Abstract
Prior research suggests that women diagnosed and treated for gestational diabetes mellitus (GDM) gain less total gestational weight than normoglycemic women. Our study finds that race/ethnicity modifies this association. Relative to normoglycemic women, non-Hispanic white women with GDM gain less weight but non-Hispanic black and Hispanic women gain more weight.
Keywords: Gestational diabetes, Weight gain, Minority health
Introduction
Compared to women without gestational diabetes mellitus (GDM), women with GDM have higher pre-pregnancy body mass indices [1] and gain more weight during their first trimester [2, 3]. Yet institutional chart reviews of women treated for GDM have found that these women experience less weight gain following GDM diagnosis [3, 4] and less total gestational weight gain (GWG) [3-5] than their normoglycemic counterparts. Thus, diagnosis and treatment of GDM may have a beneficial “side effect” of controlling GWG [3, 6]. Larger studies are needed to better understand the association between GDM diagnosis and GWG, particularly among African-American and Hispanic women who are at higher risk than non-Hispanic white women for developing GDM and type 2 diabetes after GDM [7, 8]. The purpose of this project was to (1) describe the association between GDM diagnosis and total GWG in a statewide database and (2) determine if the association is modified by race/ethnicity.
Material and Methods
We conducted a retrospective cohort study of non-Hispanic white (NHW), non-Hispanic black (NHB) and Hispanic adult women (age 18 and above) delivering an infant between 2005 and 2011in Tennessee using maternal data recorded in state birth certificate files [9, 10]. Women who self-identified as Hispanic were categorized as Hispanic regardless of their racial identification. We excluded women with missing pre-pregnancy weight or delivery weight data as well as women with pre-gestational diabetes. The dependent variable of interest was GWG (calculated as delivery weight minus pre-pregnancy weight). The independent variable of interest was GDM status. Additional covariates included a priori in the analyses were race/ethnicity, pre-pregnancy BMI, age, highest education level achieved, payment source for the delivery, parity and tobacco use. Age and pre-pregnancy BMI were included as flexible smooth variables to account for non-linear associations.
First, we used a multiple linear regression model to describe the association between GDM and GWG adjusting for all of the covariates (model 1). Next, we included the cross-product term of race/ethnicity and GDM status to determine if race/ethnicity modified the association (model 2). Finally, we calculated adjusted GWG for women with and without GDM in the full sample and stratified by race/ethnicity using parameter estimates obtained from each model. All analyses were conducted using R-software v. 3.1.0 (R statistical software, Institute for Statistics and Mathematics, Vienna, Austria) [11].
Results
We identified 531,638 women who met the study criteria. Approximately 5% of the study sample was diagnosed with GDM. Seventy-two percent of women identified themselves as NHW, 20% as NHB, and 8% as Hispanic.
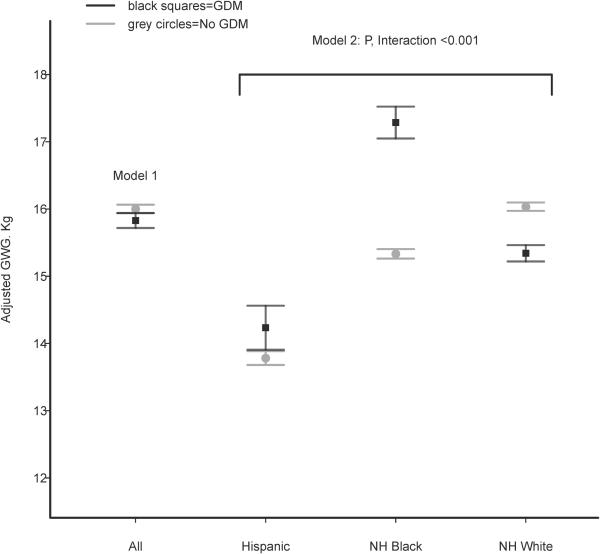
Women with GDM had less mean GWG than women without GDM (13.1 ± 9.0 kg versus 14.5 ± 8.0 kg). After adjusting for covariates women with GDM were found to gain 0.17 kg less than women without GDM (95%CI −0.27, −0.08) in model 1. However, we observed a statistically significant differential association of GDM status with GWG by race/ethnicity (p<0.001 for interaction). In model 2, NHW women with GDM gained 0.69 kg less than NHW women without GDM (95% CI −0.80, −0.58) but NHB women with GDM gained 1.95 kg more than NHB women without GDM (95%CI 1.72, 2.19) and Hispanic women with GDM gained 0.45 kg more than Hispanic women without GDM (95%CI 0.12, 0.78). Figure 1 displays adjusted GWG estimates for women with and without GDM in the full sample and according to race/ethnicity.
Figure 1. Adjusted GWG in Women With and Without GDM by Race/Ethnicity.
Legend: GWG=gestational weight gain, GDM=gestational diabetes mellitus, grey circles=women without GDM, black squares=women with GDM. Model 1 calculates GWG by adjusting for maternal race/ethnicity, pre-pregnancy BMI, maternal age, highest maternal education level achieved, payment source for the delivery, parity and tobacco use. Model 2 calculates GWG according to maternal race/ethnicity by adjusting for all of the covariates in Model 1 as well as the cross-product of maternal race/ethnicity and GDM status.
Discussion
We found that GDM-affected NHW women gain less weight than their normoglycemic counterparts, supporting the previously proposed hypothesis that treatment of GDM promotes behaviors leading to decreased GWG such as diet modification, intensive self-monitoring, and frequent follow up with healthcare providers [3, 6]. However, for NHB and Hispanic women we found that GDM-affected individuals gained significantly more weight than their normoglycemic peers.
Race/ethnicity may modify the association between GDM diagnosis and GWG for several reasons. First, African-American and Hispanic women are at increased risk for inadequate GWG as well as excessive GWG [12]. Therefore, observing relatively greater GWG for women with GDM may be due, in part, to inadequate GWG among women without GDM from these groups. Second, NHB and Hispanic women with GDM may have greater GWG prior to the diagnosis of GDM such that a decrease in weight gain following GDM diagnosis cannot overcome the early pregnancy weight gains. Prior research notes that the association between early pregnancy weight gain and risk of GDM is stronger for nonwhite women [2]. Third, racial/ethnic differences in GDM management or in the uptake of recommended lifestyle modifications could contribute to our findings; previous studies have not examined these possibilities making them important areas for future work.
Our findings are limited by the potential for misclassification bias that is inherent to analyses of vital statistics data. Prior research suggests that the recall bias associated with self-report of pre-pregnancy weight leads to misclassification of pre-pregnancy BMI and GWG category in birth certificate data [13]; however, misclassification of GWG category was not found to differ significantly by race/ethnicity. Misclassification bias could also occur with reporting of GDM diagnosis by hospital staff. Prior studies have found GDM diagnoses to be underreported on the birth certificate compared to the medical record but it is unknown if misclassification differs by race/ethnicity [14].
Despite its limitations, this exploratory analysis identifies a need to specifically include NHB and Hispanic women in future prospective studies of GWG in women with GDM. A growing body of evidence supports a positive association between GWG, postpartum weight retention, and risk of type 2 diabetes, particularly for women with GDM [15-17]. Therefore, an early opportunity to prevent type 2 diabetes in women with GDM is to prevent excessive GWG. Further research is needed to determine if NHB and Hispanic women with GDM could benefit from supplemental strategies to control GWG beyond usual care for GDM.
Highlights.
In the US, the association between GDM and GWG varies by race/ethnicity
Non-Hispanic white women with GDM experience less GWG than those without GDM
Non-Hispanic black women with GDM experience greater GWG than those without GDM
Hispanic women with GDM experience greater GWG than those without GDM
Acknowledgments
Dr. Rosette Chakkalakal is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This research was supported in part by the Vanderbilt Center for Diabetes Translational Research (P30DK092986) which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- 1.Bennett WL, et al. Changes in weight and health behaviors after pregnancies complicated by gestational diabetes mellitus: the CARDIA study. Obesity (Silver Spring) 2013;21(6):1269–75. doi: 10.1002/oby.20133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597–604. doi: 10.1097/AOG.0b013e3181cfce4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morisset AS, et al. Weight gain measures in women with gestational diabetes mellitus. J Womens Health (Larchmt) 2011;20(3):375–80. doi: 10.1089/jwh.2010.2252. [DOI] [PubMed] [Google Scholar]
- 4.Dornhorst A, et al. Calorie restriction for treatment of gestational diabetes. Diabetes. 1991;40(Suppl 2):161–4. doi: 10.2337/diab.40.2.s161. [DOI] [PubMed] [Google Scholar]
- 5.Lauszus FF, Paludan J, Klebe JG. Birthweight in women with potential gestational diabetes mellitus--an effect of obesity rather than glucose intolerance? Acta Obstet Gynecol Scand. 1999;78(6):520–5. [PubMed] [Google Scholar]
- 6.Kim C, Ferrara A. Gestational diabetes during and after pregnancy. Springer; Dordrecht New York: 2010. pp. xviii–394. [Google Scholar]
- 7.Lawrence JM, et al. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care. 2008;31(5):899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 8.Xiang A, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011;54(12):3016–3021. doi: 10.1007/s00125-011-2330-2. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention 2003 Revisions of the U.S. Standard Certificates of Live Birth and Death and the Fetal Death Report. [cited 2014 June 1]; Available from: http://www.cdc.gov/nchs/nvss/vital_certificate_revisions.htm. [Google Scholar]
- 10.Tennessee Department of Health Health Statistics, Vital Statistics. [cited 2014 June 1]; Available from: http://health.tn.gov/statistics/vital.htm.
- 11.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 12.Headen IE, et al. Racial-ethnic differences in pregnancy-related weight. Adv Nutr. 2012;3(1):83–94. doi: 10.3945/an.111.000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodnar LM, et al. Validity of birth certificate-derived maternal weight data. Paediatr Perinat Epidemiol. 2014;28(3):203–12. doi: 10.1111/ppe.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lydon-Rochelle MT, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193(1):125–34. doi: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, et al. Prepregnancy body mass index and weight change on postpartum diabetes risk among gestational diabetes women. Obesity (Silver Spring) 2014;22(6):1560–7. doi: 10.1002/oby.20722. [DOI] [PubMed] [Google Scholar]
- 16.Mannan M, Doi SA, Mamun AA. Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev. 2013;71(6):343–52. doi: 10.1111/nure.12034. [DOI] [PubMed] [Google Scholar]
- 17.Nehring I, et al. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94(5):1225–31. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]