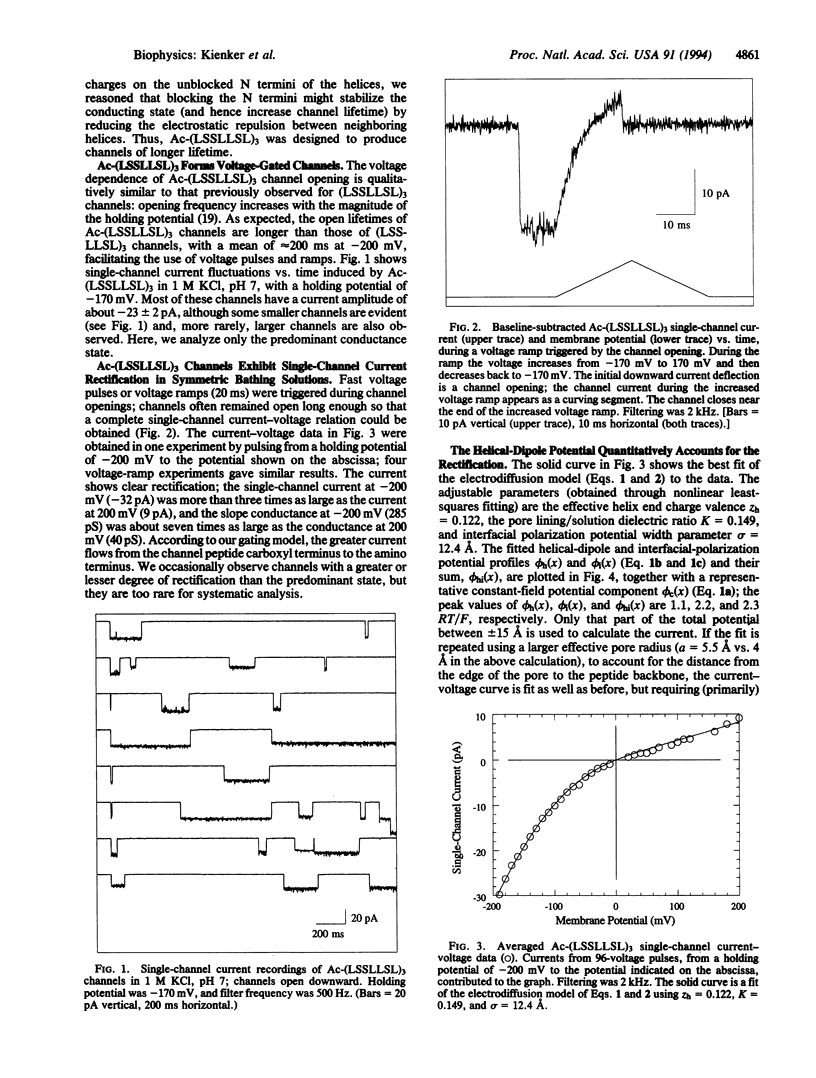
Abstract
We are designing simple peptide ion channels as model systems for the study of the physical principles controlling conduction through ion-channel proteins. Here we report on an uncharged peptide, Ac-(Leu-Ser-Ser-Leu-Leu-Ser-Leu)3-CONH2, designed to form an aggregate of parallel, amphiphilic, membrane-spanning alpha-helices around a central water-filled pore. This peptide in planar lipid bilayers forms ion channels that show single-channel current rectification in symmetric 1 M KCl. The current at a given holding membrane potential is larger than the current measured through the same channel when the potential is reversed. Based on our hypothesized gating mechanism, the larger currents flow from the peptide carboxyl terminus toward the amino terminus. We present an ionic electrodiffusion model based on the helical-dipole potential and the dielectric interfacial polarization energy, which with reasonable values for dipole magnitude and dielectric constants, accurately replicates the current-voltage data.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busath D., Szabo G. Gramicidin forms multi-state rectifying channels. Nature. 1981 Nov 26;294(5839):371–373. doi: 10.1038/294371a0. [DOI] [PubMed] [Google Scholar]
- CRICK F. H. C. Is alpha-keratin a coiled coil? Nature. 1952 Nov 22;170(4334):882–883. doi: 10.1038/170882b0. [DOI] [PubMed] [Google Scholar]
- Chung L. A., Lear J. D., DeGrado W. F. Fluorescence studies of the secondary structure and orientation of a model ion channel peptide in phospholipid vesicles. Biochemistry. 1992 Jul 21;31(28):6608–6616. doi: 10.1021/bi00143a035. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. J Gen Physiol. 1987 Jun;89(6):959–983. doi: 10.1085/jgp.89.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Zaleske D. J. Stereochemical considerations for constructing alpha-helical protein bundles with particular application to membrane proteins. Biochem J. 1977 Apr 1;163(1):45–57. doi: 10.1042/bj1630045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin J. T., Providence L. L., Koeppe R. E., 2nd, Andersen O. S. Energetics of heterodimer formation among gramicidin analogues with an NH2-terminal addition or deletion. Consequences of missing a residue at the join in the channel. J Mol Biol. 1993 Jun 20;231(4):1102–1121. doi: 10.1006/jmbi.1993.1355. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Sodium permeability in toad nerve and in squid nerve. J Physiol. 1960 Jun;152:159–166. doi: 10.1113/jphysiol.1960.sp006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. Energetics of charge-charge interactions in proteins. Proteins. 1988;3(1):32–52. doi: 10.1002/prot.340030104. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Krishtalik L. I., Tae G. S., Cherepanov D. A., Cramer W. A. The redox properties of cytochromes b imposed by the membrane electrostatic environment. Biophys J. 1993 Jul;65(1):184–195. doi: 10.1016/S0006-3495(93)81050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear J. D., Wasserman Z. R., DeGrado W. F. Synthetic amphiphilic peptide models for protein ion channels. Science. 1988 May 27;240(4856):1177–1181. doi: 10.1126/science.2453923. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov A. A., Ukrainskii I. I. O vozmozhnoi roli alpha-spiral'nykh belkov v protsessakh perenosa zariada. Dokl Akad Nauk SSSR. 1979;244(3):751–754. [PubMed] [Google Scholar]
- Parsegian V. A. Ion-membrane interactions as structural forces. Ann N Y Acad Sci. 1975 Dec 30;264:161–171. doi: 10.1111/j.1749-6632.1975.tb31481.x. [DOI] [PubMed] [Google Scholar]
- Tibbitts T. T., Caspar D. L., Phillips W. C., Goodenough D. A. Diffraction diagnosis of protein folding in gap junction connexons. Biophys J. 1990 May;57(5):1025–1036. doi: 10.1016/S0006-3495(90)82621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A. The alpha-helix as an electric macro-dipole. Adv Biophys. 1976:1–63. [PubMed] [Google Scholar]



