Abstract
Infections with avian influenza viruses (AIV) of low and high pathogenicity (LP and HP) and Newcastle disease virus (NDV) are commonly reported in domestic ducks in many parts of the world. However, it’s not clear if co-infections with these viruses affect the severity of the diseases they produce, the amount of virus shed, and transmission of the viruses. In this study we infected domestic ducks with a virulent NDV virus (vNDV) and either a LPAIV or a HPAIV by giving the viruses individually, simultaneously, or sequentially two days apart. No clinical signs were observed in ducks infected or co-infected with vNDV and LPAIV, but co-infection decreased the number of ducks shedding vNDV and the amount of virus shed (P <0.01) at 4 days post inoculation (dpi). Co-infection didn’t affect the number of birds shedding LPAIV, but more LPAIV was shed at 2 dpi (P <0.0001) from ducks inoculated with only LPAIV compared to ducks co-infected with vNDV. Ducks that received the HPAIV with the vNDV simultaneously survived fewer days (P <0.05) compared to the ducks that received the vNDV two days before the HPAIV. Co-infection also reduced transmission of vNDV to naïve contact ducks housed with the inoculated ducks. In conclusion, domestic ducks can become co-infected with vNDV and LPAIV with no effect on clinical signs but with reduction of virus shedding and transmission. These findings indicate that infection with one virus can interfere with replication of another, modifying the pathogenesis and transmission of the viruses.
Keywords: Avian influenza virus, Newcastle disease virus, domestic ducks, co-infections, viral interference
1. Introduction
Avian influenza virus (AIV) and Newcastle disease virus (NDV) are two of the most economically important viruses affecting poultry worldwide (Alexander, 1995). These viruses transmit from their natural reservoirs, wild birds, to domestic birds initially producing subclinical infections and occasionally upper respiratory disease and drops in egg production (Swayne, et al., 2013). More virulent forms of the viruses can arise and cause high mortality and great economic losses in poultry. Both, AIV and NDV are single-stranded, negative-sense RNA viruses. AIV’s are type A Orthomyxoviruses and are classified as low pathogenicity (LP) and high pathogenicity (HP) viruses based on their virulence in chickens and the presence of multiple basic amino acids at the cleavage site of the hemagglutinin (HA) protein (Swayne, et al., 2013). NDV’s, also known as avian Paramyxovirus 1 (APMV1), are members of the genus Avulavirus in the Paramyxoviridae family (Miller and Koch, 2013). NDV’s also vary in the type and severity of the disease they produce, and different pathotypes based on virulence in chicken and the sequences surrounding the protease cleavage site of the fusion (F) protein, have been described in poultry (Alexander and Senne, 2008; Miller and Koch, 2013). The original classification of NDV isolates into 1 of 3 virulence groups by chicken embryo and chicken inoculation as virulent (velogenic), moderately virulent (mesogenic), or of low virulence (lentogenic) has been abbreviated for regulatory purposes. Velogens and mesogens are now classified as virulent NDV (vNDV), the cause of Newcastle disease, whereas infections with lentogenic strains are the low virulence NDV widely used as live vaccines (Miller and Koch, 2013). The diseases produced by AIV and NDV remain one of the major problems affecting existing or developing poultry industries in many countries. Importantly, disease from vNDV and HPAIV are clinically indistinguishable.
Domestic ducks are economically important poultry, especially in Asian countries. Domestic ducks act as intermediate hosts of AIV between wild ducks and terrestrial poultry, with LPAIV’s of many subtypes being isolated from domestic ducks (Huang et al., 2010; Kim et al., 2013). Historically, ducks naturally or experimentally infected with AIV’s, including HPAIV’s, only develop subclinical to mild disease. This dogma has been challenged since many Asian lineage H5N1 HPAIV’s since 2002 have produced severe disease and mortality in ducks (Pantin-Jackwood and Swayne, 2009). Although waterfowl are a reservoir of NDV, the epidemiology of NDV in domestic ducks remains unclear. NDV has been isolated from domestic ducks in countries reporting endemic ND (Liu et al., 2009). Similar to AIV, genetically varied NDV found in domestic ducks suggests they may act as reservoir of different NDV genotypes and play a role in NDV epidemiology (Hu et al., 2010; Lee et al., 2009; Liu et al., 2009; Zhang et al., 2011b). In general, ducks show few if any clinical signs after NDV infection with strains lethal to chickens (Aldous et al., 2010; Anis et al., 2013; Dai et al., 2013; Otim Onapa et al., 2006; Tsai et al., 2004; Zhang et al., 2011a). However, some studies report NDV strains capable of causing clinical disease in ducks (Shi et al., 2011; Dai et al., 2014).
Natural co-infections of NDV and LPAIV have been documented numerous times in wild waterfowl and in domestic poultry (Couacy-Hymann et al., 2012; Dormitorio et al., 2009; Hanson et al., 2005; Molia et al., 2011; Rosenberger et al., 1974; Roussan et al., 2008; Shortridge, 1980). However, little is known on the interactions between these two viruses when simultaneously infecting poultry species including domestic ducks. We have previously demonstrated differences in virus shedding when chickens and turkeys were co-infected with a low virulence NDV and a LPAIV (Costa-Hurtado et al., 2014). Similarly, co-infection of mallard ducks with low virulence wild bird isolates of NDV and LPAIV did not affect the ability of the ducks to become infected with either virus but a minor effect on virus shedding was found (França et al., 2014).
Domestic ducks likely become co-infected with low and high virulence NDV, LPAIV and HPAIV in countries where these viruses circulate in poultry. It’s not clear if co-infections exacerbate the diseases caused by these viruses, or if infection with one virus would interfere with infection by another. An effect of co-infection on virus replication could affect virus shedding and consequently transmission of the viruses to other hosts. This is information is important because it helps understand the epidemiology of these viruses in field situations aiding in the control of AI and NDV. The objective of this study was to examine co-infection of domestic ducks with a virulent NDV and a LPAIV or a HPAIV by infecting the ducks simultaneously or sequentially with the viruses. Pathogenesis (clinical signs, lesions), duration and titer of virus shed, seroconversion, and transmission were evaluated.
2. Materials and Methods
2.1. Viruses
The following viruses were obtained from the Southeast Poultry Research Laboratory (SEPRL) virus repository: virulent NDV (vNDV): APMV-1/duck/Vietnam (Long Bien)/78/2002; LPAIV: A/Mallard/OH/421/1987 H7N8; and HPAIV: A/duck/VN/NCVD-672/2011 (H5N1). The APMV-1/duck/Vietnam, Long Bien/78/2002, was initially isolated from ducks in a Vietnamese poultry market and belongs to genotype VIId. This virus produces severe disease and death in chickens (Susta et al., 2011). The LPAIV is a wild duck isolate that has an infectious dose of 101 EID50 for ducks (E. Spackman, unpublished data). The HPAIV belongs to HA clade 2.3.2.1B and is highly virulent for ducks (Cha et al., 2013). The viruses were propagated in embryonating chicken eggs (ECE) as previously described (Senne, 2008). Allantoic fluid was diluted in brain heart infusion (BHI) medium (BD Bioscience, Sparks, MD) in order to obtain an inoculum with 106–7.5 50% egg infectious dose (EID50) per bird in 0.1 ml. A sham inoculum was made using sterile allantoic fluid diluted 1:300 in brain heart infusion (BHI) medium (BD Bioscience, Sparks, MD). The experiment was performed in biosecurity level-3 enhanced (BSL-3E) and animal BSL-3E facilities at the SEPRL, United States Department of Agriculture, Agricultural Research Service, and procedure were reviewed by the SEPRL institutional biosecurity committee.
2.2. Birds
Pekin ducks (A. platyrhynchos var. domestica) were obtained at 1 day of age from a commercial hatchery. Serum samples were collected from 15 ducks to ascertain that the birds were serologically negative to NDV and AIV. At two weeks of age the ducks were housed in self-contained isolation units ventilated under negative pressure with inlet and exhaust HEPA-filtered air, and maintained under continuous lighting. Feed and water were provided with ad libitum access. Birds were cared for in accordance to an SEPRL’s Institutional Animal Care and Use Committee approved animal use protocol.
2.3 Experimental design
Ducks were separated into a control group and nine virus-inoculated groups (Table 1). All treatment groups contained nine birds and were inoculated by the intraocular and intranasal (choanal cleft) routes. Each virus or sham inoculum was administered in 0.1 ml split between the conjunctival sac of the right eye and the choana. The virus doses administered were: 107 EID50 of NDV, 107.5 EID50 of LPAIV, and 106 EID50 of HPAIV. The viruses were given alone, simultaneously, or sequentially (the second virus two days after the first virus) (Table 1). The ducks were observed daily for signs of illness for 10 days. Body weights and temperatures were taken at the time of virus exposure (day 0), and at 2 days post inoculation (dpi) with the single or combined viruses, or 2 days after receiving the second virus when given sequentially. Oropharyngeal (OP) and cloacal (CL) swabs were collected from all virus-inoculated ducks at 2, 4, 6, and 8 days dpi (the dpi were counted from exposure to the second virus with groups sequentially inoculated), to assess virus shedding. Two ducks from each group were euthanized at 2 dpi and tissues were collected in 10% neutral buffered formalin to evaluate microscopic lesions and the extent of virus replication in tissues as described previously (Pantin-Jackwood and Swayne, 2007; Susta et al., 2011). Three naïve contact ducks were added to each group at this time to examine for virus transmission. Ducks that showed severe neurological signs, stopped eating or drinking, or remained recumbent, were euthanized and counted dead as for the next day for mean death time calculations. Surviving ducks were bled at 10 dpi for serology and euthanized by the intravenous (IV) administration of sodium pentobarbital (100 mg/kg body weight).
Table 1.
Experimental design
| Groups | Day of inoculation | Days of sampling | |||
|---|---|---|---|---|---|
|
| |||||
| Day 0 | Day 2 | OP and CL swabs | Necropsy (n=2) | Serology | |
| Addition of contacts (n=3) | |||||
| 1 (controls) | - | - | 2, 6, 10 | 2 | 10 |
| 2 | vNDV | - | 2, 4, 6, 8 | 2 | 10 |
| 3 | LPAIV | - | 2, 4, 6, 8 | 2 | 10 |
| 4 | HPAIV | - | 2, 4, 6, 8 | 2 | 10 |
| 5 | vNDV + LPAIV | - | 2, 4, 6, 8 | 2 | 10 |
| 6 | vNDV +HPAIV | - | 2, 4, 6, 8 | 2 | 10 |
| 7 | vNDV | LPAIV | 4, 6, 8, 10* | 4** | 10 |
| 8 | vNDV | HPAIV | 4, 6, 8, 10* | 4** | 10 |
| 9 | LPAIV | vNDV | 4, 6, 8, 10* | 4** | 10 |
| 10 | HPAIV | vNDV | 4, 6, 8, 10* | 4** | 10 |
These time points correspond to 2, 4, 6, 8 days after inoculation with the second virus and 4, 6, 8, and 10 days after inoculation with the first virus.
This time point corresponds to 2 days after inoculation with the second virus.
2.4. Quantitative real-time RT-PCR
OP and CL swabs were collected in 2 mL of BHI broth with a final concentration of gentamicin (1,000 μg/mL), penicillin G (10,000 units/mL), and amphotericin B (20 IU/mL) and kept frozen at −70°C until processed. Viral RNA was extracted using Trizol LS reagent (Invitrogen, Calsbad, CA) and the MagMAX AI/ND Viral RNA Isolation Kit (Ambion, Austin, TX, USA). Quantitative real time RT-PCR (qRT-PCR) for AIV and NDV detection was performed as previously described (Costa-Hurtado et al., 2014). qRT-PCR reactions targeting the influenza virus M gene (Spackman et al., 2002) and NDV M gene (Wise et al., 2004) were conducted using AgPath-ID one-step RT-PCR Kit (Ambion, Austin, TX, USA) and the ABI 7500 Fast Real-Time PCR system (Applied Biosystem, Calsbad, CA, USA). The RT step conditions for both primer sets were 10 min at 45°C and 95°C for 10 min. The cycling conditions for AIV were 45 cycles of 15 s, 95°C; 45 s, 60°C; and for NDV were 40 cycles of 10 s, 94°C; 30 s, 56°C; 10 s, 72°C. The calculated qRT-PCR lower detection limit for LPAIV, HPAIV and vNDV was 100.8EID50/mL, 101.0EID50/mL, and 100.94EID50/mL, respectively. A standard curve for virus quantification was established with RNA extracted from dilutions of the same titrated stock of the challenge virus, and results also reported as EID50/mL equivalents.
2.5. Serology
Hemagglutination inhibition (HI) assays were performed to quantify antibody responses to virus infection as previously described (OIE, 2012), with serum collected from ducks at 10 dpi (8 dpi in groups that were exposed to the viruses sequentially). HI titers were reported as reciprocal log2 titers, with a 3 log2 (a titer of 1:8) titer considered the minimum positive titer.
2.6. Statistical analyses
Data were analyzed using Prism v.5.01 software (GraphPad Software Inc. La Jolla, CA, USA). The survival rate data was analyzed using the Mantel-Cox Log-Rank test. One-way ANOVA with Tukey post-test was used to analyze body weights, body temperatures, and virus titers in swabs. For statistical purposes, all qRT-PCR negative oropharyngeal and cloacal swabs were given a numeric value of 100.8EID50/mL, 101.0EID50/mL and100.94 EID50/mL for LPAIV, HPAIV, and vNDV, respectively. These values represent the lowest detectable level of virus in these samples based on the methods used. Statistical significance was set at P <0.05 unless otherwise stated.
3. Results
3.1. Clinical signs
No morbidity or mortality was observed in the groups of ducks inoculated with vNDV or LPAIV, given individually or in combination. All ducks inoculated with the HPAIV died in less than four days post HPAIV inoculation, regardless of simultaneous or sequential co-infection with vNDV (Table 2). The MDT was calculated based on the number of dead ducks per day after HPAIV inoculation. Ducks that received the HPAIV with the vNDV simultaneously had significantly shorter survival times than the ducks that received the vNDV two days before the HPAIV. Clinical signs of infection with HPAIV were found in all the groups inoculated with this virus and included lethargy, anorexia, prostration, and neurological signs, similar to previously reported with H5N1 HPAIV infection in ducks (Pantin-Jackwood and Swayne, 2007). Apart from the delay in deaths in the group inoculated with vNDV two days before the HPAIV, no difference in the severity of clinical signs was observed between the groups.
Table 2.
Survival, body temperatures, body weights and HI titers of ducks inoculated with vNDV, LPAIV and HPAIV, given single or combined.
| Groups | Day of inoculationa
|
# dead/ total (MDT)b | Body temperaturec 2 dpi | Body weightsc 2 dpi | HI titersd
|
||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | NDV | AIV | ||||
| 1 | - | - | 0/9 | 107.0±0.6A | 694±55AB | ND | ND |
| 2 | vNDV | - | 0/9 | 107.5±0.5A | 638±81AB | 7/7 (5.7+.9) | ND |
| 3 | LPAIV | - | 0/9 | 107.7±0.8A | 705±12A | ND | 7/7 (3.8+.3) |
| 4 | HPAIV | - | 9/9 (2.3)AB | 103.6±4.3B | 576±27B | ND | ND |
| 5 | vNDV +LPAIV | - | 0/9 | 107.6±0.5A | 682±49AB | 7/7 (5.8+.6) | 6/7 (3) |
| 6 | vNDV +HPAIV | - | 9/9 (2.1)A | 107.9±2.6A | 672±73AB | ND | ND |
| 7 | vNDV | LPAIV | 0/9 | 108.0±0.8A | 838±79A | 7/7 (5.2+.7) | 0/7 |
| 8 | vNDV | HPAIV | 9/9 (3.2)B | 109.5±0.8B | 748±113A | ND | ND |
| 9 | LPAIV | vNDV | 0/9 | 107.6±0.8A | 867±130A | 7/7 (5.5+.5) | 4/7 (3.2+.5) |
| 10 | HPAIV | vNDV | 9/9 (2.3)AB | ND | - | ND | ND |
Two-week-old Pekin ducks were inoculated with 107 EID50 of vNDV, 107.5 EID50 of LPAIV, and 106 EID50 of HPAIV administered in 0.1 ml split between the right eye and the choana. The viruses were given alone, simultaneously, or sequentially (the second virus 2 days after the first).
Mean death time calculated after exposure to the HPAIV. Survival in groups with different uppercase letters is significantly different (P<0.05).
Body temperature (F) and weights in groups with different uppercase letters are significantly different (P<0.05).
Mean HI titers (log2) in ducks. Serum samples were taken at 8 or 10 days after infection with LPAIV, vNDV, or both. The number of birds with positive HI titers is shown. ≥ threshold of positivity/total number of sera tested (mean ± SEM).
ND = no data
Body temperatures were taken 2 days after inoculation with the single viruses or viruses given simultaneously, and 2 days after receiving the second virus in groups that were inoculated sequentially. A significant reduction in body temperature was found at 2 dpi in ducks inoculated with the HPAIV alone when compared to the other groups, which corresponds with a moribund physical condition (Table 2). Ducks inoculated with the vNDV followed by the HPAIV, had significantly higher body temperatures at 2dpi. These ducks were still not moribund and hence the fever. Three of four ducks from the group simultaneously infected with vNDV and the HPAIV also presented with fever and one duck had low body temperature. The rest of the ducks infected with the vNDV and /or the LPAIV had only slight variations in body temperature, but were not significantly different from the controls. The lowest body weights were observed in the ducks inoculated with the HPAIV, which were anorexic.
3.2. Gross lesions, microscopic lesions and viral antigen staining in tissues
No gross lesions were observed in any of the birds inoculated with vNDV, LPAIV, or co-infected with both, when examined at necropsy on 2 dpi. However, microscopic lesions were present and consistent with LPAIV and vNDV infection or non-specific inflammation. NDV and AIV antigen staining was rare in tissues collected from LPAIV and vNDV-inoculated ducks and were confined to the nasal and trachea epithelial cells and infiltrating macrophages (not shown). Because of the minimal viral staining observed with these viruses, no conclusions could be reached on differences in virus replication in tissues between single virus-infected ducks and co-infected ducks.
Gross lesions were observed and were similar in all ducks inoculated with HPAIV regardless of co-infection with vNDV, and consisted of dehydration, empty intestines, splenomegaly, and thymus atrophy. Also, nasal discharge, cyanotic bill and toes, dilated and flaccid heart with increased pericardial fluid, renal pallor, and congested brain were commonly observed. Widespread AIV viral antigen staining was observed in tissues collected from ducks inoculated with the HPAIV similar to previous reports with H5N1 HPAI (Pantin-Jackwood and Swayne, 2009). No difference in the intensity or distribution of virus staining or in the severity of lesions was found between ducks infected only with HPAIV or co-infected with vNDV.
3.3. Viral shedding
Oropharyngeal (OP) and cloacal (CL) viral shedding was examined by qRT-PCR and the results are shown in Tables 3 and 4 and Figures 1, 2 and 3. All virus-inoculated ducks became infected with the viruses given as determined by the detection of the viruses in OP swabs or by seroconversion (Tables 3 and 4; Figures 1–3). The total number of positive swabs, the viral titers, and the duration of virus shedding varied among the treatment groups.
Table 3.
Number of ducks positive for NDV RNA in oropharyngeal (OP) and cloacal (CL) swabs in single and co-infected groups. In groups sequentially infected, the day post-inoculation (dpi) is based on the last virus given.
| Virus detected | Swab | Group | Days post inoculation
|
|||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | 2 | 4 | 6 | 8 | Total b | ||
| NDV | OP | vNDV | 5/9 | 7/7 | 2/7 | 2/7 | 16/30 | |
| LPAIV | ND | ND | ND | ND | - | |||
| HPAIV | ND | ND | ND | ND | - | |||
| vNDV+LPAIV | 3/9 | 4/7 | 0/7 | 0/7 | 7/30 | |||
| vNDV+HPAIV | 0/4 | ND | ND | ND | 0/4 | |||
| vNDV | LPAIV | 7/9 | 2/7 | 1/7 | 1/7 | 11/30 | ||
| vNDV | HPAIV | 1/7 | 2/2 | ND | ND | 3/9 | ||
| LPAIV | vNDV | 5/9 | 6/7 | 2/7 | 1/7 | 14/30 | ||
| HPAIV | vNDV | ND | ND | ND | ND | - | ||
|
| ||||||||
| CL | vNDV | 3/9 | 5/7 | 1/7 | 1/7 | 10/30 | ||
| LPAIV | ND | ND | ND | ND | - | |||
| HPAIV | ND | ND | ND | ND | - | |||
| vNDV+LPAIV | 3/9 | 3/7 | 1/7 | 1/7 | 8/30 | |||
| vNDV+HPAIV | 0/4 | ND | ND | ND | 0/4 | |||
| vNDV | LPAIV | 3/9 | 0/7 | 0/7 | 0/7 | 3/30 | ||
| vNDV | HPAIV | 2/7 | 0/2 | ND | ND | 2/9 | ||
| LPAIV | vNDV | 3/9 | 2/7 | 1/7 | 2/7 | 8/30 | ||
| HPAIV | vNDV | ND | ND | ND | ND | - | ||
Number of positive birds/total number of birds sampled at each time point.
total number of positive swabs/total number of swabs.
ND = No data
Table 4.
Number of ducks positive for AIV RNA in oropharyngeal (OP) and cloacal (CL) swabs in single and co-infected groups. In groups sequentially infected, the day post-inoculation (dpi) is based on the last virus given.
| Virus detected | Swab | Group | Days post inoculation
|
|||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | 2 | 4 | 6 | 8 | Total b | ||
| AIV | OP | vNDV | ND | ND | ND | ND | - | |
| LPAIV | 9/9 | 7/7 | 7/7 | 7/7 | 30/30 | |||
| HPAIV | 2/2 | ND | ND | ND | 2/2 | |||
| vNDV+LPAIV | 8/9 | 7/7 | 7/7 | 7/7 | 30/30 | |||
| vNDV+HPAIV | 4/4 | 0/0 | 0/0 | 0/0 | - | |||
| vNDV | LPAIV | 9/9 | 7/7 | 7/7 | 7/7 | 30/30 | ||
| vNDV | HPAIV | 5/5 | 2/2 | ND | ND | 7/7 | ||
| LPAIV | vNDV | 9/9 | 7/7 | 7/7 | 7/7 | 30/30 | ||
| HPAIV | vNDV | ND | ND | ND | ND | - | ||
|
| ||||||||
| CL | vNDV | ND | ND | ND | ND | - | ||
| LPAIV | 9/9 | 7/7 | 7/7 | 7/7 | 30/30 | |||
| HPAIV | 2/2 | ND | ND | ND | 2/2 | |||
| vNDV+LPAIV | 7/9 | 7/7 | 7/7 | 7/7 | 28/30 | |||
| vNDV+HPAIV | 4/4 | ND | ND | ND | - | |||
| vNDV | LPAIV | 3/9 | 7/7 | 7/7 | 7/7 | 24/30 | ||
| vNDV | HPAIV | 2/6 | 2/2 | ND | ND | 4/8 | ||
| LPAIV | vNDV | 1/9 | 7/7 | 7/7 | 7/7 | 22/30 | ||
| HPAIV | vNDV | ND | ND | ND | ND | - | ||
Number of positive birds/total number of birds sampled at each time point.
Total number of positive swabs/total swabs.
ND = No data
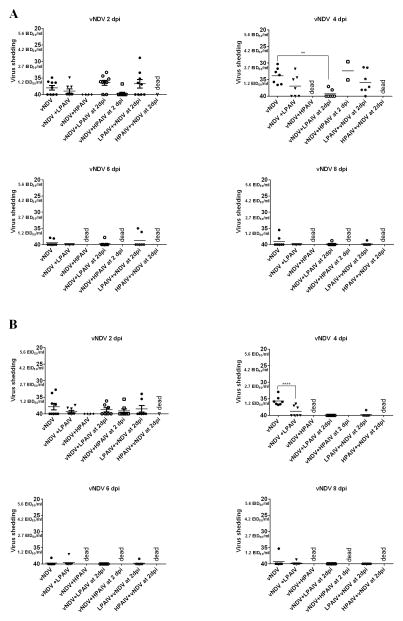
Fig. 1.
vNDV OP (A) and CL (B) shedding in ducks. Mean cycle threshold (Ct) values and equivalent mean embryo infectious dose per ml (EID50/ml) titers of vNDV detected in swabs at different time points after inoculation. In groups sequentially infected, the days post-inoculation (dpi) is based on the last virus given. Significant difference for number of positive ducks by qRT-PCR compared to single virus infected groups, (**, P < 0.01; **** P <0.0001).
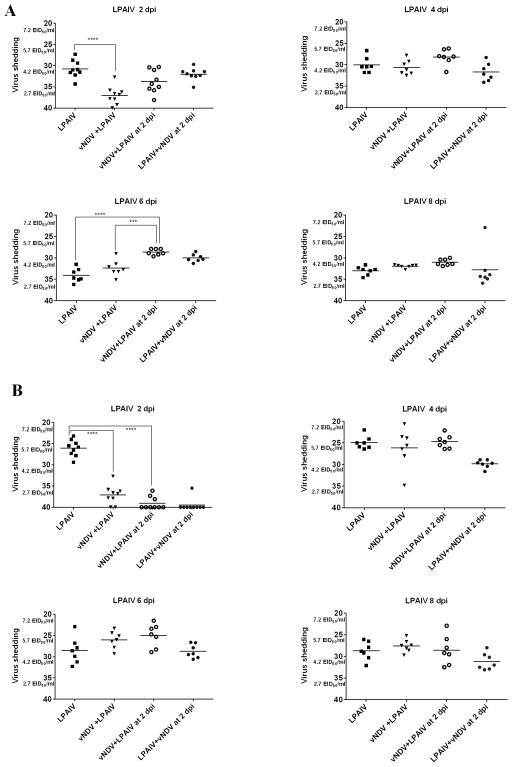
Fig. 2.
LPAIV OP (A) and CL (B) shedding in ducks. Mean Ct values and equivalent EID50/ml titers of AIV detected in OP swabs at different time points after inoculation. In groups sequentially infected, the days post-inoculation (dpi) is based on the last virus given. Significant difference for number of positive ducks by qRT-PCR compared to single virus infected groups, (*** P <0.001; **** P<0.0001).
Fig. 3.
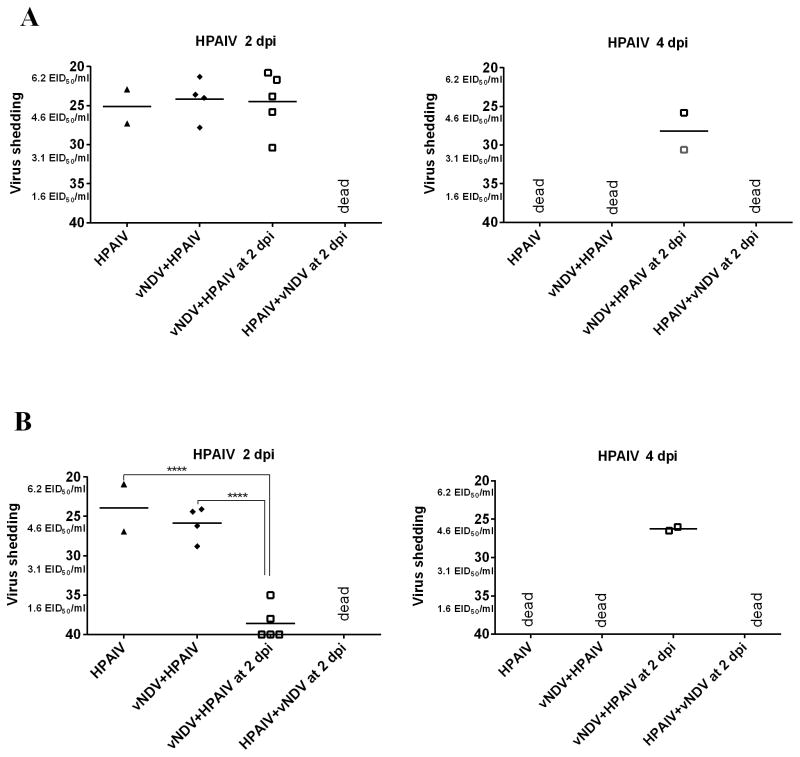
HPAIV OP (A) and CL (B) shedding in ducks. Mean Ct values and equivalent EID50/ml titers of AIV detected in OP swabs at different time points after inoculation. In groups sequentially infected, the days post-inoculation (dpi) is based on the last virus given. Significant difference for number of positive ducks by qRT-PCR compared to single virus-infected groups, (**** P<0.0001).
vNDV shedding
By 4 dpi, all ducks (7 of 7) inoculated with vNDV alone shed virus by the OP route and 5 of 7 by the CL route, with fewer birds shedding virus at 6 and 8 dpi (Table 3, Figure 1A and B). Only 4 of 7 ducks from the group simultaneously co-infected with LPAIV were shedding NDV by the OP route at 4 dpi (3 of 7 ducks were shedding virus by the CL route) and there was a significant difference in the proportion of ducks shedding virus when compared to the group inoculated only with the vNDV. Ducks from co-infected groups inoculated with HPAIV (simultaneously or sequentially) died in less than 4 days. However, none of the surviving ducks from the group that received vNDV and HPAIV simultaneously, and only 2 of 7 from the sequentially inoculated group, were shedding vNDV. No effect on the number of ducks shedding vNDV was found in the groups that received vNDV and LPAIV sequentially, with most of the ducks shedding virus by the OP route.
There was also a significant difference in the amount of OP virus shed between the vNDV single virus-infected ducks at 4dpi and the ducks that received LPAIV two days after the vNDV, (P<0.05), but this difference is most likely because the titers for the second group correspond to 6 dpi after receiving vNDV. A significant difference in CL virus titers was found between the vNDV single inoculated group and the group simultaneously co-infected with LPAIV at 4 dpi (P<0.0001). No differences were observed among the different groups at 6 and 8 dpi in the number of ducks shedding vNDV or in the virus titers.
LPAIV shedding
Although all ducks inoculated with LPAIV, co-infected or not with vNDV, shed virus by both the OP and CL routes during the entire study (Table 4), differences were observed in the titers of virus shed (Figure 2). Lower LPAIV OP and CL titers were observed at 2 dpi in ducks simultaneously infected with vNDV compared to ducks only inoculated with LPAIV (P<0.0001). Lower CL LPAIV titers were also observed at 2 dpi in ducks inoculated simultaneously with vNDV or 2 days after vNDV as well as the ducks inoculated with LPAIV followed by the vNDV (P<0.0001). This last group was not included in the statistical analysis because it corresponds to 4 dpi of the LPAIV. Interestingly, the ducks inoculated 2 days earlier with vNDV had higher LPAIV OP titers compared to single and simultaneously co-infected ducks at 6 dpi (P<0.001, P<0.0001). No differences in LPAIV titers were observed for the rest of the groups at 6 dpi, and for any of the groups at 4 and 8 dpi.
HPAIV shedding
As for ducks shedding HPAIV, no difference in virus titers were found among all surviving ducks at the different time points, with the exception of the group that received the HPAIV two days after the vNDV, which were shedding less virus (P <0.0001) by the CL route than the ducks inoculated only with HPAIV or simultaneously with vNDV.
3.4. Serology
HI assays were used to test for antibodies against NDV and the AIV viruses (Table 2). Because serum samples were taken the same day for single, simultaneously and sequentially exposed birds (10 dpi for the first virus and 8 dpi for the second), not all groups are strictly comparable. Nevertheless, all ducks seroconverted to vNDV with no significant differences in titers among the treatment groups. Not all ducks infected with LPAIV had detectable antibodies and, in general, the positive titers were low. Ducks inoculated with vNDV followed by the LPAIV were negative for LPAIV antibodies, as were one out of seven ducks in the simultaneously co-infected group and three out of seven ducks in the groups that received LPAIV followed by vNDV. No serology is reported for any of the groups inoculated with HPAIV since all ducks died.
3.5. Transmission to contacts
To assess virus transmission, naïve ducks were added to the experimental groups 2 days after inoculation (2 dpi after the second virus in sequentially inoculated groups). All contact ducks in the single-inoculated vNDV group and in the group that received the vNDV two days after LPAIV became infected with vNDV, as demonstrated by virus shedding and/or seroconversion (Table 5). However, contact transmission of vNDV was not observed in naïve ducks in the group simultaneously infected with vNDV and LPAIV, nor one duck in the group that received LPAIV two days after vNDV. All contact ducks became infected with LPAIV in the LPAIV group and the groups that were co-infected with vNDV, but seven did not seroconvert. HPAIV was transmitted to contact ducks in all the groups inoculated with this virus, and all contact ducks died. The contact ducks from the group inoculated with HPAIV 2 days after vNDV survived 2 days longer than the contact ducks in the single HPAIV-inoculated group and the group simultaneously co-infected with vNDV and HPAIV. In the groups that were inoculated with vNDV two days after HPAIV, no contact ducks were added because all were dead at 2 dpi.
Table 5.
Survival, number of ducks shedding virus during the study, and seroconversion of naïve contact ducks after introduced into isolators with the ducks directly virus-inoculated.
| Groups | Day of inoculation
|
# dead/ total (MDT)a | #ducks shedding vNDV | #ducks with vNDV antibodies | #ducks shedding AIV | #ducks with AIV antibodies | |
|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | ||||||
| 1 | - | - | 0/3 | ND | ND | ND | ND |
| 2 | vNDV | - | 0/3 | 3/3 | 3/3 | ND | ND |
| 3 | LPAIV | - | 0/3 | ND | ND | 3/3 | 3/3 |
| 4 | HPAIV | - | 3/3 (2.3) | ND | ND | 3/3 | ND |
| 5 | vNDV +LPAIV | - | 0/3 | 0/3 | 0/3 | 3/3 | 2/3 |
| 6 | vNDV +HPAIV | - | 3/3 (2) | ND | ND | 3/3 | ND |
| 7 | vNDV | LPAIV | 0/3 | 0/3 | 2/3 | 3/3 | 0/3 |
| 8 | vNDV | HPAIV | 3/3 (4.6) | 0/3 | ND | 3/3 | ND |
| 9 | LPAIV | vNDV | 0/3 | 1/3 | 3/3 | 3/3 | 0/3 |
| 10 | HPAIV | vNDV | ND | ND | ND | ND | ND |
Mean death time. Survival after introduction of contact ducks in respective group (2 days after inoculation with the single or simultaneously given viruses, and 2 days after the second virus was given for the group sequentially inoculated).
ND = No data
4. Discussion
The goal of this study was to examine the effect of co-infection with vNDV and LPAIV or vNDV and HPAIV in domestic ducks. To our knowledge, this is the first study to do so. Even though these viruses co-circulate in poultry in many parts of the world and domestic ducks are considered reservoirs and source of these viruses for other poultry species, NDV and AIV co-infections in domestic ducks are not well understood. AIV and NDV co-infections have been studied in vitro using cell cultures or chicken embryos, and interference between these viruses has been demonstrated, with one virus inhibiting the growth of the other (Bang, 1949; Carr, 1960; Ge et al., 2012; Shortridge and King, 1983). Contrary to in vitro or in ovo settings, in vivo experiments examine the overall effect of co-infections by incorporating the complexity of the whole organism, including target cells and immune responses (Costa-Hurtado et al., 2014).
We found that domestic ducks can become co-infected with vNDV and LPAIV or HPAIV, with patterns of virus shedding different than those observed when infected with each virus individually. Similar results in chickens, turkeys, and mallard ducks have been reported in co-infections with low virulence NDV and LPAIV (Costa-Hurtado et al., 2014; França, et al., 2014). In a previous co-infection study, we demonstrated that chickens and turkeys became infected when inoculated with a lentogenic NDV and a LPAIV, with a significant effect on virus replication in birds inoculated with the two viruses when compared to birds inoculated with only one of the viruses (Costa-Hurtado et al., 2014). The virus interactions observed depended on the bird species, the virus, and the timing of inoculation. In spite of the differences in virus replication, co-infection of NDV and LPAIV in chickens and turkeys did not increase or decrease the severity of clinical signs. Similarly, in the present study no effect on clinical signs was found in vNDV and LPAIV co-infections, however an increase in death time was seen in some ducks co-infected with vNDV and HPAIV, indicating that vNDV interfered with HPAIV replication, thus delaying the onset of mortality.
All AIV-inoculated ducks, co-infected with vNDV or not, became infected with the LPAIV and the HPAIV. With the LPAIV, higher titers were shed by the cloacal route than by the OP route as expected with wild duck isolates (Spackman et al., 2010). A clear effect of co-infection was found at 2 dpi, in which simultaneously infected ducks shed a lower amount of LPAIV by the OP and CL route than single-infected ducks. Reduced LPAIV CL viral titers were also observed in the ducks that received the LPAIV two days after the vNDV. However, by 4 dpi co-infected groups shed similar amount of virus than single infected birds indicating a transient effect. Interestingly, ducks that received the virus sequentially had higher titers at 6 dpi than single infected ducks, probably because by then the replication of the vNDV had decreased, so the effect of viral interference was less. Serology conducted at 10 dpi (8 dpi for ducks sequentially infected) indicated that some co-infected ducks had low AIV HI titers or were under the limit of detection. Similar absent or low titers were found in contact ducks for those groups, however, the virus did transmit to these ducks since all contact ducks were shedding virus. While there was no significant difference in the HPAIV OP virus titers when the groups are compared at 2 dpi, there was significantly lower amounts of virus shed by the CL route in ducks infected with HPAIV two days after the vNDV.
A decreased number of ducks shed vNDV when simultaneously co-infected with LPAIV or HPAIV compared to ducks infected with vNDV alone. The vNDV used in this study was a high virulence NDV strain known to induce severe disease in chickens (Susta et al., 2011), but as shown in this study, it did not replicate to high titers in ducks. Nevertheless, ducks still became infected and transmitted the virus to all contact ducks in the single virus-infected group and the group that received the vNDV after the LPAIV. However, contact ducks in the simultaneously co-infected group didn’t become infected with vNDV, indicating that co-infection affected the transmission of this virus.
For both AIV and NDV, co-infection affected the titers of virus shed, and in the case of vNDV, the number of birds shedding. The effect on viral replication caused by one virus over another is known as viral interference, a phenomenon in which a cell infected by a virus does not permit multiplication of a second homologous or heterologous superinfecting virus (Dianzani, 1975). Viral interference can occur by different mechanisms including: competing by attachment interference therefore reducing or blocking receptor sites for the superinfecting virus; competing intracellularly for replication host machinery; and virus-induced interferon interference (Kimura et al., 1976). Replication of one virus might be affected by previous replication in the same site of another virus that has already activated antiviral immune responses including immunomodulators or recruitment of immune cells. Other studies examining co-infection of LPAIV and NDV with other respiratory viruses of poultry demonstrated that co-infections can either exacerbate clinical disease, or, like in our study, affect virus replication by lowering viral titers, serological conversion and virus transmission (Gelb et al., 2007; Haghighat-Jahromi et al., 2008; Hanson et al., 1956; Raggi and Lee, 1963; Turpin et al., 2002).
The specific mechanisms of AIV-NDV viral interference and the role of co-infections in the spread of AIV and NDV remain to be determined. Because our study was performed under controlled conditions, it does not entirely reflect the field situation where ducks are exposed to many more infectious and non-infectious disease agents. A better understanding of AIV and NDV co-infections and the many factors affecting co-infections in the field will help to better understand the pathogenesis and transmission of these viruses in domestic ducks.
Highlights.
Ducks can be co-infected with Newcastle disease and low or highly pathogenic avian influenza viruses
Co-infection of domestic ducks with NDV and AIV reduced virus shedding and transmission
Co-infection with NDV and LPAIV did not affect clinical signs
Ducks that received the vNDV two days before the HPAIV survived longer than co-infected the same day
Infection with one virus interfered with replication of the other, modifying pathogenesis
Acknowledgments
The authors appreciate the assistance provided by Aniko Zsak, Tim Olivier, Dawn Williams-Coplin, Rami Cha, Kira Moresco, Ronald Graham, and Roger Brock in conducting these studies. This work has been funded by the Agriculture Research Service CRIS Project 6612-32000-048 and with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200700007C.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldous EW, Seekings JM, McNally A, Nili H, Fuller CM, Irvine RM, Alexander DJ, Brown IH. Infection dynamics of highly pathogenic avian influenza and virulent avian paramyxovirus type 1 viruses in chickens, turkeys and ducks. Avian Pathology. 2010;39:265–273. doi: 10.1080/03079457.2010.492825. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. The epidemiology and control of avian influenza and Newcastle disease. Journal of Comparative Pathology. 1995;112:105–126. doi: 10.1016/s0021-9975(05)80054-4. [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Senne DA. Newcastle disease. In: Saif YM, FAM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. Diseases of Poultry. 12. Iowa State University Press; Ames, Iowa, USA: 2008. pp. 75–116. [Google Scholar]
- Anis Z, Morita T, Azuma K, Ito H, Ito T, Shimada A. Comparative study on the pathogenesis of the generated 9a5b Newcastle disease virus mutant isolate between chickens and waterfowl. Veterinary Pathology. 2013;50:638–647. doi: 10.1177/0300985812467470. [DOI] [PubMed] [Google Scholar]
- Bang FB. Cell blockade in Newcastle disease of chickens and chicken embryos. The Journal of Experimental Medicine. 1949;89:141–154. doi: 10.1084/jem.89.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JH. Inoculation time differentials for expression of interference of Newcastle disease virus by swine influenza virus in chick embryos. Transactions of the Kansas Academy of Science. Kansas Academy of Science. 1960;63:141–146. [PubMed] [Google Scholar]
- Cha RM, Smith D, Shepherd E, Davis CT, Donis R, Nguyen T, Nguyen HD, Do HT, Inui K, Suarez DL, Swayne DE, Pantin-Jackwood M. Suboptimal protection against H5N1 highly pathogenic avian influenza viruses from Vietnam in ducks vaccinated with commercial poultry vaccines. Vaccine. 2013;31:4953–60. doi: 10.1016/j.vaccine.2013.08.046. [DOI] [PubMed] [Google Scholar]
- Costa-Hurtado M, Afonso CL, Miller PJ, Spackman E, Kapczynski DR, Swayne DE, Shepherd E, Smith D, Zsak A, Pantin-Jackwood M. Virus interference between H7N2 low pathogenic avian influenza virus and lentogenic Newcastle disease virus in experimental co-infections in chickens and turkeys. Veterinary Research. 2014;45:1. doi: 10.1186/1297-9716-45-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couacy-Hymann E, Kouakou AV, Kouame CK, Kouassi AL, Koffi YM, Godji P, Nana P, Tarnagda Z, Akoua-Koffi C. Surveillance for avian influenza and Newcastle disease in backyard poultry flocks in Cote d’Ivoire, 2007–2009. Rev Sci Tech. 2012;31:821–828. doi: 10.20506/rst.31.3.2158. [DOI] [PubMed] [Google Scholar]
- Dai Y, Liu M, Cheng X, Shen X, Wei Y, Zhou S, Yu S, Ding C. Infectivity and pathogenicity of Newcastle disease virus strains of different avian origin and different virulence for mallard ducklings. Avian Diseases. 2013;57:8–14. doi: 10.1637/10298-070212-Reg.1. [DOI] [PubMed] [Google Scholar]
- Dai Y, Cheng X, Liu M, Shen X, Li J, Yu S, Zou J, Ding C. Experimental infection of duck origin virulent Newcastle disease virus strain in ducks. Veterinary Research. 2014;10:164. doi: 10.1186/1746-6148-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani F. Viral interference and interferon. La Ricerca in clinica e in laboratorio. 1975;5:196–213. doi: 10.1007/BF02908284. [DOI] [PubMed] [Google Scholar]
- Dormitorio TV, Giambrone JJ, Guo K, Hepp GR. Detection and characterization of avian influenza and other avian paramyxoviruses from wild waterfowl in parts of the southeastern United States. Poultry Science. 2009;88:851–855. doi: 10.3382/ps.2008-00337. [DOI] [PubMed] [Google Scholar]
- França M, Howerth E, Carter DL, Byas A, Poulson R, Afonso CL, Stallknecht DE. Co-infection of mallards with low virulence Newcastle disease virus and low pathogenicavian influenza virus. Avian Pathology. 2014;43:96–104. doi: 10.1080/03079457.2013.876530. [DOI] [PubMed] [Google Scholar]
- Ge S, Zheng D, Zhao Y, Liu H, Liu W, Sun Q, Li J, Yu S, Zuo Y, Han X, Li L, Lv Y, Wang Y, Liu X, Wang Z. Evaluating viral interference between Influenza virus and Newcastle disease virus using real-time reverse transcription-polymerase chain reaction in chicken eggs. Virology Journal. 2012;9:128. doi: 10.1186/1743-422X-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb J, Jr, Ladman BS, Licata MJ, Shapiro MH, Campion LR. Evaluating viral interference between infectious bronchitis virus and Newcastle disease virus vaccine strains using quantitative reverse transcription-polymerase chain reaction. Avian Diseases. 2007;51:924–934. doi: 10.1637/7930-020807-REGR.1. [DOI] [PubMed] [Google Scholar]
- Haghighat-Jahromi M, Asasi K, Nili H, Dadras H, Shooshtari AH. Coinfection of avian influenza virus (H9N2 subtype) with infectious bronchitis live vaccine. Archives of Virology. 2008;153:651–655. doi: 10.1007/s00705-008-0033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson BA, Swayne DE, Senne DA, Lobpries DS, Hurst J, Stallknecht DE. Avian influenza viruses and paramyxoviruses in wintering and resident ducks in Texas. Journal of Wildlife Diseases. 2005;41:624–628. doi: 10.7589/0090-3558-41.3.624. [DOI] [PubMed] [Google Scholar]
- Hanson LE, White FH, Alberts JO. Interference between Newcastle disease and infectious bronchitis viruses. American Journal of Veterinary Research. 1956;17:294–298. [PubMed] [Google Scholar]
- Hu B, Huang Y, He Y, Xu C, Lu X, Zhang W, Meng B, Yan S, Zhang X. Avian influenza virus and Newcastle disease virus (NDV) surveillance in commercial breeding farm in China and the characterization of Class I NDV isolates. Veterinary Microbiology. 2010;144:82–86. doi: 10.1016/j.vetmic.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Huang K, Bahl J, Fan XH, Vijaykrishna D, Cheung CL, Webby RJ, Webster RG, Chen H, Smith GJ, Peiris JS, Guan Y. Establishment of an H6N2 influenza virus lineage in domestic ducks in southern China. Journal of Virology. 2010;84:6978–6986. doi: 10.1128/JVI.00256-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KI, Choi JG, Kang HM, To TL, Nguyen TD, Song BM, Hong MS, Choi KS, Kye SJ, Kim JY, Lee HS, Lee YJ. Geographical distribution of low pathogenic avian influenza viruses of domestic poultry in Vietnam and their genetic relevance with Asian isolates. Poultry Science. 2013;92:2012–2023. doi: 10.3382/ps.2013-03105. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Norrby E, Nagata I, Ito Y, Shimokata K. Homologous interference induced by a temperature-sensitive mutant derived from an HVJ (Sendai virus) carrier culture. The Journal of General Virology. 1976;33:333–343. doi: 10.1099/0022-1317-33-2-333. [DOI] [PubMed] [Google Scholar]
- Lee EK, Jeon WJ, Kwon JH, Yang CB, Choi KS. Molecular epidemiological investigation of Newcastle disease virus from domestic ducks in Korea. Veterinary Microbiology. 2009;134:241–248. doi: 10.1016/j.vetmic.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang X, Wu S, Hu S, Peng Y, Xue F, Liu X. Surveillance for a virulent Newcastle disease viruses in domestic ducks (Anas platyrhynchos and Cairina moschata) at live bird markets in Eastern China and characterization of the viruses isolated. Avian Pathology. 2009;38:377–391. doi: 10.1080/03079450903183637. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Koch G. Newcastle disease, Other Avian Paramyxoviruses and Avian Metapneumovirus Infections. In: Swayne DE, GJ, McDougald LR, Nolan LK, Suarez DL, Nair V, editors. Diseases of Poultry. 13. Wiley-Blackwell; 2013. pp. 89–138. [Google Scholar]
- Molia S, Samake K, Diarra A, Sidibe MS, Doumbia L, Camara S, Kante S, Kamissoko B, Diakite A, Gil P, Hammoumi S, de Almeida RS, Albina E, Grosboisa V. Avian influenza and Newcastle disease in three risk areas for H5N1 highly pathogenic avian influenza in Mali, 2007–2008. Avian Diseases. 2011;55:650–658. doi: 10.1637/9775-050911-Reg.1. [DOI] [PubMed] [Google Scholar]
- OIE, World Organisation for Animal Health. Manual of diagnostic tests and vaccines for terrestrial animals: mammals, birds and bees. 2012 http://www.oie.int/international-standard-setting/terrestrial-manual/access-online. [PubMed]
- Otim Onapa M, Christensen H, Mukiibi GM, Bisgaard M. A preliminary study of the role of ducks in the transmission of Newcastle disease virus to in-contact rural free-range chickens. Tropical Animal Health and Production. 2006;38:285–289. doi: 10.1007/s11250-006-4309-4. [DOI] [PubMed] [Google Scholar]
- Pantin-Jackwood MJ, Swayne DE. Pathobiology of Asian highly pathogenic avian influenza H5N1 virus infections in ducks. Avian Diseases. 2007;51:250–259. doi: 10.1637/7710-090606R.1. [DOI] [PubMed] [Google Scholar]
- Pantin-Jackwood MJ, Swayne DE. Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev Sci Tech. 2009;28:113–136. [PubMed] [Google Scholar]
- Raggi LG, Lee GG. Infectious bronchitis virus interference with growth of Newcastle disease virus. I. Study of interference in chicken embryos. Avian Diseases. 1963;7:106–122. [PubMed] [Google Scholar]
- Rosenberger JK, Krauss WC, Slemons RD. Isolation of Newcastle disease and type-A influenza viruses from migratory waterfowl in the Atlantic flyway. Avian Diseases. 1974;18:610–613. [PubMed] [Google Scholar]
- Roussan DA, Haddad R, Khawaldeh G. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poultry Science. 2008;87:444–448. doi: 10.3382/ps.2007-00415. [DOI] [PubMed] [Google Scholar]
- Senne D. Virus propagation in embryonating eggs. In: Dofour-Zavala L, Swayne DE, Gilsson JR, Pearson JE, Jackwood MW, Reed WM, Woolcock PR, editors. A laboratory manual for the isolation, identification and characterization of avian pathogens. 5. American Association of Avian Pathologists; Jacksonville, FL: 2008. pp. 204–208. [Google Scholar]
- Shi SH, Huang Y, Cui SJ, Cheng LF, Fu GH, Li X, Chen Z, Peng CX, Lin F, Lin JS, Su JL. Genomic sequence of an avian paramyxovirus type 1 strain isolated from Muscovy duck (Cairina moschata) in China. Archives of Virology. 2011;156:405–412. doi: 10.1007/s00705-010-0866-y. [DOI] [PubMed] [Google Scholar]
- Shortridge KF. Isolation of ortho- and paramyxoviruses from domestic poultry in Hong Kong between November 1977 and October 1978 and comparison with isolations made in the preceding two years. Research in Veterinary Science. 1980;28:296–301. [PubMed] [Google Scholar]
- Shortridge KF, King AP. Cocultivation of avian orthomyxoviruses and paramyxoviruses in embryonated eggs: implications for surveillance studies. Applied and Environmental Microbiology. 1983;45:463–467. doi: 10.1128/aem.45.2.463-467.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, Gelb J, Jr, Preskenis LA, Ladman BS, Pope CR, Pantin-Jackwood MJ, McKinley ET. The pathogenesis of low pathogenicity H7 avian influenza viruses in chickens, ducks and turkeys. Virology Journal. 2010;7:331. doi: 10.1186/1743-422X-7-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susta L, Miller PJ, Afonso CL, Brown CC. Clinicopathological characterization in poultry of three strains of Newcastle disease virus isolated from recent outbreaks. Veterinary Pathology. 2011;48:349–360. doi: 10.1177/0300985810375806. [DOI] [PubMed] [Google Scholar]
- Swayne DE, Suarez DL, Sims LD. Influenza. In: Swayne DE, Glisson JR, McDougald LR, Nair V, Nolan LK, Suarez DL, Nair V, editors. Diseases of Poultry. 13. Willey-Blackwell; Ames, Iowa: 2013. pp. 181–218. [Google Scholar]
- Tsai HJ, Chang KH, Tseng CH, Frost KM, Manvell RJ, Alexander DJ. Antigenic and genotypical characterization of Newcastle disease viruses isolated in Taiwan between 1969 and 1996. Veterinary Microbiology. 2004;104:19–30. doi: 10.1016/j.vetmic.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Turpin EA, Perkins LE, Swayne DE. Experimental infection of turkeys with avian pneumovirus and either Newcastle disease virus or Escherichia coli. Avian Diseases. 2002;46:412–422. doi: 10.1637/0005-2086(2002)046[0412:EIOTWA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wise MG, Suarez DL, Seal BS, Pedersen JC, Senne DA, King DJ, Kapczynski DR, Spackman E. Development of a real-time reverse-transcription PCR for detection of newcastle disease virus RNA in clinical samples. Journal of Clinical Microbiology. 2004;42:329–338. doi: 10.1128/JCM.42.1.329-338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang X, Zhao C, Liu D, Hu Y, Zhao J, Zhang G. Phylogenetic and pathotypical analysis of two virulent Newcastle disease viruses isolated from domestic ducks in China. PloS one. 2011a;6:e25000. doi: 10.1371/journal.pone.0025000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhao L, Wang X, Zhang D, Zhao J, Zhang G. Serologic and virologic survey for evidence of infection with velogenic Newcastle disease virus in Chinese duck farms. Avian Diseases. 2011b;55:476–479. doi: 10.1637/9649-010611-ResNote.1. [DOI] [PubMed] [Google Scholar]