupling: variations on the theme of calcium- activated exocytosis involving cellular and extracellular sources of calcium. CIBA Foundation Symposium; N. Holland: Elsevier; 1978. pp. 61–90. [DOI] [PubMed] [Google Scholar]
- 45.Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-alpha and IL-6 production through NFkB in peritoneal mast cells. Biochemica et Biophysica Acta. 2003;1643:75–83. doi: 10.1016/j.bbamcr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Kang OH, Choi JG, Lee JH, Kwon DY. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules. 2010;15(1):385–98. doi: 10.3390/molecules15010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller-Ladner U, Gay RE, Gay S. Role of nuclear factor kappaB in synovial inflammation. Curr Rheumatol Rep. 2002;4(3):201–7. doi: 10.1007/s11926-002-0066-1. [DOI] [PubMed] [Google Scholar]
- 48.Butterfield JH, Weiler DA, Hunt LW, Wynn SR, Roche PC. Purification of tryptase from a human mast cell line. J Leukocyte Biol. 1990;47:409–19. doi: 10.1002/jlb.47.5.409. [DOI] [PubMed] [Google Scholar]
- 49.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–50. [PubMed] [Google Scholar]
- 50.Jin M, Son KH, Chang HW. Luteolin-7-O-glucoside suppresses leukotriene C(4) production and degranulation by inhibiting the phosphorylation of mitogen activated protein kinases and phospholipase Cgamma1 in activated mouse bone marrow-derived mast cells. Biol Pharm Bull. 2011;34(7):1032–6. doi: 10.1248/bpb.34.1032. [DOI] [PubMed] [Google Scholar]
- 51.Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–44. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10(1):21–7. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang M, Kempuraj D, Papadopoulou N, Kourelis T, Donelan J, Manola A, et al. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-kappaB activation. J Mol Endocrinol. 2009;42(5):397–405. doi: 10.1677/JME-08-0120. [DOI] [PubMed] [Google Scholar]
- 54.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140(10):1892–8. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han SY, Bae JY, Park SH, Kim YH, Park JH, Kang YH. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J Nutr. 2013;143(5):632–9. doi: 10.3945/jn.112.173302. [DOI] [PubMed] [Google Scholar]
- 56.Theoharides TC. Extracellular mitochondrial ATP, suramin, and autism? Clin Ther. 2013;35(9):1454–6. doi: 10.1016/j.clinthera.2013.07.419. [DOI] [PubMed] [Google Scholar]
- 57.Theoharides TC. Is a subtype of autism “allergy of the brain”? Clin Ther. 2013;35(5):584–91. doi: 10.1016/j.clinthera.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K, et al. Mast cell activation and autism. Biochim Biophys Acta. 2012;1822(1):34–41. doi: 10.1016/j.bbadis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Lee JY, Kim JM, Kim CJ. Flavones derived from nature attenuate the immediate and late-phase asthmatic responses to aerosolized-ovalbumin exposure in conscious guinea pigs. Inflamm Res. 2013 doi: 10.1007/s00011-013-0670-8. [DOI] [PubMed] [Google Scholar]
- 60.Shim SY, Park JR, Byun DS. 6-Methoxyluteolin from Chrysanthemum zawadskii var. latilobum suppresses histamine release and calcium influx via down-regulation of FcepsilonRI alpha chain expression. J Microbiol Biotechnol. 2012;22(5):622–7. doi: 10.4014/jmb.1111.11060. [DOI] [PubMed] [Google Scholar]
- 61.Tominari T, Hirata M, Matsumoto C, Inada M, Miyaura C. Polymethoxy flavonoids, nobiletin and tangeretin, prevent lipopolysaccharide-induced inflammatory bone loss in an experimental model for periodontitis. J Pharmacol Sci. 2012;119(4):390–4. doi: 10.1254/jphs.11188sc. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z, Zheng S, Li L, Jiang H. Metabolism of flavonoids in human: a comprehensive review. Curr Drug Metab. 2014;15(1):48–61. doi: 10.2174/138920021501140218125020. [DOI] [PubMed] [Google Scholar]
- 63.Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci U S A. 2008;105(21):7534–9. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Oh JM, Heo P, Shin JY, Kong B, Shin J, et al. Polyphenols differentially inhibit degranulation of distinct subsets of vesicles in mast cells by specific interaction with granule-type-dependent SNARE complexes. Biochem J. 2013;450(3):537–46. doi: 10.1042/BJ20121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.