Abstract
Erythropoietin (Epo) and Epo-receptor (EpoR) signaling, in addition to its classical role in erythropoiesis, exhibit a protective response in non-hematopoietic tissues. Mice with EpoR expression restricted to only hematopoietic tissues (ΔEpoRE), become obese, have low energy expenditure, and are glucose intolerant and insulin resistant. In the arcuate nucleus of the mouse hypothalamus, EpoR expression co-localizes in proopiomelanocortin (POMC) neurons. In vivo high-dose Epo treatment increases hypothalamus POMC, reduces food intake and fat mass accumulation. Here we report that Epo treatment also decreases plasma concentration of the pituitary derived POMC peptide, adrenocorticotropic hormone (ACTH). Conversely, ΔEpoRE mice show reduced hypothalamus POMC and high plasma concentrations of ACTH. In the pituitary, POMC is synthesized in the corticotroph cells, and here we examine Epo effect on pituitary POMC expression using the AtT-20 mouse corticotroph pituitary cell line. In AtT-20 cells, enzyme immunoassay analysis showed that Epo inhibits ACTH secretion. This effect is post-translational, as Epo treatment did not affect POMC mRNA expression but increased intracellular levels of ACTH peptide. Moreover, Epo reduced the basal intracellular calcium (Ca2+) levels, suggesting an effect in the Ca2+-signaling pathway. In summary, our studies suggest a novel regulatory pathway of ACTH secretion in the pituitary via EpoR-signaling. The higher plasma ACTH level in ΔEpoRE mice also suggests a possible mechanism of deregulated pituitary function with loss of Epo-signaling.
Keywords: Erythropoietin, adrenocorticotropic hormone, pituitary gland
1. Introduction
The Hypothalamic-Pituitary-Adrenal (HPA) axis is a neuroendocrine system involved in regulating stress-response, physical activity, energy balance, and circadian rhythm [1]. The cascade of the HPA axis is initiated as a result of biological stress and involves three steps: (i) production and release of corticotropin-releasing hormone (CRH) from the hypothalamus, (ii) subsequent CRH signaling in the anterior pituitary to synthesize proopiomelanocortin (POMC) which is proteolytically cleaved to form adrenocorticotropic hormone (ACTH), and finally (iii) secreted ACTH in the blood stream stimulating the adrenal gland to secrete hormones like cortisol. Evidence suggests that the HPA axis deregulation is involved in the pathogenesis of human obesity [2, 3]. In both lean and obese subjects, visceral fat distribution has been associated with increased HPA axis activity after physical and psychological stressors [2, 3]. Prolonged stress may result in chronic hyperactivation of the HPA axis, which can progressively cause visceral fat accumulation and insulin resistance [4]. Patients with metabolic syndromes seem to have HPA axis hyperactivity and a functional hypercortisolism [5, 6]. More evidence for the involvement of the HPA axis in the regulation of body weight and body fat distribution is found in Cushing's disease, that is caused by increased secretion of ACTH from an anterior pituitary tumor, which leads to rapid weight gain, particularly fat gain in trunk and face but not in the limbs [7].
Erythropoietin (Epo), a cytokine most well known for its function in red blood cell production, is produced primarily in the fetal liver, adult kidney, and also from astrocytes and neuronal cells in the CNS [8-11]. Chronic kidney disease patients who do not produce enough Epo develop anemia and have to undergo dialysis with concurrent recombinant Epo treatment. Renal failure patients with Epo deficiency show several complications including endocrine organ failure, and reduced kidney function is associated with pituitary-hypothalamus axis dysfunction. More specifically, renal failure patients undergoing hemodialysis show significantly elevated ACTH plasma levels compared to healthy volunteers [12]. Moreover, Epo treatment of these patients significantly decreases plasma ACTH, cortisol, and somatotropic hormone levels [12, 13].
Studies in animal models demonstrated a role for Epo and Epo receptor (EpoR)-signaling in metabolic regulation. Epo treatment decreased body weight, fat mass, and blood glucose in obese mice in a dose-dependent manner [14] and provided protection in mouse models of type I and type II diabetes [15]. Using a mouse model of EpoR expression restricted to only hematopoietic cells (ΔEpoRE) [16], we showed, that Epo activity in non-hematopoietic tissues is important for controlling weight gain, insulin sensitivity, accumulation of white fat, and energy expenditure [17]. In the arcuate nucleus of wild-type (WT) hypothalamus, EpoR expression co-localizes with POMC expression, and the ΔEpoRE mice show reduced POMC expression in the hypothalamus [17]. Similarly, high-dose Epo treatment of WT-mice induced POMC expression in the hypothalamus and significantly decreased food intake [14, 17]. Interestingly, the HPA axis also controls energy expenditure by influencing several mechanisms that regulate energy intake [18, 19].
Pathogenesis of obesity and metabolic syndrome occurs through multiple mechanisms and is still poorly understood. In the present study we investigate how Epo may regulate the pituitary function with respect to synthesis and secretion of ACTH, a POMC cleavage product. We find that in contrast to the hypothalamus, in pituitary cells Epo does not affect expression of POMC gene products, but regulates the secretion of ACTH. We also observe that Epo regulates this secretion by controlling the intracellular Ca2+ homeostasis of the pituitary cells. Our results identify Epo as a critical regulator of ACTH secretion in addition to CRH.
2. Results
2.1 Epo and EpoR-signaling regulate plasma ACTH levels
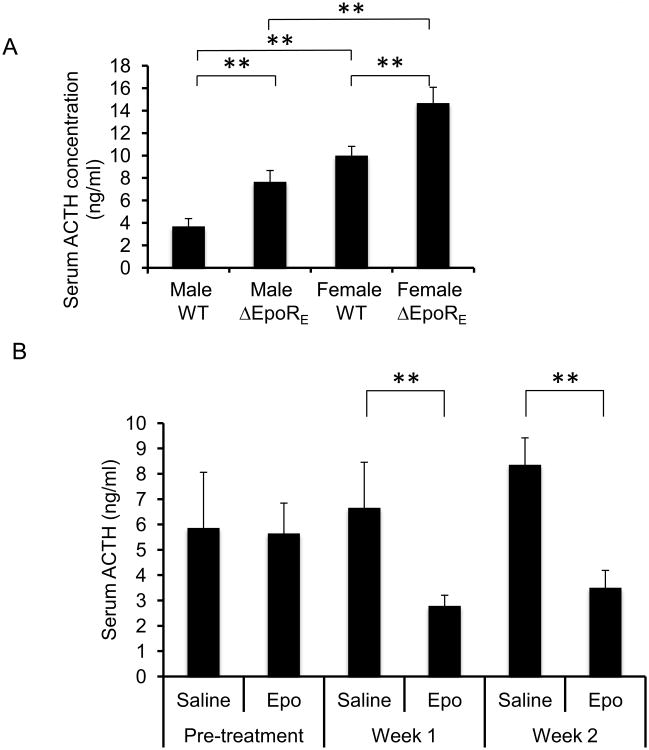
Knockout of the EpoR gene in mice results in embryonic death due to anemia [20, 21]. This lethal phenotype can be rescued by erythroid cell-specific expression of EpoR [16]. Mice with EpoR expression restricted to hematopoietic tissue (ΔEpoRE mice) show an unexpected decrease in energy expenditure, are glucose intolerant, insulin resistant, and develop obesity even on normal chow diet [17]. Conversely, Epo treatment of wild-type (WT) mice increases energy expenditure, and decreased fat mass gain on high fat-diet (HFD) [17]. To determine if pituitary function of the ΔEpoRE mice is affected, serum ACTH levels were measured in both male and female mice and compared to WT controls (Fig. 1A). ΔEpoRE mice showed higher serum ACTH levels (2-fold in males and 1.5-fold in females), compared to WT controls. Interestingly, female WT and ΔEpoRE mice showed significantly higher serum ACTH levels compared to male WT and ΔEpoRE mice respectively (Fig. 1A). Furthermore, subcutaneous administration of Epo in WT mice reduced serum ACTH levels at both week 1 and week 2 of treatment compared to saline-treatment (Fig. 1B). These data provide evidence that Epo administration and EpoR-signaling affects serum ACTH levels.
Figure 1. EpoR signaling regulates serum ACTH levels in young mice.
(A) Serum ACTH measurements in 3 weeks-old male and female wild-type (WT) and ΔEpoRE mice (n=4 in each group). (B) Serum ACTH levels in 3 weeks-old female mice before (pre-treatment) and after week 1 and week 2 of Epo or control saline treatment (n=4 in each group). Error bars indicate standard deviation and significance is indicated by ** p<0.01.
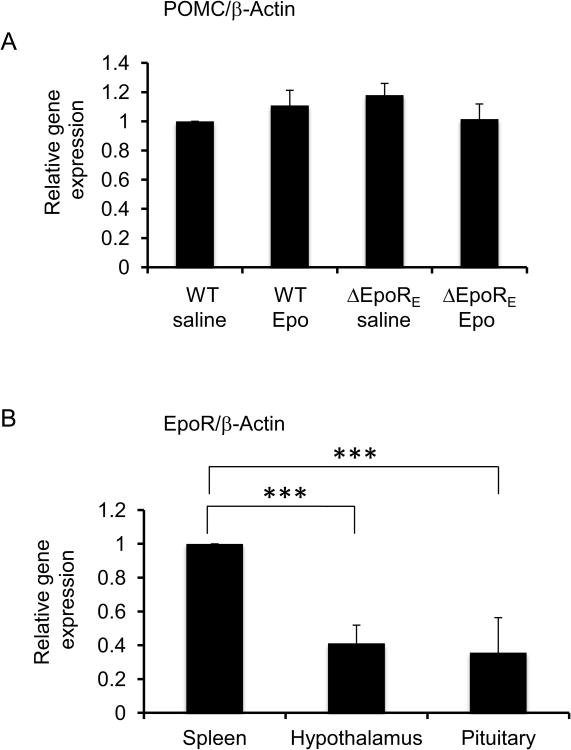
Interestingly, Epo treatment of WT and ΔEpoRE mice did not affect POMC mRNA levels (Fig. 2A). The POMC polypeptide is proteolytically cleaved into ACTH, and the fact that POMC mRNA levels are not affected by Epo treatment or lack of EpoR signaling, suggests post-translational regulation by Epo leads to differences in serum ACTH levels. Moreover, EpoR mRNA expression could be detected in the mouse pituitary gland and was similar to the elevated level of expression in the hypothalamus (Fig. 2B) compared with other non-hematopoietic tissues [17]. EpoR expression is greatest in hematopoietic tissues, bone marrow, and spleen, and hypothalamus and the pituitary gland levels of EpoR mRNA were both at one-half the level in the spleen.
Figure 2. EpoR and POMC gene expression in the murine pituitary gland.
(A) POMC mRNA levels in the pituitary gland of 3 weeks-old female WT and ΔEpoRE mice treated with Epo or saline for two weeks, relative to levels in WT saline treated group and adjusted to β-Actin mRNA levels. (B) EpoR mRNA levels in the hypothalamus and the pituitary gland of WT mice at 3 weeks age relative to levels in the spleen and adjusted to β-Actin mRNA levels. For each group, n=4 and error bars indicate standard deviation and significance is indicated by ***p<0.001.
2.2 Epo regulates ACTH secretion from the AtT-20 corticotrophic cell line
To study how Epo may directly regulate ACTH secretion in the pituitary, the mouse pituitary cell line AtT-20 was used. The AtT-20 cells synthesize the POMC precursor to ACTH and correctly glycosylate and cleave it to make mature ACTH that is then secreted. To study how Epo might affect ACTH synthesis and secretion, AtT-20 cells were treated with different doses of Epo (0-100 U/ml) for 24 hours, and intracellular ACTH levels were measured at the end of the treatment by using Western blotting (Fig. 3A). Compared to untreated AtT-20 cells, Epo was able to increase intracellular ACTH level at 5 U/ml with an optimum dose of 10 U/ml. Downstream phosphorylation of Stat3 protein also followed the same pattern showing optimum effect at an Epo dose of 10 U/ml (Fig. 3B). However, Epo-induced Stat5 phosphorylation was not detected by Western blotting at this dose range (data not shown). Moreover, Epo treatment at 10 U/ml did not affect the mRNA levels of POMC (Fig. 3C), suggesting that the increase in intracellular ACTH could be due to a decrease in secretion of ACTH. Indeed, ACTH measurement in the cell culture supernatant showed a dose dependent decrease at 5 and 10 U/ml of Epo (Figure 3D). Treatment at 20 U/ml was less effective than 10 U/ml Epo in reducing supernatant ACTH whereas 40 and 100 U/ml doses were not effective. This suggests that Epo can inhibit the secretion of ACTH from AtT-20 cells leading to an accumulation of intracellular ACTH without affecting the mRNA levels of POMC.
Figure 3. Epo regulates ACTH secretion from the murine pituitary cell line AtT-20.
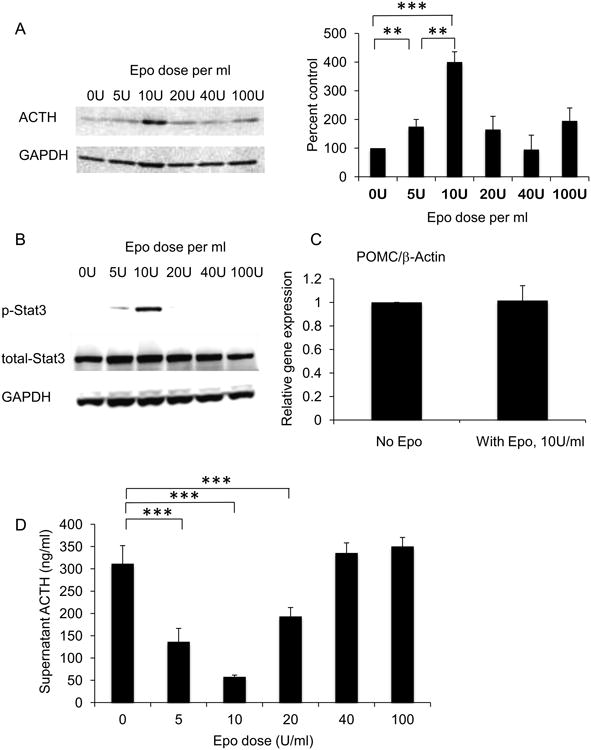
(A) AtT-20 cells were treated with Epo at 0, 5, 10, 20, 40, 100 U/ml for 24 hours and intracellular ACTH protein levels were measured by Western blotting, and quantitative densitometry was done using GAPDH loading control. (B) Epo treatment of AtT-20 cells for 30 minutes at same doses was done to detect phosphorylated Stat3 (p-Stat3), total Stat3, and GAPDH loading control by Western blotting. (C) POMC mRNA levels in AtT-20 cells treated with 10 U/ml of Epo for 24 hours, relative to untreated cells (No Epo) adjusted to β-Actin mRNA levels. (D) ACTH levels in cell culture supernatant of AtT-20 cells treated with 0, 5, 10, 20, 40, 100 U/ml Epo for 24 hours. Significance is indiacted by **p<0.01 and ***p<0.001.
2.3 Decrease of intracellular calcium (Ca2+) by Epo in AtT-20 cells
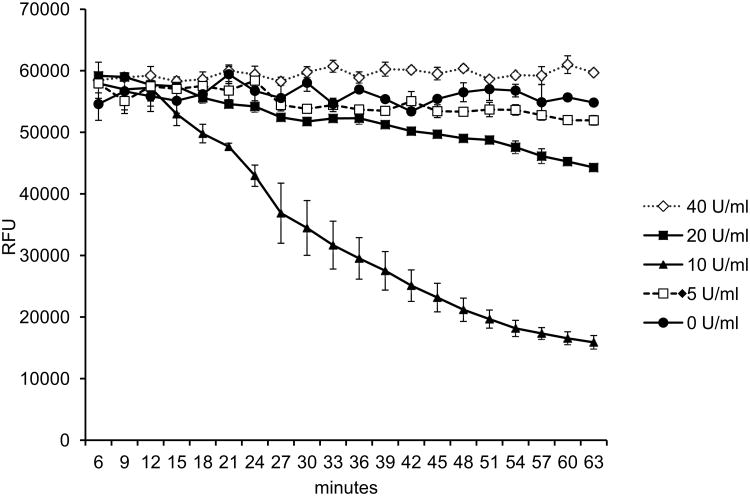
ACTH secretion from anterior pituitary corticotrophs is stimulated by an increase in ionized Ca2+ influx into the cells. A change in the cytosolic Ca2+ levels regulates the ACTH secretion from the AtT-20 cells. [22, 23]. One question pertinent to the present study is whether the actions of Epo on ACTH secretion are mediated by regulating the Ca2+ influx into the AtT-20 cells. To test this, intracellular Ca2+ levels were assessed by Fluo-4 Ca2+ assay in AtT-20 cells treated with Epo at several doses from 0-40 U/ml. The addition of Epo decreased the basal cytosolic free levels of AtT-20 cells (Fig. 4). An Epo dose of 10 U/ml was most effective in reducing the Ca2+ levels and decreased intracellular Ca2+ level to 25% at 60 minutes, while 20 U/ml of Epo showed a small reduction in intracellular Ca2+ to 75% at 60 minutes. Other Epo doses showed little or no differences in any significant difference in intracellular Ca2+ compared to untreated AtT-20 cells (0 U/ml). This indicates that Epo at an optimum dose of 10 U/ml reduces the basal cytosolic Ca2+ content in AtT-20 cells, which possibly leads to a reduction in ACTH secretion from the treated cells.
Figure 4. Decrease of cytosolic Ca2+ levels in AtT-20 cells due to Epo treatment.
Cytosolic Ca2+ levels measured in AtT-20 cells treated with Epo at 0, 5, 10, 20, 40 U/ml and denoted as relative fluorescence unit (RFU) of the Fluo-4 dye. Measurement was initiated from 6 minutes after Epo treatment until 63 minutes, and average of 3 readings are shown for each data point. Error bars indicate standard deviation of three readings and the trace is representative of three similar experiments.
3. Discussion
The present work identifies an Epo-mediated regulation of serum ACTH levels, through control of ACTH secretion from the anterior pituitary gland. Abnormal ACTH level in human is closely related to development of metabolic syndrome and obesity. Cushing's syndrome caused by excess production of ACTH due to pituitary tumor, share clinical features to metabolic syndrome [24, 25]. In line with this, patients with metabolic syndrome seem to have anterior pituitary gland hyperactivity [5, 6]. Conversely, in obese individuals, levels of cortisol and other glucocorticoid hormones, which are stimulated by ACTH, have been reported as normal or even low by some studies [26, 27]. This could be explained by an obesity-related altered peripheral metabolism of cortisol due to neuroendocrine abnormality, or partly explained by enhanced metabolic clearance of cortisol [28], due to a combination of increased 5α-reductase activity and impaired regeneration of cortisol from cortisone by 11β-HSD1 in the liver, which results in the increased cortisol urinary metabolites excretion [29].
The mouse model of hematopoietic tissue-restricted EpoR gene expression (ΔEpoRE) becomes obese with increased visceral fat and sub-cutaneous fat, develops insulin resistance and glucose intolerance with age, and shows disrupted energy homeostasis [17]. EpoR expression is high in the hypothalamus and colocalizes in the POMC neurons of the arcuate nucleus, and ΔEpoRE mice have lower hypothalamus POMC expression that contributes to this phenotype [17]. POMC is the precursor peptide for ACTH in the pituitary gland and the present study shows that EpoR-signaling does not affect the pituitary expression of the POMC gene, but affects the secretion of the processed ACTH product from the anterior pituitary gland. This is in contrast to the regulatory mechanism seen in the hypothalamus, where Epo and EpoR-signaling had a more direct effect on POMC gene expression. Moreover, we found a gender-specific difference in serum ACTH levels with female mice showing a higher level compared to males (Fig. 1 A), which has been previously reported in rodents [30].
We found that the level of EpoR mRNA expression in the mouse pituitary gland is comparable to that in the hypothalamus. Interestingly, past studies have reported significantly higher basal plasma ACTH levels in patients with chronic renal failure, as compared to healthy individuals [12, 31]. We find this particularly relevant given that the peri-tubular region of kidney is a primary site of Epo production [37] and our data suggest that a compromise in Epo production or EpoR-signaling might consequently increase basal ACTH levels. Moreover, previous clinical studies with hemodialyzed patients undergoing long-term Epo treatment show a reduction in plasma ACTH levels after 3 months, compared to the group not receiving Epo [12, 13]. Although such phenomena are likely related to physiological stress responses, we believe that such a scenario is not mutually exclusive from our hypothesis and that these studies support our findings that Epo negatively regulates plasma ACTH levels.
To further study the mechanism by which Epo regulates secretion from the pituitary gland, we used the mouse AtT-20 cell line. The AtT-20 cells provide a homogenous corticotropic cell line that makes such mechanistic studies more feasible. AtT-20 cells constitutively secrete ACTH that is further stimulated in response to a variety of secretagogues like CRH [32], and serve as a model system for study of regulated and constitutive secretory pathways. Secretion of ACTH is controlled by calcium (Ca2+) signaling pathways and increases with rising intracellular Ca2+ levels [22, 23]. Epo has been previously shown to inhibit Ca2+-induced dopamine release from pheochromocytoma PC12 cells [33]. Conversely, Epo stimulation of myoblast C2C12 cells increases intracellular Ca2+, resulting in a decrease in terminal differentiation [34]. In the AtT-20 cells, we found an Epo-dependent decrease in intracellular Ca2+ content which could be a possible mechanism by which Epo suppresses ACTH release in AtT-20 cells. Somatostatin, a known inhibitor of ACTH secretion in AtT-20 cells also act similarly via inhibition of voltage-dependent Ca2+ channels and decrease of basal cytosolic Ca2+ channels. Interestingly, this effect of Epo was seen optimally at 10 U/ml, is reduced at 20 U/ml dose, and is undetectable at higher dose of 40 and 100 U/ml. Epo treatment of AtT-20 cells at high doses does not induce Stat3 phosphorylation (Fig. 3B), has no affect on intracellular (Fig. 3A) and secreted ACTH levels (Fig. 3D), and does not reduce intracellular Ca2+ levels (Fig. 4), suggesting that such high dose Epo does not elicit any response. It is possible that this could be due to a saturation of receptor-ligand interaction at higher doses that prevents homodimerization of the receptor and blocks downstream signaling, although other inhibitory mechanisms can also play a role [34].
In summary, our data suggest that Epo regulates pituitary secretion of ACTH by controlling the basal intracellular Ca2+ levels. Epo, most well known for its hematopoietic functions, is also produced in the brain by astrocytes [11, 35, 36] and has been known to provide neuroprotective function in the brain [37]. Recent findings in our lab have shown that in addition, Epo function in the hypothalamus also controls metabolic characteristics such as feeding and energy expenditure [17]. Our present work shows that Epo has a wider role in neuroendocrine regulation via its function in the pituitary gland and may have a wider implication in obesity and development of metabolic syndrome.
4. Experimental Procedures
4.1 Animals
The ΔEpoRE mice containing the TgEpoRE transgene (GATA-1 locus hematopoietic regulatory domain driving mouse EpoR cDNA) have been previously described [16]. Age- and sex-matched C57BL/6 wild-type (WT) mice were obtained from NCI, Frederick. For Epo treatment, (3000 U/kg body weight; Epoetin alpha, Amgen), mice received subcutaneous injection three-times per week. Blood was collected from mice by mandibular bleeding between 1 PM and 3 PM for all the measurements to avoid diurnal variation. For serum preparation, blood was allowed to clot for 30 to 40 minutes at room temperature, and then centrifuged at 2000×g for 10 minutes. All animals were kept on a 12-hour light/dark cycle and fed normal chow diet. Studies were conducted in accordance to the National Institutes of Health guidelines under institution approved animal protocol.
4.2 Cell culture methods
AtT-20 mouse pituitary cell line was obtained from American Type Culture Collection (ATCC) and cultured according to ATCC guidelines. The base medium used was F-12K media supplemented with 2.5% fetal bovine serum and 15% horse serum.
4.3 ACTH measurement in serum and cell culture supernatant
The ACTH level was measured by an enzyme immunoassay using a commercially available kit (Phoenix Pharmaceuticals) according to manufacturer's protocol.
4.4 Western blotting
AtT-20 cells were homogenized, incubated in lysis buffer (50mM Tris-Hcl buffer, pH7.4, 1% NP40, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate) with phosphatase and protease inhibitors (Roche), on ice for 30 minutes, and cell debris were removed by centrifugation. Protein concentration was measured using Bio-Rad Protein Assay Dye reagent and BSA standards. Sample proteins (50 μg for each lane) were elecrophoretically separated, transferred and blotted using XCell SureLock Mini-Cell system (Invitrogen) and visualized using protein-specific antibodies and the Supersignal West Dura Extended duration Substrate (Thermo Scientific). The antibodies used were chicken polyclonal to POMC (ab14064, Abcam) that detected ACTH at 4.5 kDa size, rabbit polyclonal to phosphorylated Stat3 (9131, Cell Signaling Technology), rabbit monoclonal to Stat3 (4904, Cell Signaling Technology), and rabbit polyclonal to GAPDH (ab9485, Abcam) for loading control.
4.5 Real-time PCR gene expression analysis
Total RNA was extracted from pituitary gland or AtT-20 cells using the RNease Lipid tissue kit (Qiagen) and 2 μg was reverse transcribed using Multiscribe Reverse Transcriptase (ABI) for quantitative PCR assays. Relative gene expression was done using probe-based Taqman PCR using EpoR, POMC and β-Actin primers and probe. The sequences are as follows: EpoR forward primer, GCTCCGGGATGGACTTCA; EpoR reverse primer, GAGCCTGGTGCAGGCTACAT; EpoR probe, CATACCAGCTCGAGGGTGAGTCACGAAAG; POMC forward primer, AACCTGCTGGCTTGCATC; POMC reverse primer, GACCCATGACGTACTTCCG; POMC probe, AGACGCCCGTGTTTCCTGGC; β-Actin forward primer, ATGCTCCCCGGGCTGTAT; β-Actin reverse primer, TCACCCACATAGGAGTCCTTCTG; β-Actin probe, CGCCCTAGGCACCAGGGTGTGAT.
4.6 Intracellular Calcium measurements
Intracellular calcium (Ca2+) measurement in AtT-20 cells was done using the Fluo-4 NW Ca2+ assay kit (Invitrogen) with 125,000 cells in each well of a 96-well plate. Dye fluorescence was measured starting from 6 minutes after Epo treatment until 63 minutes, using a spectrum of excitation at 494 nm and emission at 516 nm using a Victor 3 Multilabel counter Model 1420 (Perkin Elmer).
4.7 Statistical Analysis
The data are expressed mean ± standard deviation. Comparisons between two groups were made using two-tailed non-paired Student's t-test. All in vitro experiments were performed in triplicate and repeated at least 3 times; a representative experiment was selected for figures. Statistical significance of differences were determined using a minimal level of significance at p<0.05.
Highlights.
Erythropoietin (Epo) treatment decreases serum concentration of ACTH in mice.
Lack of Epo receptor signaling in mice increases serum concentrations of ACTH.
In AtT-20 mouse pituitary cells, Epo post-translationally inhibits ACTH secretion.
Epo also reduces basal intracellular calcium (Ca2+) levels in AtT-20 cells.
This suggests a novel regulatory pathway of pituitary ACTH secretion by Epo.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Diabetes and Digestive and Kidney Diseases and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicolaides NC, et al. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci. 2014;1318:71–80. doi: 10.1111/nyas.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vicennati V, et al. Stress-related development of obesity and Cortisol in women. Obesity (Silver Spring) 2009;17(9):1678–83. doi: 10.1038/oby.2009.76. [DOI] [PubMed] [Google Scholar]
- 3.Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9(6):787–93. doi: 10.1016/j.coph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41(8):871–82. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr Metab (Lond) 2005;2(1):3. doi: 10.1186/1743-7075-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira CD, et al. 11beta-Hydroxysteroid dehydrogenase type 1: relevance of its modulation in the pathophysiology of obesity, the metabolic syndrome and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(10):869–81. doi: 10.1111/j.1463-1326.2012.01582.x. [DOI] [PubMed] [Google Scholar]
- 7.Hankin M, Theile HM, Steinbeck AW. An evaluation of laboratory tests for the detection and differential diagnosis of Cushing's syndrome. Clin Endocrinol (Oxf) 1977;6(3):185–96. doi: 10.1111/j.1365-2265.1977.tb03314.x. [DOI] [PubMed] [Google Scholar]
- 8.Marti HH, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8(4):666–76. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 9.Bernaudin M, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19(6):643–51. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Juul SE, et al. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43(1):40–9. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Masuda S, et al. A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem. 1994;269(30):19488–93. [PubMed] [Google Scholar]
- 12.Kokot F, et al. Function of endocrine organs in hemodialyzed patients of long-term erythropoietin therapy. Artif Organs. 1995;19(5):428–35. doi: 10.1111/j.1525-1594.1995.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 13.Kokot F, et al. Influence of erythropoietin treatment on function of the pituitary-adrenal axis and somatotropin secretion in hemodialyzed patients. Clin Nephrol. 1990;33(5):241–6. [PubMed] [Google Scholar]
- 14.Foskett A, et al. The effects of erythropoietin dose titration during high-fat diet-induced obesity. J Biomed Biotechnol. 2011;2011:373781. doi: 10.1155/2011/373781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi D, et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med. 2010;207(13):2831–42. doi: 10.1084/jem.20100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N, et al. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100(7):2279–88. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- 17.Teng R, et al. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat Commun. 2011;2:520. doi: 10.1038/ncomms1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zellner DA, et al. Food selection changes under stress. Physiol Behav. 2006;87(4):789–93. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Martens MJ, et al. Effects of single macronutrients on serum Cortisol concentrations in normal weight men. Physiol Behav. 2010;101(5):563–7. doi: 10.1016/j.physbeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Lin CS, et al. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10(2):154–64. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, et al. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83(1):59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 22.Tse A, Lee AK, Tse FW. Ca2+ signaling and exocytosis in pituitary corticotropes. Cell Calcium. 2012;51(3-4):253–9. doi: 10.1016/j.ceca.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Mamillapalli R, Wysolmerski J. The calcium-sensing receptor couples to Galpha(s) and regulates PTHrP and ACTH secretion in pituitary cells. J Endocrinol. 2010;204(3):287–97. doi: 10.1677/JOE-09-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan SA, et al. 11beta-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc Natl Acad Sci U S A. 2014;111(24):E2482–91. doi: 10.1073/pnas.1323681111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geibel M, et al. Ablation of TrkB signalling in CCK neurons results in hypercortisolism and obesity. Nat Commun. 2014;5:3427. doi: 10.1038/ncomms4427. [DOI] [PubMed] [Google Scholar]
- 26.Rask E, et al. Tissue-specific changes in peripheral Cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab. 2002;87(7):3330–6. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- 27.Stewart PM, et al. Cortisol metabolism in human obesity: impaired cortisone-->cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84(3):1022–7. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- 28.Anagnostis P, et al. Clinical review: The pathogenetic role of Cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94(8):2692–701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 29.Rask E, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86(3):1418–21. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki-Sekino A, et al. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–37. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Clodi M, et al. Adrenal function in patients with chronic renal failure. Am J Kidney Dis. 1998;32(1):52–5. doi: 10.1053/ajkd.1998.v32.pm9669424. [DOI] [PubMed] [Google Scholar]
- 32.Zhang N, et al. MicroRNA 375 mediates the signaling pathway of corticotropin-releasing factor (CRF) regulating pro-opiomelanocortin (POMC) expression by targeting mitogen-activated protein kinase 8. J Biol Chem. 2013;288(15):10361–73. doi: 10.1074/jbc.M112.425504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami M, et al. Erythropoietin inhibits calcium-induced neurotransmitter release from clonal neuronal cells. Biochem Biophys Res Commun. 2000;279(1):293–7. doi: 10.1006/bbrc.2000.3926. [DOI] [PubMed] [Google Scholar]
- 34.Ogilvie M, et al. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275(50):39754–61. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- 35.Marti HH, et al. Detection of erythropoietin in human liquor: intrinsic erythropoietin production in the brain. Kidney Int. 1997;51(2):416–8. doi: 10.1038/ki.1997.55. [DOI] [PubMed] [Google Scholar]
- 36.Bernaudin M, et al. Neurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30(3):271–8. [PubMed] [Google Scholar]
- 37.Zhang Y, et al. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014;15(6):10296–333. doi: 10.3390/ijms150610296. [DOI] [PMC free article] [PubMed] [Google Scholar]