Abstract
Importance
Delirium, an acute disorder with high morbidity and mortality, is often preventable through multi-component non-pharmacologic strategies. The efficacy of these strategies for preventing subsequent adverse outcomes has been limited to small studies.
Objective
Evaluate available evidence on multi-component non-pharmacologic delirium interventions in reducing incident delirium and preventing poor outcomes associated with delirium.
Data Sources
PubMed, Google Scholar, ScienceDirect and Cochrane Database of Systematic Reviews from January 1, 1999–December 31, 2013.
Study Selection
Studies examining the following outcomes were included: delirium incidence, falls, length of stay, rate of discharge to a long-term care institution, change in functional or cognitive status.
Data Extraction and Synthesis
Two experienced physician reviewers independently and blindly abstracted data on outcome measures using a standardized approach. The reviewers conducted quality ratings based on the Cochrane Risk of Bias criteria for each study.
Main Outcomes and Measures
We identified 14 interventional studies. Results for outcomes of delirium, falls, length of stay and institutionalization data were pooled for meta-analysis but heterogeneity limited meta-analysis of results for outcomes of functional and cognitive decline. Overall, eleven studies demonstrated significant reductions in delirium incidence (Odds Ratio 0.47, 95% Confidence Interval 0.38–0.58). The four randomized or matched (RMT) studies reduced delirium incidence by 44% (95% CI 0.42–0.76). Rate of falls decreased significantly among intervention patients in four studies (OR 0.38, 95% CI 0.25–0.60); in the two RMTs, the fall rate was reduced by 64% (95% CI 0.22–0.61). Lengths of stay and institutionalization rates also trended towards decreases in the intervention groups, mean difference −0.16 days shorter (95% CI −0.97–0.64) and odds of institutionalization 5% lower (OR 0.95, 95% CI 0.71–1.26) respectively. Among the higher quality RMTs, length of stay trended −0.33 days shorter (95% CI −1.38–0.72) and odds of institutionalization trended 6% lower (95% CI 0.69–1.30).
Conclusions and Relevance
Multi-component non-pharmacologic delirium prevention interventions are effective in reducing delirium incidence and preventing falls, with trend towards decreasing length of stay and avoiding institutionalization. Given the current focus on prevention of hospital-based complications and improved cost-effectiveness of care, this meta-analysis supports the use of these interventions to advance acute care for older persons.
Keywords: Delirium, delirium prevention, non-pharmacologic intervention, multi-component intervention, Hospital Elder Life Program, systematic review, meta-analysis
Introduction
Delirium is an acute confusional state marked by inattention and global cognitive dysfunction. It is multi-factorial and develops due to interactions between risk factors and noxious insults (1). Common yet under-diagnosed, delirium is particularly prevalent among hospitalized elderly, occurring in 29–64% and contributes to over $164 billion in healthcare costs in the United States annually (1, 2). Delirium significantly increases risk for falls, functional decline, dementia, prolonged length of stay and institutionalization (3). The strong correlation between delirium and hospital-related falls has led to calls for delirium prevention quality metrics to improve hospital care and prevent falls in older persons (4). Importantly, at least 30–40% of delirium cases are preventable (1, 5, 6). Surprisingly, most hospitals do not have delirium prevention protocols or their protocols are inconsistently implemented, with variable adherence (1, 7).
Recent systematic reviews and clinical guidelines have recommended targeted multi-component non-pharmacologic intervention strategies for the prevention of delirium (8, 9). The Hospital Elder Life Program (HELP) is the original evidence-based approach targeted to delirium risk factors, which is widely known and disseminated (5, 10, 11). HELP utilizes an interdisciplinary team and trained volunteers to implement practical interventions, including reorientation, early mobilization, therapeutic activities, hydration, nutrition, sleep strategies, and hearing and vision adaptations. HELP has been cost-effective and successful in preventing delirium and functional decline (2, 12, 13). Subsequently, studies evaluated modified HELP models (12, 14, 15). New multi-component non-pharmacologic interventions have been developed, targeting peri-operative patients (16–18) or utilizing volunteers, family members and nurses in delivery of interventions (19, 20). However, differing outcomes were examined across studies, and systematic examination of their effectiveness has not been conducted. Most recently, there have been systematic reviews and guidelines (7, 8) but these underscored the limitation of small samples, heterogeneous outcomes and variable adherence. There is need for more definitive review to expedite dissemination in practice and spur further research into areas of uncertain outcomes.
Thus, the primary aims of our study were to: (1) perform a systematic review of all studies related to multi-component non-pharmacologic interventions for delirium prevention; and (2) conduct quantitative meta-analyses evaluating impact of these interventions on important clinical outcomes. Secondary aims were to evaluate whether quality of studies with respect to risk of bias impacted effectiveness.
Methods
Literature Search
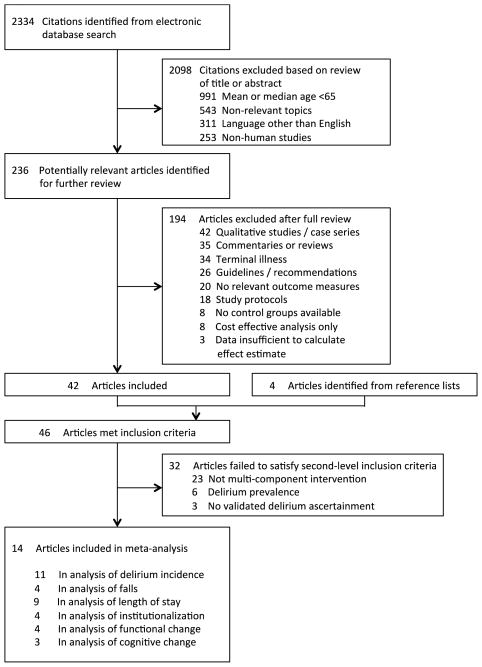
We conducted a comprehensive systematic literature review to identify all studies related to delirium prevention from January 1999–December 2013. Databases searched included PubMed, Google Scholar, ScienceDirect and the Cochrane Database of Systematic Reviews. For search terms, we used a combination of keyword terms and specific phrases representing delirium prevention, targeted multi-component intervention, multi-component intervention, non-pharmacologic intervention, and Hospital Elder Life Program. Review articles were examined for secondary references and all bibliographies from retrieved articles were screened for other relevant studies. Studies were included in the review if they met the following inclusion criteria: original articles, median or mean age of subjects ≥65 years, relevant topics given search terms, published in English, and including human subjects. Studies were excluded after full review for the following criteria: qualitative studies, case series, commentaries, reviews, guidelines/recommendations, no relevant outcomes measures, study protocols, no control groups, or cost-effective analysis. Studies involving terminally ill patients or insufficient data were also excluded. The remaining articles were evaluated and excluded if they failed second-level inclusion criteria: (1) multi-component non-pharmacologic interventions, (2) delirium incidence not prevalence, and (3) validated delirium instrument for ascertainment (Figure 1).
Figure 1. Literature Identification, Review and Selection for Inclusion in Meta-analysis.
The study followed the approaches outlined by the PRISMA flow diagram and checklist, Meta-analysis of Observational Studies in Epidemiology (MOOSE) consensus statement and the Cochrane Handbook for Systematic Reviews of Interventions. Databases searched included PubMed, Google Scholar, ScienceDirect and the Cochrane Database of Systematic Reviews from January 1999 – December 2013. For search terms, we used a combination of controlled vocabulary and keyword terms representing delirium prevention, delirium intervention, targeted multi-component intervention, multi-component intervention, non-pharmacologic intervention, and Hospital Elder Life Program. Utilizing our systematic literature search strategy, 2334 articles were found. Of these, 2098 were excluded based on our screening criteria for relevance, language, age range, or non-human study subjects. A further 189 were excluded after full review yielding 46 articles which met our initial screening criteria. Upon further review, 32 of these did not meet our second-level inclusion criteria which required delirium prevention (not treatment), validated delirium assessment methods, multi-component non-pharmacologic interventions.
Study Selection
The study followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram and checklist (21, 22). The initial search yielded 2334 articles published between January 1999–December 2013 (Figure 1). After exclusion based on screening criteria (relevance, language, age range, non-human study subjects), the number of articles was narrowed to 236. More than half of these articles (119) were not interventional studies. Based on this initial screen, augmented by article reference lists, a total of 46 articles were selected for full review by two independent clinical reviewers. 32 articles did not meet second-level criteria which required delirium prevention (not treatment), validated delirium assessment methods and multi-component non-pharmacologic interventions. Thus, 14 original articles were selected for inclusion in the meta-analysis, encompassing 12 unique intervention trials (Figure 1). The 2 additional studies included addressed different outcomes in different study subgroups. Bogardus et al. examined function and cognition post-discharge in a subgroup of Inouye et al.’s study (5, 23); Stenvall et al. focused on falls among Lundstrom et al. study participants (17, 18).
Outcome Measures
Primary outcomes examined in this study were incident delirium and falls. Incident delirium, defined as new onset delirium during hospitalization, was measured with validated delirium instruments. Of the 14 articles, 12 used the Confusion Assessment Method (24) and two used the Delirium Observation Screening (17, 18, 25). Falls were defined as total number per 1000 patient-days; presented values were recalculated to adhere to these units.
The secondary outcomes examined in this study include length of stay, institutionalization, and functional or cognitive decline. Length of stay was defined, according to standard usage, as total number of calendar days the patient was in the hospital, from date of arrival in emergency room to date of discharge. Institutionalization was defined as new placement in senior residential or nursing home facility upon discharge, for long-term care. Cognitive status was evaluated by mean difference in Mini Mental Status Examination (MMSE) scores between admission and discharge. Changes in functional status were measured by changes in Activities of Daily Living Scale (Lawton Scale) in two studies (5, 26) and Barthel’s Index in two studies (16, 20). For one study (5, 23), admission and 6 months post-discharge functional scores were used. Functional change was evaluated using standardized mean differences in order to compare results across studies utilizing different scoring systems. Standardized mean differences take the difference between functional scores and divides it by standard deviation of scores.
Quality Assessment
We examined the quality of studies included in the meta-analysis using the six domains of the Cochrane Collaboration’s Tool for Assessing Risk of Bias (21). These included random or balanced allocation method, allocation concealment, blinding of participants/personnel/outcome assessors, completeness of outcome data, lack of selective outcome reporting, or other sources of bias.
Data Collection
A standardized data extraction protocol was developed with input from experts in delirium (JY, SKI), multi-component interventions (JY, SKI), geriatrics (TTH, JY, EO, SKI) and systematic reviews/meta-analysis (JY, EO, TT). Two reviewers (JY, TTH) independently extracted and cross-checked data from all articles, assessing study quality using standard criteria. Two additional reviewers (EO, SKI) conducted spot-checks to confirm accuracy of extracted data and resolve any discrepancies.
The 14 articles were abstracted for reference (primary author, publication year), study characteristics (design, duration, setting, country of study, number of patients), and patient characteristics (mean age, gender, type). For each outcome (delirium incidence, falls, institutionalization, length of stay, functional or cognitive change), the reviewers extracted means with standard deviations, numbers of occurrences/total number in sample, odds ratios or relative risks and associated 95% confidence intervals, as applicable. Finally, quality ratings were conducted, as described above. When essential data were not reported, corresponding authors were contacted up to three times.
Statistical Analysis
Following standard procedures (21, 22), we performed meta-analyses on the 14 papers. Intervention trials that utilized formal methods for balanced allocation between treatment and control arms through randomization or prospective individual matching designs, were combined into the group we refer to as randomized or matched trials (RMTs). We considered them separately from other interventional studies (non-RMTs) that did not use such rigorous designs. We made this decision to include RCTs with matched, blinded trials because the small number of RCTs precluded meta-analysis separately. We also judged that the robust methodology and balanced allocation with prospective matching and blinded outcome assessment made some studies of comparable quality to RCTs and combinable without excessive heterogeneity.
For proportions and rates (e.g., incidence of delirium, institutionalizations, falls), odds ratio and 95% confidence intervals were estimated according to intention-to-treat principles. For statistically significant effects, we calculated number needed to treat (NNT) from the risk difference, using the inverse of pooled absolute risk. For continuous data (e.g., length of stay, functional and cognitive change), means with standard deviations and mean differences were used for outcomes pooled on the same scale (e.g., days of hospital stay, MMSE) and standardized mean difference was used for outcomes pooled on different scales (e.g., various functional status measures).
The study results considered for inclusion into meta-analysis were assessed for heterogeneity using chi-square statistic Q, with p <0.1 the threshold indicator for heterogeneity of effects. In addition, I2 was used to estimate the proportion of total variation due to heterogeneity across studies. Values of I2 <25% were regarded as low heterogeneity and fixed-effect models for meta-analysis were used. Values of I2 25%-75% represented moderate heterogeneity and random-effects model were applied. Values of I2 >75% represented high heterogeneity and meta-analysis was not considered appropriate for interpretation. All statistical analyses for meta-analysis were performed using Review Manager (RevMan) software [Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012].
To assess associations between study quality and effectiveness of interventions, we used linear regression analysis to determine at the study level whether there was a relationship between continuous Cochrane Risk of Bias score (range: 0–6) and multiplicative increase in odds (i.e. odds ratio) of incident delirium with intervention versus control. We additionally divided studies into lower and higher quality subgroups based on Cochrane score of <3 vs. ≥4, and performed independent meta-analyses of studies falling into each of these two categories.
All statistical tests performed were two-sided, and statistical significance was indicated by p-value <0.05 or a 95% confidence interval that excluded the null.
Results
Study characteristics
The analytic sample for the present study included 14 articles. Six studies involved randomized or matched trials (RMTs) and eight non-RMTs (Table 1). Two non-randomized trials involved high quality study designs, with prospective individual matching and rigorously blinded outcome assessment (5, 23). Among the eight non-RMTs, three had non-matched concurrent controls and five used historical controls. Overall, approximately 4267 patients at 12 sites (acute medical and surgical wards in academic and community hospitals) were involved; average age was 79.7 years old. All studies involving non-acute care settings ended up excluded based on our criteria. Of the 14 studies, 9 involved HELP adaptations or included at least 4 of the 6 evidence-based interventions from HELP (Table 1).
Table 1.
Characteristics of Studies
| Study | Study Design | Study Duration (months) | Patient Type (setting)/ n | Mean Age (years) | Quality Measures | Interventions |
|---|---|---|---|---|---|---|
| Andro 2011 (France) (34) | Historically controlled (non-RMT) | 18 | Medical / 256 | 84.7 | 1/6 (O) | 5/6 (C, E, H, V, W) |
| Babine 2013 (USA) (14) | Historically controlled (non-RMT) | 3 | Medical / 516 | 70+ | 1/6 (O) | 6/6 (C, E, H, P, V, W) |
| Bo 2009 (Italy) (35) | Nonrandomized controlled trial (non-RMT) | 4 | Medical / 252 | 82.4 | 3/6 (I, O, X) | 4/6 (C, E, P, W) |
| Bogardus 2003 (USA) (23) | Nonrandomized controlled trial - matched/blinded (RMT) | 36 | Medical – Geriatric / 705 | 80 | 5/6 (B, I, O, S, X) | 6/6 (C, E, H, P, V, W) |
| Caplan 2007 (Australia) (20) | Historically controlled (non-RMT) | 5 / 5 | Medical – Geriatric / 37 | 84.7 | 3/6 (I, O, S) | 4/6 (C, H, V, W) |
| Chen 2011 (Taiwan) (16) | Historically controlled (non-RMT) | 20 | Surgical / 179 | 73.0 | 3/6 (B, I, O) | 2/6 (E, C) |
| Holt 2013 (England) (36) | Historically controlled (non-RMT) | 12 | Medical – Geriatric / 362 | 85.4 | 4/6 (B, I, O, X) | 5/6 (C, E, H, V, W) |
| Inouye 1999 (USA) (5) | Nonrandomized controlled trial - matched/blinded (RMT) | 36 | Medical / 852 | 79.7 | 5/6 (B, I, O, S, X) | 6/6 (C, E, H, P, V, W) |
| Jeffs 2013 (Australia) (37) | Randomized controlled trial (RMT) | 30 | Medical / 648 | 79.3 | 6/6 (A, B, I, O, S, X) | 2/6 (C, E) |
| Kratz 2008 (USA) (38) | Nonrandomized controlled trial (non-RMT) | 36 | Medical- Surgical / 137 | 70+ | 1/6 (O) | 6/6 (C, E, H, P, V, W) |
| Lundstrom 2007 (Sweden) (17) | Randomized controlled trial (RMT) | 32 | Surgical/ 199 | 82.2 | 5/6 (A, I, O, S, X) | 1/6 (E) |
| Martinez 2012 (Chile) (39) | Randomized controlled trial (RMT) | 9 | Medical / 287 | 78.2 | 6/6 (A, B, I, O, S, X) | 3/6 (C, H, V) |
| Stenvall 2007 (USA) (18) | Randomized controlled trial - single blinded (RMT) | 32 | Surgical / 199 | 82.2 | 5/6 (A, B, I, S, X) | 3/6 (E, H, P) |
| Vidan 2009 (Spain) (26) | Nonrandomized controlled (non-RMT) | 18 | Medical – Geriatric / 542 | 84.0 | 1/6 (O) | 6/6 (C, E, H, P, V, W) |
Quality Measures: Allocation concealment (A), Blinding of participants/personnel/outcome assessors (B), Completeness of outcome data (I), Selective outcome reporting (O), Random sequence generation/balanced allocation (S), Other sources of bias (X)
Evidence-based Non-pharmacologic Interventions: Cognition/Orientation (C), Early mobility (E), Hearing (H), Sleep-wake cycle preservation (P), Vision (V), Hydration (W)
n = sample size; RMT = Randomized or Matched Trial
Incidence of delirium
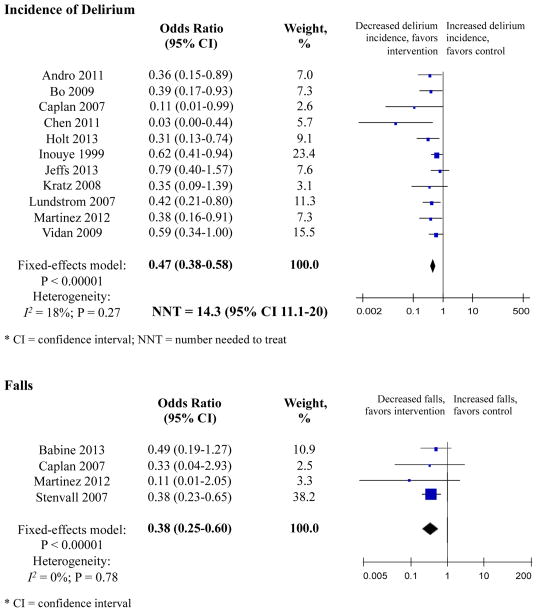
Eleven studies measured delirium (Table 2). Overall, meta-analysis involving 3751 patients showed that odds of delirium were 53% lower in the intervention group compared with controls (OR 0.47, 95% CI 0.38–0.58) (Figure 2). The NNT in the combined sample was 14.3 (95% CI 11.1–20.0) (Figure 2).
Table 2.
Meta-analyses of impact of multicomponent non-pharmacologic interventions
| Intervention | Control | References | Odds Ratios (95% CI) | I2, % | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcome Events | Total Patients | Outcome Events | Total Patients | ||||
| Delirium Incidence | |||||||
| • RMTs | 83 | 977 | 137 | 1009 | 5, 17, 37, 39 | 0.56 (0.42–0.76) | 0 |
| • Non-RMTs | 46 | 752 | 164 | 1013 | 16, 20, 26, 34, 35, 36, 38 | 0.37 (0.27–0.53) | 20 |
| • Combined | 129 | 1729 | 301 | 2022 | 0.47 (0.38–0.58) | 18 | |
|
| |||||||
| Falls | |||||||
| • RMTs | 18 | 245 | 64 | 240 | 18, 39 | 0.36 (0.22–0.61) | 0 |
| • Non-RMTs | 6 | 274 | 31 | 279 | 14, 20 | 0.46 (0.19–1.10) | 0 |
| • Combined | 24 | 519 | 95 | 519 | 0.38 (0.25–0.60) | 0 | |
|
| |||||||
| Institutionalization | |||||||
| • RMTs | 185 | 694 | 190 | 731 | 17, 23 | 0.94 (0.69–1.30) | 0 |
| • Non-RMTs | 19 | 168 | 27 | 231 | 20, 36 | 0.79 (0.25–2.51) | 56 |
| • Combined | 120 | 557 | 132 | 619 | 0.95 (0.71–1.26) | 0 | |
|
| |||||||
| Mean Difference (95% CI) | |||||||
|
| |||||||
| Length of Stay | |||||||
| • RMTs | 977 | 1009 | 5, 17/18, 37, 39 | −0.33 (−1.38–0.72) | 65 | ||
| • Non-RMTs | 561 | 811 | 16, 20, 26, 35, 36 | 0.01 (−1.72–1.73) | 69 | ||
| • Combined | 1538 | 1820 | −0.16 (−0.97–0.64) | 63 | |||
|
| |||||||
| Functional Status | |||||||
| • RMTs | 426 | 426 | 5 | 0.00 (−0.13−0.13) | 0 | ||
| • Non-RMTs | 118 | 98 | 16, 20, 26 | 0.79 (−0.26, 1.84) | 96 | ||
| • Combined | 544 | 524 | 0.57 (−0.03, 1.18) | 96 | |||
|
| |||||||
| Cognitive Status | |||||||
| • RMTs | 426 | 426 | 5 | −0.20 (−0.83–0.43) | 0 | ||
| • Non-RMTs | 188 | 470 | 16, 20 | 2.23 (−0.78, 5.24) | 75 | ||
| • Combined | 714 | 896 | 0.97 (−0.46–2.41) | 83 | |||
RMT = Randomized or Matched Trials
Figure 2. Primary Outcomes.
Incidence of Delirium
Eleven studies measured incidence of delirium. Three RMTs and five non-RMTs demonstrated significant reductions in the incidence of delirium. Overall, the meta-analysis involving 3751 patients showed that the odds of delirium were 53% lower in the intervention group compared with controls (OR 0.47, 95% CI 0.38–0.58). The number needed to treat in the combined sample was 14.3 (95% CI 11.1–20.0). There was low heterogeneity, I2 = 18% with p < 0.00001. Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased delirium incidence. RMT indicates randomized or matched trials; CI indicates confidence interval.
Falls
Four studies examined the number of falls per patient-days. Individually, only Stenvall et al., an RMT, demonstrated significant reductions in the number of falls. Combined, the meta-analysis involving 1038 patients showed that the odds of falling were 62% lower among intervention subjects (OR 0.38, 95% CI 0.25–0.60). This represents the equivalent of 4.26 falls prevented per 1000 patient-days – or 2.79 falls per 1000 patient-days among intervention subjects compared to 7.05 falls per 1000 patient-days among control subjects. There was low heterogeneity, I2 = 0.00% with p < 0.0001. Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased rate of falls. RMT indicates randomized or matched trials; CI indicates confidence interval.
Stratified by study type, multi-component non-pharmacologic interventions lowered odds of delirium by 44% (RR 0.56, 95% CI 0.42–0.76) among 977 intervention patients included in four RMTs and by 63% (OR 0.37, 95% CI 0.27–0.53) among 752 intervention patients included in seven non-RMTs (Appendix Figure 1). The NNT was 20.0 patients (95% CI 12.5–33.3) among RMTs and 11.1 patients (95% CI 8.3–16.7) among non-RMTs (Appendix Figure 1).
Falls
Four studies examined the number of falls per patient-days (Table 2). Combined, meta-analysis involving 1038 patients showed that odds of falling were 62% lower among intervention subjects (OR 0.38, 95% CI 0.25–0.60) (Figure 2). This represents the equivalent of 4.26 falls prevented per 1000 patient-days or 2.79 falls per 1000 patient-days among intervention subjects compared to 7.05 falls per 1000 patient-days among controls.
Stratified by study type, multi-component non-pharmacologic interventions lowered odds of falling significantly among 245 intervention patients included in two RMT studies (OR 0.36, 95% CI 0.22–0.61) (Appendix Figure 2). This represents 8.53 falls prevented per 1000 patient-days or 4.34 falls per 1000 patient days among intervention subjects compared to 12.87 falls per 1000 patient days among controls. The odds of falling trended lower among 274 intervention patients included in two non-RMT studies (OR 0.46, 95% CI 0.19–1.10) (Appendix Figure 2). This represents the equivalent of 2.34 falls prevented per 1000 patient-days, or 1.35 falls per 1000 patient days among intervention subjects compared to 3.69 falls per 1000 patient days among controls.
Length of stay
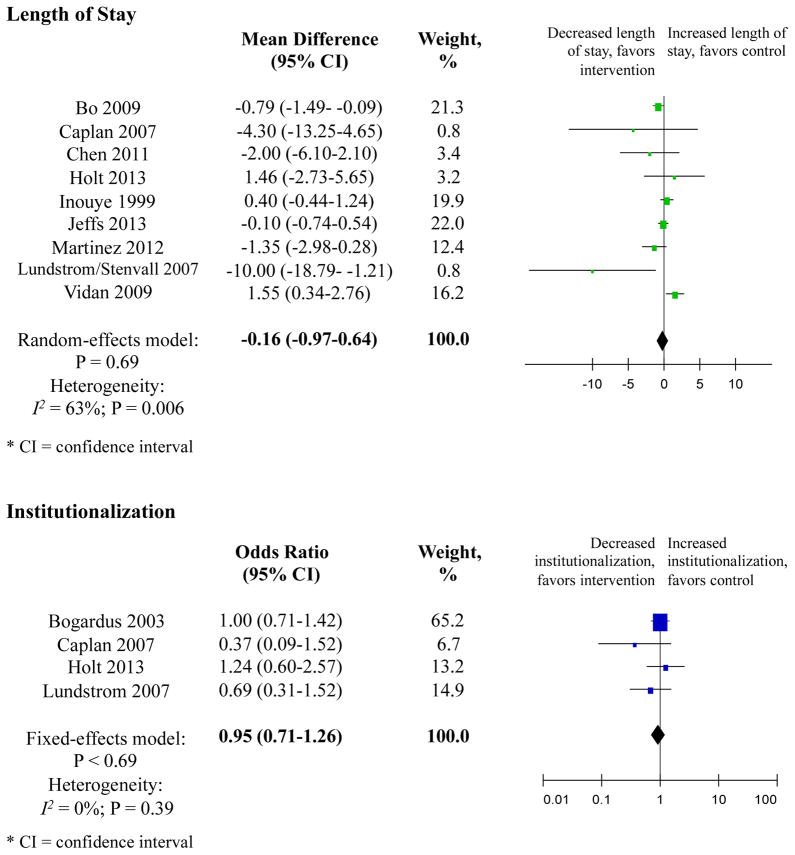
Nine studies measured length of stay (Table 2). Overall, meta-analysis involving 3358 patients showed that mean difference was −0.16 days shorter in the intervention group with a trend towards significance (95% CI −0.97–0.64) (Figure 3).
Figure 3. Secondary Outcomes.
Length of Stay
Nine studies measured length of stay. Individually, only two studies, Bo et al., a non-RMT, and Lundstrom 2007, an RMT, demonstrated significant reductions in length of stay. Overall, the meta-analysis involving 3358 patients showed that the mean difference was −0.16 days shorter in the intervention group with a trend towards significance (95% CI −0.97–0.64). Heterogeneity was moderate, I2 = 64% with p = 0.006). Weighting was assigned according to the inverse of the variance. Mean differences less than 0 indicate decreased length of stay, in days. RMT indicates randomized or matched trials; CI indicates confidence interval.
Institutionalization
Four studies examined the rate of institutionalization in a senior residential or nursing home facility post-hospital discharge. Overall, the meta-analysis involving 1176 patients showed that the odds of discharge to long-term care were 5% lower (OR 0.95, 95% CI 0.71–1.26) in the intervention group, but the results did not achieve statistical significance. Heterogeneity was low, I2 = 0.00% with p = 0.69 among intervention subjects, but there was little to no significant effect. Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased rate of institutionalization. RMT indicates randomized or matched trials; CI indicates confidence interval.
Stratified by study type, multi-component non-pharmacologic interventions decreased length of stay by −0.33 days (95% CI −1.38–0.72) among 977 intervention patients included in four RMTs (Appendix Figure 3). Length of stay was increased by 0.01 days (95% CI −1.72–1.73) among 561 intervention patients included in five non-RMTs (Appendix Figure 3). Neither of these stratified analyses achieved statistical significance.
Institutionalization
Four studies examined rate of institutionalization post-hospital discharge (Table 2). Overall, meta-analysis involving 1176 patients showed that odds of discharge to long-term care were 5% lower (OR 0.95, 95% CI 0.71–1.26) in the intervention group, but the results did not achieve statistical significance (Figure 3).
Stratified by study type, trends are consistent with an odds ratio for institutionalization among 120 intervention patients in two RMTs 0.94 in favor of multi-component non-pharmacologic interventions, but were not statistically significant (95% CI 0.69–1.30) (Appendix Figure 4). The odds ratio for institutionalization among 132 patients involved in two non-RMTs was 0.79 in favor of targeted interventions, but the results did not achieve statistical significance (95% CI 0.25–2.51) (Appendix Figure 4).
Functional change
Four studies measured change in functional status, one high quality RMT (5) and three non-RMTs (Table 2). Random-effects models were used due to high heterogeneity of these studies. Combined, standard mean difference for functional improvement among 1068 patients was 0.57 in favor of multi-component non-pharmacologic interventions, but not statistically significant (95% CI −0.03–1.18). Heterogeneity was high, I2=96% with p <0.00001, and thus the pooled results must be interpreted with caution (Appendix Figure 5).
Cognitive change
Three studies measured change in cognition, with one high quality RMT (5) and two non-RMTs (Table 2). Random effects models were used due to high heterogeneity of these studies. Combined, mean difference for cognitive improvement among 1610 patients was 0.97 in favor of multi-component non-pharmacologic interventions, but did not achieve statistical significance (95% CI −0.46–2.41). In addition, heterogeneity was high, I2=83% with p=0.002, again indicating that pooled results must be interpreted with caution (Appendix Figure 5).
Relationship between Quality Ratings and Effectiveness
We observed limited, statistically non-significant evidence of any association between study quality and effectiveness of interventions in preventing incident delirium. Per unit increase on the Cochrane measure, the decrease attributable to intervention in odds of incident delirium or falls was reduced by 4% (R2=0.025) and 10% (R2=0.438) respectively. Results did not change when lower and higher-quality studies were compared. The odds ratio for delirium incidence among higher quality studies was 0.53 (95% CI 0.39–0.71) compared with 0.38 (95% CI 0.23–0.64) among lower quality studies, p=0.28. When examining whether higher quality yielded better outcomes for falls, length of stay and institutionalization outcomes, the subgroup differences were also not statistically significant (p=0.65, 1.00 and 0.18 respectively). Thus, study quality ratings were not highly correlated with effectiveness.
Discussion
This systematic review and meta-analysis of 14 studies involving 12 unique interventions demonstrate that multi-component non-pharmacologic interventions for delirium prevention are highly effective in decreasing occurrence of both delirium and falls during hospitalization in older persons. The impact of intervention strategies on delirium prevention across 11 studies is striking, with a >50% odds reduction that was highly significant (OR=0.47, 95% CI, 0.38–0.58). In 2008, there were 13.2 million hospital discharges of older patients in the United States with a mean hospital stay of 5.5 days (27). Based on our results, approximately one million cases of delirium in the hospital could have been prevented by multi-component non-pharmacologic interventions each year, resulting in Medicare cost savings of approximately $10,000 per case prevented or $10 billion (U.S.) per year (1, 2).
The impact on fall prevention is a novel and important finding, with >60% odds reduction (OR=0.38, 95% CI, 0.25–0.60). Since delirium is the leading contributor to hospital falls (1, 8), the prevention of falls with these interventions is a consistent and compelling result. Furthermore, given their status as Medicare no-pay conditions, fall prevention has become a top priority among U.S. hospitals (28). If 4.26 falls can be avoided with multi-component non-pharmacologic interventions per 1000 patient-days, 326,996 falls can be prevented annually with these interventions. This translates into an additional $4.5–6.7 billion Medicare dollars saved annually from preventable falls (29, 30).
Few intervention strategies have proven effective for fall prevention in the hospital. Most fall interventions have focused on identifying fall risk and implementing various alarms that limit patient mobility. While minimally effective for preventing falls, these approaches result in unintended consequences of decreased physical and cognitive functioning (28). Notably, 12 of the 14 studies examined in this meta-analysis include exercise interventions designed to enhance mobility. The well-documented effectiveness of these strategies for fall prevention is worthy of special emphasis.
While a trend toward benefit existed, lack of significant association between delirium interventions and length of stay and institutionalization is unsurprising given multiple complex influences on these outcomes, including multi-morbidity and psychosocial factors (supports, finances, caregiver preferences), all of which make ultimate disposition unpredictable. Furthermore, because sample sizes were small, our meta-analyses may have been underpowered to detect true differences.
The small number of studies examining functional and cognitive decline, and their substantial heterogeneity, limited our ability to examine these outcomes. In addition, the preferred outcome with delirium prevention should be functional and cognitive stability (i.e., maintenance), not improvement or decline. Only one study examined function and cognition at admission and six months post-discharge (23). Performing cognitive and functional reassessments upon discharge when patients may still be delirious and acutely deconditioned is suboptimal, and waiting until they have returned to a stable condition should be the preferred approach.
Our study has several noteworthy strengths. Meta-analysis allowed us to extend conclusions beyond populations contained in a single study, particularly given the variable and limited number of studies and the small study sizes available for review. We used a comprehensive search strategy and systematic review method (21, 22). With over 4200 study participants in pooled analyses, there was improved power for meta-analysis of study results. We limited heterogeneity and rigorously controlled for potential sources of bias by adhering to clear, predetermined selection criteria and evaluating the quality of our selected studies based on the Cochrane Collaboration’s Risk of Bias guidelines (21). Our stratified models for delirium incidence, institutionalization and falls confirmed that observed outcome associations were robust across study designs. Heterogeneity analysis allowed us to account for study factors that could be influencing our outcomes of interest across multiple trials. Use of quality ratings facilitated our evaluation of the studies; however, quality scores did not correlate significantly with effectiveness.
Several limitations of our study are worthy of comment. The final number of included studies is small and many had limited sample sizes. Less than one-third of the interventions evaluated were randomized controlled trials (29%, 4 of 14), the gold standard for evidence-based practice. Blinding was difficult to achieve in non-pharmacologic intervention studies by the unit-wide nature of many interventions. Thus, data available for synthesis may have been limited, restricting the strength of our conclusions. In particular, there were only 4 studies examining the outcome of falls, 2 of which were historically controlled studies with smaller sample sizes. Importantly, selective reporting bias in the literature would have resulted in our meta-analysis reflecting an over-estimation of the impact on falls. Despite similarities in research questions and interventions across studies, there remained a moderate degree of heterogeneity for all studies examining length of stay, functional and cognitive decline, and for non-RMTs examining institutionalization. This heterogeneity, which likely stems from variations in study designs, sample characteristics, sample sizes, and outcome measures used, limits the interpretation of our pooled estimates. Despite these limitations, the findings of this meta-analysis are highly clinically relevant for the hospitalized geriatric population.
A few studies were not included in our meta-analysis despite being well designed and influential in the field of delirium prevention. These papers were excluded based on our predetermined inclusion criteria. Marcantonio et al. (2001) and Milisen et al. (2001) were excluded because they primarily involved consultation for preventive management of delirium, not multi-component non-pharmacologic interventions (6, 19). Cole at al. (2002), Naughton et al. (2005) and Zaubler et al. (2013) published work on effective multi-component non-pharmacologic interventions but included delirious patients in their studies. Thus, these were not considered primary prevention studies since incident delirium rates could not be calculated (3, 31–33).
In conclusion, this meta-analysis suggests that multi-component non-pharmacologic interventions are effective in decreasing incident delirium and preventing falls, potentially saving over $16 billion annually in the United States alone. Therefore, these multi-component non-pharmacologic strategies hold great promise to impact two of the most important and prevalent conditions affecting seniors during hospitalization. Our systematic review and meta-analysis demonstrate that multi-component non-pharmacologic interventions decrease the substantial healthcare and societal burden of delirium and falls, improving quality of life for these patients and their families.
Supplementary Material
Appendix Figure 1. Incidence of Delirium
Stratified by study type, multi-component non-pharmacologic interventions lowered the odds of delirium by 44% (RR 0.56, 95% CI 0.42–0.76) among 977 intervention patients in four RMTs and by 63% (OR 0.37, 95% CI 0.27–0.53) among 752 patients in seven non-RMTs. Heterogeneity was low, I2 = 0.00% with p < 0.0001 for the three RMTs and I2 = 20% with p < 0.0001 for the non-RMTs. The number needed to treat among RMTs was 20.0 patients (95% CI 12.5–33.3); the number needed to treat among non-RMTs was 11.1 patients (95% CI 8.3–16.7). Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased delirium incidence. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 2. Falls
Stratified by study type, multi-component non-pharmacologic interventions significantly decreased the odds of falling for study participants among 245 intervention patients in the two RMT studies (OR 0.36, 95% CI 0.22–0.61). This represents the equivalent of 8.53 falls prevented – or 4.34 falls per 1000 patient days among intervention subjects compared to 12.87 falls per 1000 patient days among control subjects. The odds of falling also trended towards significantly lower among 274 intervention patients in two non-RMT study participants (OR 0.46, 95% CI 0.19–1.10). This represents the equivalent of 2.34 falls prevented – or 1.35 falls per 1000 patient days among intervention subjects compared to 3.69 falls per 1000 patient days among control subjects. Heterogeneity was low, I2 = 0.00% with p < 0.0001 among RMTs and I2 = 0.00% with p < 0.0007 among non-RMTs. Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased rate of falls. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 3. Length of Stay
Stratified by study type, multi-component non-pharmacologic interventions decreased the length of stay by −0.33 days (95% CI −1.38–0.72) among the 977 intervention patients in four RMTs, in favor of multi-component non-pharmacologic interventions, although there was little to no significant effect. Length of stay was increased by 0.01 days (95% CI −1.72–1.73) among the 561 intervention patients in five non-RMTs, also without statistical significance. Heterogeneity was moderate, I2 = 65% with p = 0.04 among RMTs and I2 = 69% with p = 0.01 among non-RMTs. Weighting was assigned according to the inverse of the variance. Mean differences less than 0 indicate decreased length of stay, in days. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 4. Institutionalization.
Stratified by study type, multi-component non-pharmacologic interventions decreased the odds of institutionalization by 4% (RR 0.94, 95% CI 0.69–1.30) among 120 intervention patients in two RMTs, but there was little to no significant effect. The odds ratio for institutionalization among 132 intervention patients in two non-RMTs was 0.79 in favor of targeted interventions but also not statistically significant (95% CI 0.25–2.51). Heterogeneity was low among RMTs (I2 = 0.00% with p = 0.72) but moderate among non-RMTs (I2 = 56% with p = 0.69). Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased rate of institutionalization. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 5. Functional and Cognitive change.
Analyses of the association between functional change and implementation of multi-component non-pharmacologic interventions to prevent delirium.
Four studies measured change in functional status, one RMT (Inouye et al.) and three non-RMTs. Only one study (Chen et al.) found statistically significant functional improvement with multi-component non-pharmacologic interventions. Random effects models were used due to the high heterogeneity of these studies. In the meta-analysis, the standard mean difference for functional improvement was 0.57 among 1068 patients in favor of multi-component non-pharmacologic interventions but with little or no significant effect (95% CI −0.03–1.18). Heterogeneity was high, precluding appropriate pooling of the data for meaningful analysis (I2 = 96% with p < 0.00001). Weighting was assigned according to the inverse of the variance. Standardized mean differences greater than 0 indicate increased functional status, favoring intervention. RMT indicates randomized or matched trials; CI indicates confidence interval.
Analyses of the association between cognitive change and implementation of multi-component non-pharmacologic interventions to prevent delirium.
Three studies measured change in cognition, one RMT (Inouye et al.) and two non-RMTs. One study (Caplan et al.) found statistically significant cognitive improvement with multi-component non-pharmacologic interventions. Random effects models were used due to the high heterogeneity of these studies in their cognitive outcomes. Overall, in the meta-analysis, the mean difference for cognitive improvement was 0.97 among 1610 patients in favor of multi-component non-pharmacologic interventions but with little or no significant effect (95% CI −0.46–2.41). Heterogeneity was high, precluding appropriate pooling of the data for meaningful analysis (I2 = 83% with p = 0.002). Weighting was assigned according to the inverse of the variance. Mean differences greater than 0 indicate increased cognitive scores, favoring intervention. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 5. Incidence of Delirium, Stratified by Patient Type
Stratified by patient type, multi-component non-pharmacologic interventions significantly lowered the odds of delirium by 51% (RR 0.49, 95% CI 0.38–0.63) among medical patients in 8 studies and by 58% (OR 0.42, 95% CI 0.19–0.93) among surgical patients in 4 studies. Of note, Kratz et al. included both medical and surgical patients and thus was included in analysis for both categories. Heterogeneity was low for pooled analysis of the medical patients, I2 = 0% with p < 0.00001 and moderate for pooled analysis of the surgical patients, I2 = 57% with p = 0.07. These trends are similar to the overall meta-analysis and stratification by RMTs and non-RMTs. Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased delirium incidence. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 6. Length of Stay, Stratified by Patient Type
Stratified by patient type, multi-component non-pharmacologic interventions decreased the length of stay by −0.02 days (95% CI −0.76–0.72) among the medical patients in 7 studies and by −5.01 days (95% CI −12.61–2.58) among the surgical patients in 2 studies. Heterogeneity was moderate for pooled analysis of the medical patients, I2 = 62% with p=0.01 and for pooled analysis of the surgical patients, I2 = 62% with p = 0.11. These trends are similar to the overall meta-analysis and stratification by RMTs and non-RMTs. Weighting was assigned according to the inverse of the variance. Mean differences less than 0 indicate decreased length of stay, in days. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Table 1.
Evidence-based Non-pharmacologic Delirium Prevention Interventions
| Risk Factors | Details |
|---|---|
| Cognition / Orientation |
|
| Early mobility |
|
| Hearing |
|
| Sleep-wake cycle preservation |
|
| Vision |
|
| Hydration |
|
Acknowledgments
Funding Disclosure: This study was supported in part by Grant K07AG041835 from the National Institute on Aging. Dr. Hshieh is supported by T32 Training Grant (AG000158) from the National Institute on Aging. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the design, conduct or reporting of this study.
Footnotes
This work is dedicated to the memory of Joshua Bryan Inouye Helfand.
Conflict of Interest: All the co-authors fully disclose they have no financial interests, activities, relationships and affiliations. The co-authors also declare they have no potential conflicts from the three years prior to submission of this manuscript.
Data Access and Responsibility: Drs. Hshieh, Yue and Inouye had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions: We affirm that all the co-authors listed contributed significantly to the preparation of this manuscript and approve this final version to be published.
Dr. Hshieh contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. Dr. Yue substantially contributed to the analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. Dr. Oh contributed to the analysis and interpretation of data and revising the article critically. Ms. Puelle contributed to the acquisition of data and revising the article critically. Ms. Dowal contributed to the acquisition of data and revising the article critically. Dr. Travison contributed substantially to the analysis and interpretation of data and revising the article critically. Dr. Inouye contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically as well as obtaining funding and administrative support.
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 4.Lee EA, Gibbs NE, Fahey L, Whiffen TL. Making hospitals safer for older adults: updating quality metrics by understanding hospital-acquired delirium and its link to falls. Perm J. 2013;17(4):32–6. doi: 10.7812/TPP/13-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 6.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 7.Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):375–80. doi: 10.7326/0003-4819-158-5-201303051-00003. [DOI] [PubMed] [Google Scholar]
- 8.O’Mahony R, Murthy L, Akunne A, Young J Guideline Development G. Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Intern Med. 2011;154(11):746–51. doi: 10.7326/0003-4819-154-11-201106070-00006. [DOI] [PubMed] [Google Scholar]
- 9.Greer N, Rossom R, Anderson P, MacDonald R, Tacklind J, Rutks I, et al. Delirium: Screening, Prevention, and Diagnosis - A Systematic Review of the Evidence. Washington (DC): 2011. [PubMed] [Google Scholar]
- 10.Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257–63. doi: 10.3109/07853890009011770. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Bogardus ST, Jr, Baker DI, Leo-Summers L, Cooney LM., Jr The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48(12):1697–706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 12.Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59(2):359–65. doi: 10.1111/j.1532-5415.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzo JA, Bogardus ST, Jr, Leo-Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care. 2001;39(7):740–52. doi: 10.1097/00005650-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Babine RL, Farrington S, Wierman HR. HELP(c) prevent falls by preventing delirium. Nursing. 2013;43(5):18–21. doi: 10.1097/01.NURSE.0000428710.81378.aa. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, Baker DI, Fugal P, Bradley EH, Project HD. Dissemination of the hospital elder life program: implementation, adaptation, and successes. J Am Geriatr Soc. 2006;54(10):1492–9. doi: 10.1111/j.1532-5415.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245–52. doi: 10.1016/j.jamcollsurg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrom M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178–86. doi: 10.1007/BF03324687. [DOI] [PubMed] [Google Scholar]
- 18.Stenvall M, Olofsson B, Lundstrom M, Englund U, Borssen B, Svensson O, et al. A multidisciplinary, multifactorial intervention program reduces postoperative falls and injuries after femoral neck fracture. Osteoporos Int. 2007;18(2):167–75. doi: 10.1007/s00198-006-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milisen K, Foreman MD, Abraham IL, De Geest S, Godderis J, Vandermeulen E, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523–32. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 20.Caplan GA, Harper EL. Recruitment of volunteers to improve vitality in the elderly: the REVIVE study. Intern Med J. 2007;37(2):95–100. doi: 10.1111/j.1445-5994.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Available from http://www.cochrane-handbook.org.
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Bogardus ST, Jr, Desai MM, Williams CS, Leo-Summers L, Acampora D, Inouye SK. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med. 2003;114(5):383–90. doi: 10.1016/s0002-9343(02)01569-3. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 25.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50. doi: 10.1891/rtnp.17.1.31.53169. [DOI] [PubMed] [Google Scholar]
- 26.Vidan MT, Sanchez E, Alonso M, Montero B, Ortiz J, Serra JA. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57(11):2029–36. doi: 10.1111/j.1532-5415.2009.02485.x. [DOI] [PubMed] [Google Scholar]
- 27.Wier LM, Thomson Reuters, Pfuntner A, Thomson Reuters, Steiner C., AHRQ . HCUP Statistical Brief #103. Agency for Healthcare Research and Quality; Agency for Healthcare Research and Quality; Rockville, MD: Dec, 2010. Hospital Utilization among Oldest Adults, 2008. http://www.hcupus.ahrq.gov/reports/statbriefs/sb103.pdf. [PubMed] [Google Scholar]
- 28.Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360(23):2390–3. doi: 10.1056/NEJMp0900963. [DOI] [PubMed] [Google Scholar]
- 29.Rizzo JA, Friedkin R, Williams CS, Nabors J, Acampora D, Tinetti ME. Health care utilization and costs in a Medicare population by fall status. Med Care. 1998;36(8):1174–88. doi: 10.1097/00005650-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Shumway-Cook A, Ciol MA, Hoffman J, Dudgeon BJ, Yorkston K, Chan L. Falls in the Medicare population: incidence, associated factors, and impact on health care. Phys Ther. 2009;89(4):324–32. doi: 10.2522/ptj.20070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaubler TS, Murphy K, Rizzuto L, Santos R, Skotzko C, Giordano J, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219–26. doi: 10.1016/j.psym.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Cole MG, McCusker J, Bellavance F, Primeau FJ, Bailey RF, Bonnycastle MJ, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Naughton BJ, Saltzman S, Ramadan F, Chadha N, Priore R, Mylotte JM. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. J Am Geriatr Soc. 2005;53(1):18–23. doi: 10.1111/j.1532-5415.2005.53005.x. [DOI] [PubMed] [Google Scholar]
- 34.Andro M, Comps E, Estivin S, Gentric A. Prevention of delirium in demented hospitalized patients. Eur J Intern Med. 2012;23(2):124–5. doi: 10.1016/j.ejim.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Bo M, Martini B, Ruatta C, Massaia M, Ricauda NA, Varetto A, et al. Geriatric ward hospitalization reduced incidence delirium among older medical inpatients. Am J Geriatr Psychiatry. 2009;17(9):760–8. doi: 10.1097/jgp.0b013e3181a315d5. [DOI] [PubMed] [Google Scholar]
- 36.Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721–7. doi: 10.1093/ageing/aft120. [DOI] [PubMed] [Google Scholar]
- 37.Jeffs KJ, Berlowitz DJ, Grant S, Lawlor V, Graco M, de Morton NA, et al. An enhanced exercise and cognitive programme does not appear to reduce incident delirium in hospitalised patients: a randomised controlled trial. BMJ Open. 2013;3(6) doi: 10.1136/bmjopen-2013-002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331–7. doi: 10.1097/01.NCQ.0000336673.02725.ec. [DOI] [PubMed] [Google Scholar]
- 39.Martinez FT, Tobar C, Beddings CI, Vallejo G, Fuentes P. Preventing delirium in an acute hospital using a non-pharmacological intervention. Age Ageing. 2012;41(5):629–34. doi: 10.1093/ageing/afs060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Incidence of Delirium
Stratified by study type, multi-component non-pharmacologic interventions lowered the odds of delirium by 44% (RR 0.56, 95% CI 0.42–0.76) among 977 intervention patients in four RMTs and by 63% (OR 0.37, 95% CI 0.27–0.53) among 752 patients in seven non-RMTs. Heterogeneity was low, I2 = 0.00% with p < 0.0001 for the three RMTs and I2 = 20% with p < 0.0001 for the non-RMTs. The number needed to treat among RMTs was 20.0 patients (95% CI 12.5–33.3); the number needed to treat among non-RMTs was 11.1 patients (95% CI 8.3–16.7). Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased delirium incidence. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 2. Falls
Stratified by study type, multi-component non-pharmacologic interventions significantly decreased the odds of falling for study participants among 245 intervention patients in the two RMT studies (OR 0.36, 95% CI 0.22–0.61). This represents the equivalent of 8.53 falls prevented – or 4.34 falls per 1000 patient days among intervention subjects compared to 12.87 falls per 1000 patient days among control subjects. The odds of falling also trended towards significantly lower among 274 intervention patients in two non-RMT study participants (OR 0.46, 95% CI 0.19–1.10). This represents the equivalent of 2.34 falls prevented – or 1.35 falls per 1000 patient days among intervention subjects compared to 3.69 falls per 1000 patient days among control subjects. Heterogeneity was low, I2 = 0.00% with p < 0.0001 among RMTs and I2 = 0.00% with p < 0.0007 among non-RMTs. Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased rate of falls. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 3. Length of Stay
Stratified by study type, multi-component non-pharmacologic interventions decreased the length of stay by −0.33 days (95% CI −1.38–0.72) among the 977 intervention patients in four RMTs, in favor of multi-component non-pharmacologic interventions, although there was little to no significant effect. Length of stay was increased by 0.01 days (95% CI −1.72–1.73) among the 561 intervention patients in five non-RMTs, also without statistical significance. Heterogeneity was moderate, I2 = 65% with p = 0.04 among RMTs and I2 = 69% with p = 0.01 among non-RMTs. Weighting was assigned according to the inverse of the variance. Mean differences less than 0 indicate decreased length of stay, in days. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 4. Institutionalization.
Stratified by study type, multi-component non-pharmacologic interventions decreased the odds of institutionalization by 4% (RR 0.94, 95% CI 0.69–1.30) among 120 intervention patients in two RMTs, but there was little to no significant effect. The odds ratio for institutionalization among 132 intervention patients in two non-RMTs was 0.79 in favor of targeted interventions but also not statistically significant (95% CI 0.25–2.51). Heterogeneity was low among RMTs (I2 = 0.00% with p = 0.72) but moderate among non-RMTs (I2 = 56% with p = 0.69). Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased rate of institutionalization. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 5. Functional and Cognitive change.
Analyses of the association between functional change and implementation of multi-component non-pharmacologic interventions to prevent delirium.
Four studies measured change in functional status, one RMT (Inouye et al.) and three non-RMTs. Only one study (Chen et al.) found statistically significant functional improvement with multi-component non-pharmacologic interventions. Random effects models were used due to the high heterogeneity of these studies. In the meta-analysis, the standard mean difference for functional improvement was 0.57 among 1068 patients in favor of multi-component non-pharmacologic interventions but with little or no significant effect (95% CI −0.03–1.18). Heterogeneity was high, precluding appropriate pooling of the data for meaningful analysis (I2 = 96% with p < 0.00001). Weighting was assigned according to the inverse of the variance. Standardized mean differences greater than 0 indicate increased functional status, favoring intervention. RMT indicates randomized or matched trials; CI indicates confidence interval.
Analyses of the association between cognitive change and implementation of multi-component non-pharmacologic interventions to prevent delirium.
Three studies measured change in cognition, one RMT (Inouye et al.) and two non-RMTs. One study (Caplan et al.) found statistically significant cognitive improvement with multi-component non-pharmacologic interventions. Random effects models were used due to the high heterogeneity of these studies in their cognitive outcomes. Overall, in the meta-analysis, the mean difference for cognitive improvement was 0.97 among 1610 patients in favor of multi-component non-pharmacologic interventions but with little or no significant effect (95% CI −0.46–2.41). Heterogeneity was high, precluding appropriate pooling of the data for meaningful analysis (I2 = 83% with p = 0.002). Weighting was assigned according to the inverse of the variance. Mean differences greater than 0 indicate increased cognitive scores, favoring intervention. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 5. Incidence of Delirium, Stratified by Patient Type
Stratified by patient type, multi-component non-pharmacologic interventions significantly lowered the odds of delirium by 51% (RR 0.49, 95% CI 0.38–0.63) among medical patients in 8 studies and by 58% (OR 0.42, 95% CI 0.19–0.93) among surgical patients in 4 studies. Of note, Kratz et al. included both medical and surgical patients and thus was included in analysis for both categories. Heterogeneity was low for pooled analysis of the medical patients, I2 = 0% with p < 0.00001 and moderate for pooled analysis of the surgical patients, I2 = 57% with p = 0.07. These trends are similar to the overall meta-analysis and stratification by RMTs and non-RMTs. Weighting was assigned according to the inverse of the variance. Odds ratios less than 1 indicate decreased delirium incidence. RMT indicates randomized or matched trials; CI indicates confidence interval.
Appendix Figure 6. Length of Stay, Stratified by Patient Type
Stratified by patient type, multi-component non-pharmacologic interventions decreased the length of stay by −0.02 days (95% CI −0.76–0.72) among the medical patients in 7 studies and by −5.01 days (95% CI −12.61–2.58) among the surgical patients in 2 studies. Heterogeneity was moderate for pooled analysis of the medical patients, I2 = 62% with p=0.01 and for pooled analysis of the surgical patients, I2 = 62% with p = 0.11. These trends are similar to the overall meta-analysis and stratification by RMTs and non-RMTs. Weighting was assigned according to the inverse of the variance. Mean differences less than 0 indicate decreased length of stay, in days. RMT indicates randomized or matched trials; CI indicates confidence interval.