Abstract
Background
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid produced by mast cells (MC) upon cross-linking of their high affinity receptors for IgE by antigen (Ag) that can amplify MC responses by binding to its S1P receptors. Acute MC-dependent allergic reaction can lead to systemic shock but the early events of its development in lung tissues have not been investigated, and S1P functions in the onset of allergic processes remain to be examined.
Objective
We used a highly specific neutralizing anti-S1P antibody (mAb) and an S1P receptor 2 (S1PR2) antagonist, JTE-013, to study S1P and S1PR2 signaling contributions to MC- and IgE-dependent airway allergic responses in mice within minutes after Ag challenge.
Methods
Allergic reaction was triggered by a single intraperitoneal (i.p.) dose of Ag in sensitized mice pre-treated i.p. with anti-S1P or isotype control mAb, or JTE-013 or vehicle prior to Ag challenge.
Results
Kinetics experiments revealed early pulmonary infiltration of mostly T cells around blood vessels of sensitized mice 20 minutes post-Ag exposure. Pre-treatment with anti-S1P mAb inhibited in vitro MC activation, as well as in vivo development of airway infiltration and MC activation, reducing serum levels of histamine, cytokines and the chemokines MCP-1/CCL2, MIP-1α/CCL3 and RANTES/CCL5. S1PR2 antagonism or deficiency, or MC deficiency recapitulated these results. Both in vitro and in vivo experiments demonstrated MC S1PR2 dependency for chemokine release and the necessity for signal transducer and activator of transcription 3 (Stat3) activation.
Conclusion
Activation of S1PR2 by S1P and downstream Stat3 signaling in MC regulate early T cell recruitment to antigen-challenged lungs by chemokine production.
Keywords: Airway inflammation, allergy, mast cells, recruitment, T cells, sphingosine-1-phosphate, sphingosine-1-phosphate receptor 2, Stat3
INTRODUCTION
Mast cells (MC) play a central role in both the development and exacerbation of allergic disorders 1. Cross-linking of their high affinity receptors for immunoglobulin (Ig) E (FcεRI) by antigen (Ag) activates sphingosine kinases that phosphorylate sphingosine to produce S1P, a critical sphingolipid contributing to degranulation, the release of intragranular mediators 2, 3. S1P can be exported from MC to amplify their responses by binding to S1P receptors 2, 4, 5 and recruit other immune cells, including Th2 lymphocytes 3. Thus, S1P participates in cytokine/chemokine production and inflammatory cell trafficking 6. S1P and its small molecule analogs are the subject of intense investigation in the pathogenesis of acute lung injury and asthma 7-11.
We previously reported that S1PR2 plays a critical role in regulating human mast cell functions 12, whereas S1PR1 is important for their migration to sites of inflammation 2, 3. IgE-dependent systemic MC activation is responsible for elevated circulating levels of histamine and associated pulmonary edema in mice 1, 13, and both are attenuated after inhibition or deletion of S1PR2 12. While exacerbation of systemic allergic reactions has been mostly studied for its after-the-fact effects, the early steps of its development in lung tissues have not been investigated. Because our preclinical model of MC-dependent allergic reaction triggered respiratory distress in mice 12, we hypothesized that early inflammatory events may be occurring in the acutely challenged airways that are S1PR2-dependent. S1PR2 signaling could also result in endothelial barrier dysfunction and increased permeability 14. However, the role of S1PR2 remains unclear because of its contrasting functions on various cell types 15.
We here evaluated IgE/Ag-induced pulmonary alterations upon blockade of S1PR2 signaling by neutralization of S1P, the ligand of S1PR2, with Sphingomab, a specific anti-S1P mAb and by inhibition of S1PR2 with the specific antagonist JTE-013. We show that inflammatory infiltration of T cells occurs within minutes of systemic Ag challenge and is strongly mitigated in mice pre-treated either with Sphingomab or JTE-013 in a MC-dependent model of acute allergic reaction. We further demonstrate that this effect is due in part to early production of chemokines. Moreover, Stat3 activation was induced in inflamed lungs and in MC shortly after exposure to S1P in an S1PR2-dependent manner. Thus, the acute onset of MC-dependent allergic responses features early lung T cell recruitment in mice via the S1P/S1PR2/Stat3 axis and chemokine production.
METHODS
Mice
C57BL6/J, C57BL/6x129/SVJ and C57BL/6-KitW-sh/W-sh mice were purchased from The Jackson Laboratory. S1PR2 knockout mice (Dr. R. Proia, NIH/NIDDK, Bethesda, MD) and corresponding wild-type mice were on mixed C57BL/6×129/SVJ background. All mice were used at 8-12 weeks of age and maintained in a pathogen-free facility. Studies were performed in accordance with the institutional IACUC guidelines.
Reagents and antibodies
Murine monoclonal antibody, LT1002 (Sphingomab™) directed against S1P, and LT1003 isotype-matched control (IgG1, κ) antibody were provided by Lpath Inc., San Diego, CA. Dinitrophenyl (DNP)-specific mouse IgE was a generous gift from Dr. Daniel Conrad (VCU, Richmond, VA). p-Stat3 and Stat3 antibodies were from Cell Signaling Technology (Danvers, MA). Hamster anti-mouse CD3ε and IgG isotype control, Alexa Fluor 488 conjugates, rat anti-mouse CD14 and IgG2a, κ isotype control, PE conjugates, and rat anti-mouse CD16/32 (Fc block) were purchased from eBioscience (San Diego, CA). JTE-013 was purchased from Tocris Bioscience (Minneapolis, MN) and S1P from Enzo Life Sciences (Farmingdale, NY). DNP-HSA (Ag) and ionomycin were obtained from Sigma-Aldrich (St. Louis, MO). Liberase TM and DNase I were obtained from Roche Diagnostics (Indianapolis, IN).
Human skin and mouse bone marrow-derived mast cells
Human skin mast cells (Sk-MC) and mouse bone marrow-derived mast cells (BMMC) were isolated and cultured as previously described 11, 16 and were more than 98% pure. Human MC and BMMC were sensitized overnight with 1 μg/ml or 0.5 μg/ml DNP-specific mouse IgE, washed to remove excess unbound IgE and stimulated with 30 or 20 ng/ml DNP-HSA (Ag), respectively 5. Ionomycin (1 μM) was used as positive control. Degranulation was quantified by measuring β-hexosaminidase release 5.
Cytokine and chemokine measurements
Human and mouse cytokines/chemokines were measured by ELISA following manufacturer's instruction (R & D Systems, Minneapolis, MN).
Acute allergic reaction
All injections were performed i.p. in a final volume of 100 μl, as previously described 12. Briefly, mice were injected with DNP-specific IgE and 12 h later, with Ag in PBS. In some experiments, blood samples were collected by tail vein nick just prior to Ag challenge to conduct flow cytometric analysis of circulating T cells. A small nick was made over the lateral vein of restrained mice using a scalpel blade. Blood (150-250 μl) was collected via heparinized capillary tubes into heparinized tubes. Core body temperature was measured immediately before Ag challenge and at indicated times using a rectal microprobe (Physitemp Instruments, Clifton, NJ). Mice were euthanized and blood was collected by cardiac puncture for serum analysis. Bronchoalveolar lavage (BAL) fluids were obtained by lavaging lungs twice with 600 μl PBS and stored at −80°C after centrifugation. Lungs were removed for histological examination or snap-frozen for Western blot analyses. Control mice injected with PBS showed no significant change in body temperature (±1°C) and had no detectable blood histamine.
Adoptive transfer of BMMC into MC-deficient mice
Bone marrow cells from femurs of female wild-type mice were cultured for 6 weeks to generate BMMC, as described 11. For MC reconstitution, recipient (7-8 weeks-old) female KitW-sh-/W-sh mice were injected i.p. with 5 × 106 BMMC in 200 μl of PBS 17. Eight weeks later, MC-reconstituted Kit W-sh-/W-sh mice (Rec. Kit W-sh-/W-sh) were treated with vehicle or JTE-013, prior to Ag triggering, as described 12. To assess for appropriate reconstitution, BAL was collected 90 minutes after Ag challenge and BAL tryptase determined.
Lung histology
Lungs were prepared for histological analysis as previously described 12. Briefly, after inflation, lungs were embedded in paraffin, serial sections stained with H&E, and perivascular areas were evaluated in 8-12 randomly selected sections for each sample. Investigators had no knowledge of the treatment group assignments at the time of analysis. A Nikon ECLIPSE E800M microscope (Nikon, Tokyo, Japan) equipped with a Diagnostic Instruments SPOT RT CCD camera (SPOT Imaging Solutions, Sterling Heights, MI) was used to photograph the sections. Perivascular infiltration was scored semi-quantitatively, as previously described 18 according to the following criteria: 0, normal; 1, few cells; 2, rings of inflammatory cells 1 cell layer deep; 3, rings of inflammatory cells 2 to 4 cells deep; and 4, rings of inflammatory cells 4 or more cells deep. Bars represent 100 μm.
Lung enzymatic digestion and flow cytometric analysis
Lungs were removed and mechanically dispersed. Liberase TM solution (0.22 Wunch units/ml final concentration) supplemented with DNase I (0.01 U/μl final concentration) was added to each dispersed sample. After 1h at 37°C, digested tissues were filtered through 70 μm cell strainers and centrifuged. Red blood cells (RBC) were lysed using hypotonic salt solutions. Cell suspensions were quickly filtered and centrifuged. Pellets were washed in ice-cold PBS/1%FCS for cell counting and staining. For blood samples, RBC were first lysed with BD PharmLyse buffer (BD Biosciences, San Jose, CA). Cells from individual mice were incubated prior to and during all Ab staining with Fc block. CD3 and CD14 expression analysis was performed using a standard flow cytometry protocol described previously 19. Cells gated on forward and side scatter parameters to exclude dead cells and debris were analyzed on a Beckman Coulter FC500 Flow Cytometer (Fullerton, CA) and data analyzed using the CXP software.
Transwell T cell migration assay
BMMC-conditioned cell-free supernatants were prepared using sensitized (0.5 μg mouse anti-DNP IgE/ml/2×106 BMMC, 12h, 37°C) BMMC treated with JTE-013 (1 μM) or vehicle for 30 min at 37°C prior to adding Ag (DNP-HSA, 100 ng/ml/30×106 BMMC). After 24h, supernatants were collected and stored at −80°C. Splenic CD4+ and CD8+ T cells were isolated using the EasySep kit (StemCell Technologies, Vancouver, BC, Canada) and used together for migration experiments. T cell enrichment was verified by flow cytometry. Assays were conducted using 5 μm pore size polycarbonate filters in Costar 24-well Transwell chambers (Corning, Corning, NY) with 850 μl BMMC-conditioned media in the lower chamber and 105 T cells in 200 μl in the upper chamber. Recombinant murine RANTES/CCL5 (100 ng/ml, Peprotech, Rocky Hill, NJ) and medium, DMSO vehicle or JTE-013 were used as positive and negative controls, respectfully. After 24h at 37°C, T cells in the lower chambers were counted in quadruplicate using flow cytometry after propidium iodide staining to exclude dead cells from analysis (FACScalibur, BD Biosciences). Migration was expressed as fold of control (culture medium alone).
Histamine determination
Mouse serum histamine levels were determined using an ELISA kit (Neogen, Lexington, KY).
Tryptase determination
An ELISA kit was used to measure tryptase in the supernatants of centrifuged BAL fluids according to manufacturer's recommendation (EMD Millipore, Billerica, MA).
Western analysis
Lungs or 5×106 MC were homogenized and equal amounts of proteins were analyzed by immunoblotting after transfer to nitrocellulose and blocking with 1:1,000 dilutions of anti-p-Stat3 or anti-Stat3 overnight at +4°C. After washes in TBS-0.1%Tween 20, membranes were incubated with 1:5,000 dilution of species-appropriate HRP-linked anti-IgG (Cell Signaling) and sizes of proteins of interest were determined using molecular weight standards (BioRad, Hercules, CA). Quantitation was carried out using ImageJ (National Institutes of Health). Integrated density numbers (in pixels) of p-Stat3 were normalized to total Stat3 and are means of three independent experiments.
Statistical analysis
Data are expressed as means ± S. E. M. and were analyzed by unpaired two-tailed Student's t-test for comparison of two groups and one-way analysis of variance (ANOVA) for analysis of three or more groups (Prism 6; GraphPad software). Significance for all statistical tests is shown in figures for p<0.05 (*), p<0.01 (**), p<0.001 (***) and p<0.0001 (^). Experiments were repeated at least three times in triplicates with consistent results. In vivo experiments were repeated three times and each experimental group consisted of five to six mice.
RESULTS
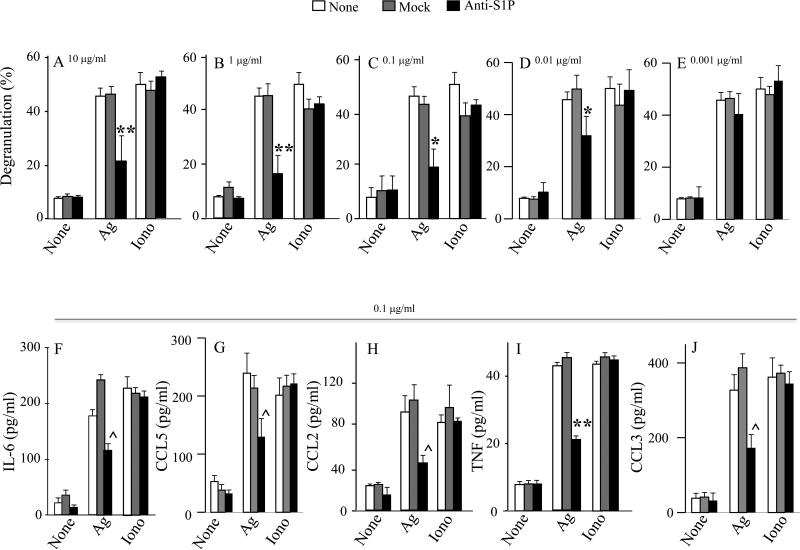
Sphingomab, a neutralizing anti-S1P mAb, significantly decreases human mast cell degranulation and cytokine/chemokine secretion
We investigated the effects of Sphingomab on in vitro MC activation. As shown in Fig 1A-D, addition of Sphingomab at concentrations ranging from 10 to 0.01 μg/ml, but not isotype-matched control mAb, or Sphingomab at 0.001 μg/ml, at time of Ag stimulation dose-dependently decreased IgE/Ag-induced degranulation as measured by beta-hexosaminidase release, without altering either spontaneous or ionomycin-induced degranulation. Since the anti S1P-mAb inhibited degranulation by 50% at 0.1 μg/ml, this concentration was selected to examine its effects on cytokine/chemokine secretion. Anti-S1P mAb treatment significantly decreased IgE/Ag-triggered IL-6 (Fig 1F), CCL5 (Fig 1G), CCL2 (Fig 1H), TNF (Fig 1I) and CCL3 (Fig 1J) secretion, without altering spontaneous or ionomycin-induced release. These results substantiate the notion that S1P released from activated MC contributes to secretion of proinflammatory mediators and this can be suppressed by neutralizing extracellular S1P.
Fig. 1.
Sphingomab, a specific anti-S1P mAb, reduces IgE/Ag-induced activation of human mast cells. Sk-MC were pretreated with anti-S1P or control (mock) prior to stimulation, at the indicated concentration. Degranulation was measured by colorimetric assay (A-E). Secretion of IL-6 (F), RANTES/CCL5 (G), MCP-1/CCL2 (H), TNF (I) and MIP-1α/CCL3 (J) were measured by ELISA. (* p < 0.05; ** p < 0.01; ^ p < 0.0001, oneway ANOVA).
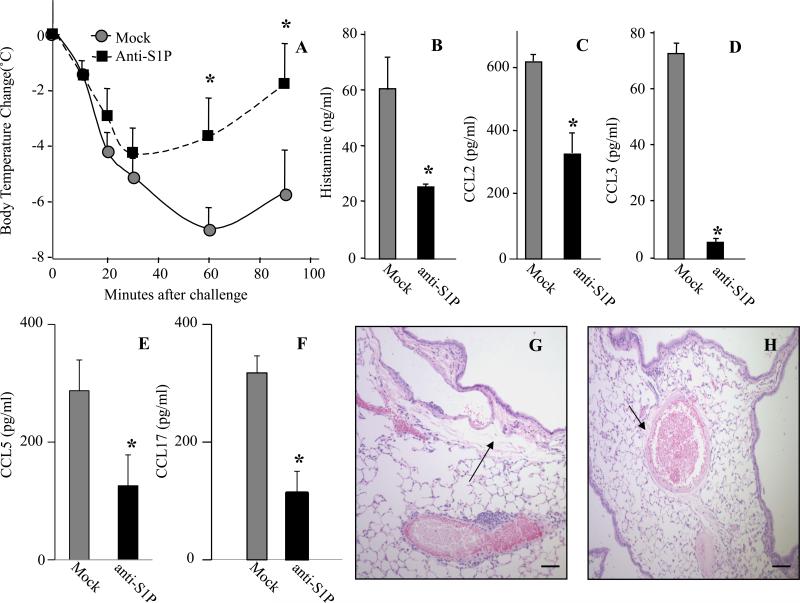
Neutralization of S1P with a specific mAb mitigates IgE-dependent airway allergic reaction
Previous studies suggest that susceptibility to anaphylaxis in mice correlates with serum S1P levels 20. Because Sphingomab neutralizes circulating levels of S1P 21, 22, we sought to examine its effects in an in vivo MC- and IgE-dependent mouse acute model of allergic reaction. To this end, prior to IgE/Ag injections, anti-S1P mAb was administered i.p., as it was previously demonstrated that over 95% of the anti-S1P mAb rapidly appeared in the bloodstream after i.p. injection of a bolus dose 21. The anti-S1P mAb-treated mice exhibited significantly reduced hypothermia, compared to mice treated with an isotype-matched control mAb (Fig 2A). Mice administered anti-S1P mAb also had markedly decreased levels of systemic histamine (Fig 2B), MCP-1/CCL2 (Fig 2C), MIP1-alpha/CCL3 (Fig 2D), RANTES/CCL5 (Fig 2E) and TARC/CCL17 (Fig 2F) 2h after Ag administration. At this time point, histopathological analysis showed extensive perivascular edema in mice pretreated with a mock mAb prior to Ag challenge (Fig 2G), which was significantly attenuated in anti-S1P mAb-treated mice (Fig 2H).
Fig. 2.
Sphingomab reduces passive systemic anaphylaxis. C57Bl/6 mice were injected i.p. with anti-S1P or isotype-matched control (mock) mAb (20 mg/kg). Twenty-four hours later, murine IgE anti-DNP mAb was administered. Mice were then re-injected i.p. with mAbs, same dose as above after 12h and antigenic challenge was performed 1h later by i.p. injection of 100 g DNP-HSA. Body temperature monitoring (A). Serum levels of histamine (B), MCP-1/CCL2 (C) MIP-1α/CCL3 (D), RANTES/CCL5 (E) and TARC/CCL17 (F) 2h after Ag challenge. Representative micrographs of H&E-stained lung tissues of mice treated with mock mAb (G) or Sphingomab (H) collected 2h after Ag challenge. (*p < 0.05; Student's t-test).
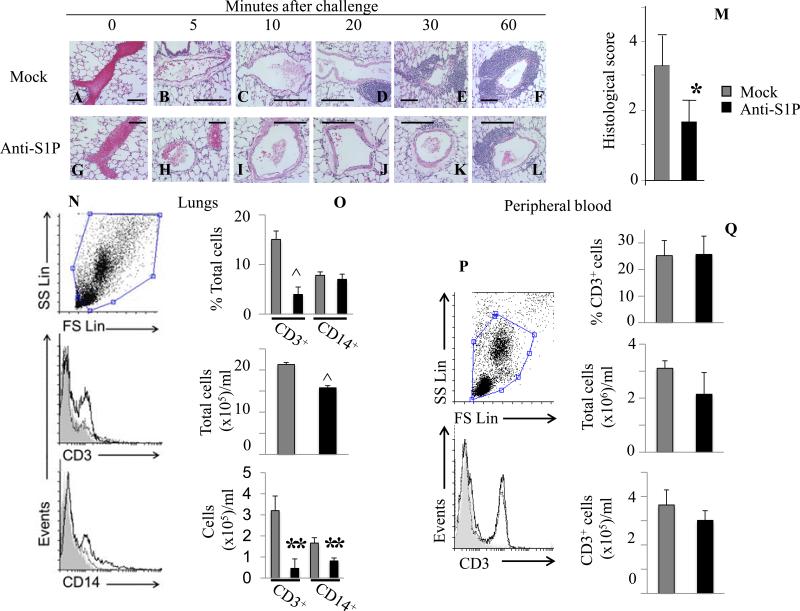
Neutralization of S1P decreases early allergic lung infiltration of T lymphocytes and macrophages
We further analyzed lung sections during the development of allergic reaction (Fig 3A-F). Surprisingly, as early as 20 min after Ag challenge, cellular infiltrates were detected around blood vessels in Ag-challenged mice treated with mock mAb (Fig 3D) and continued to intensify 30-60 min after challenge (Fig 3E and F). By contrast, mice treated with the specific anti-S1P mAb exhibited delayed and markedly attenuated perivascular infiltrations after Ag challenge (Fig 3G-L). Semi-quantitative scoring confirmed that anti-S1P mAb significantly reduced infiltration (Fig 3M). Flow cytometric analysis revealed that infiltrating cells were CD3+ T cells with fewer CD14+ monocytes/macrophages (Fig 3N-O). No eosinophils or neutrophils were detected (data not shown). S1P neutralization mitigated perivascular infiltration of both cell types (Fig 3N-O). Peripheral blood analysis prior to Ag triggering demonstrated that numbers of circulating CD3+ cells in anti-S1P-treated mice were not altered compared to mice treated with control mAb (Fig 3P-Q).
Fig. 3.
Early allergic lung infiltration is mitigated after Sphingomab pretreatment. H&E-stained lung sections of mice pretreated with a mock mAb (A-F) or with Sphingomab (G-L) as described in Fig 2. (M) Infiltration scoring 1h after Ag challenge. (N-O) Flow cytometric analysis and quantification of lung CD3+ and CD14+ cells 2h after Ag challenge. (P-Q) Flow cytometric analysis and quantification of circulating CD3+ cells prior to Ag challenge. Gray histograms represent isotype controls staining, unfilled solid lines depict mock-mAb-treated cell suspensions, and unfilled dotted lines, the anti-S1P-treated cells. Graphs represent percentages of CD3+ or CD14+ cells, total cell, CD3+ or CD14+ cell numbers. (*p < 0.05, **p < 0.01, ^ p < 0.0001; Student's t-test).
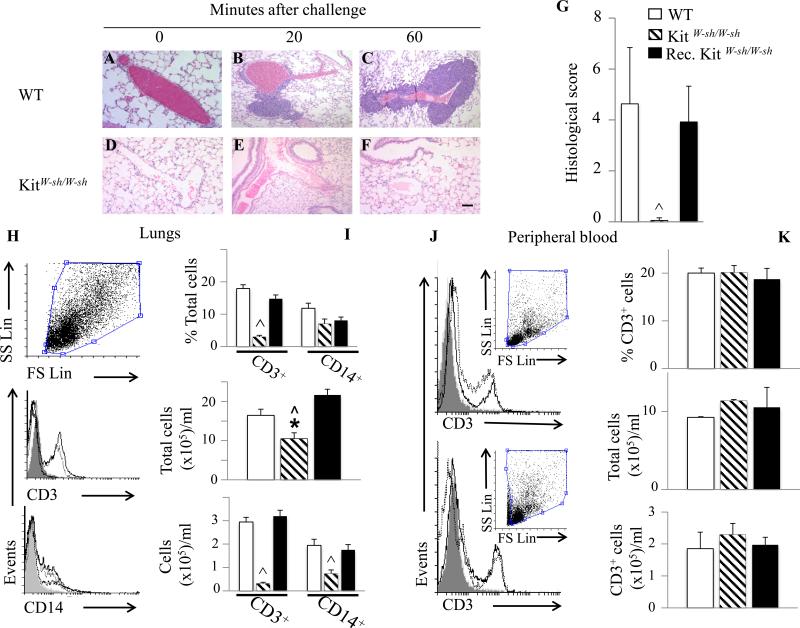
Ag-triggered lung perivascular infiltration is drastically decreased in MC-deficient KitW-sh/W-sh mice and restored in MC-reconstituted KitW-sh/W-sh mice
To investigate the role of MC in early infiltration, we utilized C57BL/6-KitW-sh/W-sh MC-deficient mice 17. Significant perivascular cell infiltration was present in WT (Fig 4A-C) but not in MC-deficient mice (Fig 4D-F), which was confirmed by semi-quantitative scoring of lung sections (Fig 4G). MC involvement was further substantiated using Ag-challenged MC-reconstituted KitW-sh/W-sh mice which displayed histological scores comparable to WT MC-sufficient mice (Fig 4G). Flow cytometry analysis demonstrated that WT mouse lungs contained large numbers of infiltrating CD3+ T cells and CD14+ macrophages which were dramatically decreased in MC-deficient mouse lungs, and restored in MC-reconstituted KitW-sh/W-sh mice (Fig 4 H-I). These results suggest an important role for MC in early infiltration of T lymphocytes and macrophages. As expected, blood samples prior to Ag challenge showed no significant differences in circulating CD3+ cells between WT, MC-deficient mice and MC-reconstituted KitW-sh/W-sh (Fig 4J-K).
Fig. 4.
Lack of IgE/Ag-induced lung perivascular infiltration in MC-deficient KitW-sh/W-sh mice and its restoration in MC-reconstituted KitW-sh/W-sh mice (Rec. KitW-sh/W-sh). (A-F) H&E-stained lung sections of MC-deficient and WT mice at the indicated times after Ag challenge. (G) Infiltration quantification 1h after Ag challenge, (H-I) Flow cytometric analysis and quantification of lung cells 1h after Ag challenge. Gray histograms represent isotype controls staining, unfilled solid lines depict WT cell suspensions, unfilled dotted lines, Rec. KitW-sh/W-sh cells and unfilled dashed lines KitW-sh/W-sh cells. Graphs represent percentages of CD3+ or CD14+ cells, total cell, CD3+ or CD14+ cell numbers (J) Flow cytometric analysis (upper panel compares WT (solid lines) to KitW-sh/W-sh cell suspensions, lower panel compares KitW-sh/W-sh (solid lines) to Rec. KitW-sh/W-sh (dotted lines) cells) and (K) quantification of circulating CD3+ cells prior to Ag challenge. (*p < 0.05; ^ p < 0.0001; one-way ANOVA).
Extracellular S1P neutralization decreases in vivo mast cell activation and levels of circulating histamine and chemokines, also observed in the absence of MC
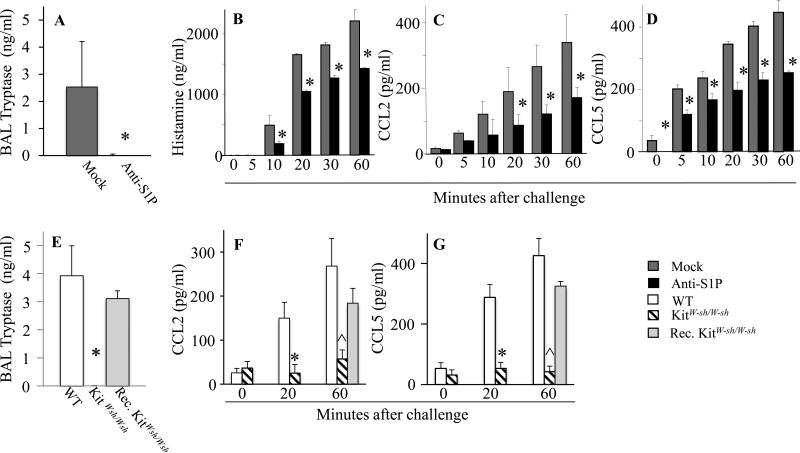
While MC account for the early airway responses to Ag challenge, it is still not clear how important these cells are for the development of the late-phase response and chronic inflammation 23. MC activation was substantiated by increased levels of tryptase in the BAL fluids of control mAb-pretreated sensitized mice 2h after Ag challenge (Fig 5A). In sharp contrast, tryptase was not detectable in BAL fluids from mice pre-treated with anti-S1P mAb (Fig 5A). As expected, tryptase was undetectable in BAL fluid from KitW-sh/W-sh mice, but was elevated in MC-reconstituted KitW-sh/W-sh mice (Fig 5B).
Fig. 5.
Effects of S1P neutralization, MC depletion or reconstitution of MC on circulating levels of proinflammatory mediators after Ag challenge. (A-D) Mice were treated with anti-S1P or mock antibody as described in Fig.2 and (A) BAL tryptase levels determined 2h after Ag challenge. Serum levels of (B) histamine, (C) MCP-1/CCL2, and (D) RANTES/CCL5. (E-G) Sensitized WT, KitW-sh/W-sh mice or Rec. KitW-sh/W-sh mice were challenged with Ag. (E) BAL tryptase levels. Serum levels of (F) MCP-1/CCL2 and (G) RANTES/CCL5. n =5-6 mice per group, repeated twice. (*p < 0.05, Student's t-test; ** p < 0.01, WT versus KitW-sh/W-sh or Rec. KitW-sh/W-sh versus KitW-sh/W-sh, one-way ANOVA; ^ p < 0.0001, WT or Rec. KitW-sh/W-sh versus KitW-sh/W-sh, one-way ANOVA).
We have previously shown that acute allergic challenge leads to elevated histamine and MCP-1/CCL2 and MIP-1α/CCL3, all of which could be ascribed to activation of MC 12. RANTES/CCL5, CCL2, and CCL3 also contribute to airway inflammation through their chemotactic effects on lymphocytes 24-27. Because MC constitute a local source of T cell-recruiting CCL3 and CCL5 27, the kinetics of histamine (Fig 5B), CCL2 (Fig 5C) and CCL5 (Fig 5D) release were monitored upon Ag challenge. All three circulating mediators were increased time-dependently up to 1h after Ag challenge. All were markedly reduced in the serum of animals pretreated with anti-S1P mAb as compared to nonspecific mAb, confirming the local as well as systemic anti-inflammatory effects of S1P neutralization in a MC-dependent acute model of allergic reaction (Fig 5B-D). Moreover, as expected, tryptase was undetectable in BAL fluid from KitW-sh/W-sh mice, but was elevated in MC-reconstituted KitW-sh/W-sh mice (Fig 5E). Importantly, serum CCL2 (Fig 5F) and CCL5 (Fig 5G) were also decreased in MC-deficient KitW-sh/W-sh mice, compared to WT or MC-reconstituted KitW-sh/W-sh mice 1h after Ag challenge.
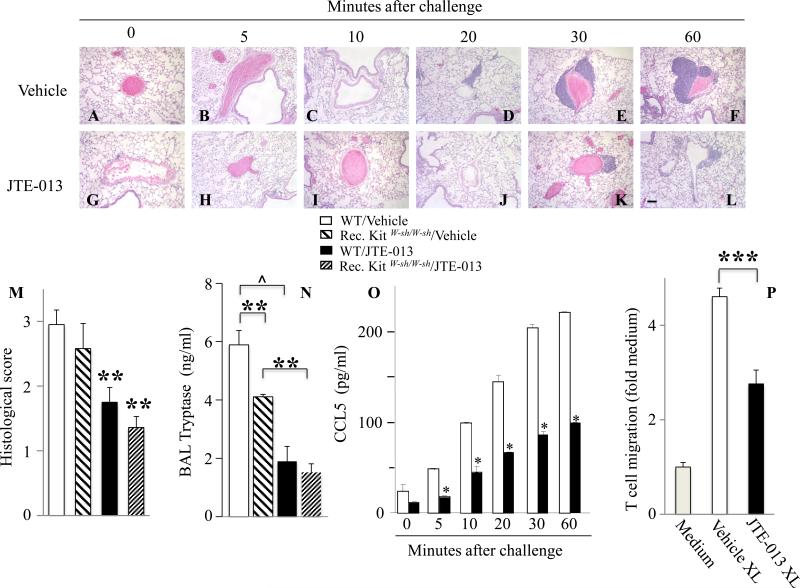
The S1PR2 antagonist JTE-013 decreases inflammatory infiltration
Next, we examined the effects of JTE-013 on pulmonary infiltration in Ag-challenged mice (Fig 6). Ag exposure triggered rapid and time-dependent cell infiltration in animals pretreated with vehicle (Fig 6A-F). However, perivascular infiltration was greatly ameliorated by JTE-013 pretreatment (Fig 6G-L). In agreement, immune cell infiltration (Fig 6M and Figs E1B-C), activation of MC as demonstrated by increased BAL tryptase (Fig 6N) as well as RANTES/CCL5 (Fig 6O and Fig E1D) and CCL2 serum levels (Fig E1D and 12) were reduced by JTE-013 treatment, in WT and in MC-reconstituted KitW-sh/W-sh mice (Figs 6M, 6N). Of note, JTE-013 treatment of sensitized MC-reconstituted KitW-sh/W-sh mice also prevented the drop in core body temperature observed upon antigenic challenge, compared to vehicle pre-treated MC-reconstituted KitW-sh/W-sh mice (Fig E1A).
Fig. 6.
JTE-013, a S1PR2 antagonist, decreases allergic lung infiltration. H&E staining of lung sections from mice sensitized with IgE and pretreated with vehicle (A-F) or JTE-013 (G-L) 30 min prior to antigenic challenge. (M) Infiltration scoring 1h after Ag challenge. (N) BAL tryptase levels. (O) Serum RANTES/CCL5 levels (*p < 0.0005, Student's t-test). (P) Transwell T cell migration assay. (Except in Fig. 6O, * p < 0.05; ** p < 0.01, *** p < 0.001, ^ p < 0.0001, one-way ANOVA).
To substantiate the notion that activated MC secrete chemokines capable of recruiting T cells in a S1PR2-dependent manner, we examined T cell migration in vitro, using a transwell assay 28. Consistent with previous studies 27, 28, T cell migration was greatly enhanced by supernatants from IgE/Ag-activated BMMC (Fig 6P). T cell chemotactic activity was significantly lower in supernatants from activated BMMC treated with JTE-013, providing further evidence of mast cell S1PR2-driven T cell chemotaxis.
Taken together, the observations that neutralizing S1P and antagonism of S1PR2 reduce the secretion of potent chemokines that recruit T lymphocytes highlight the importance of intact MC S1PR2 signaling for adequate chemokine secretion and provide a mechanistic explanation for the early T cell infiltration observed in pulmonary allergic reaction.
Functional S1P/S1PR2 axis on MC triggers Stat3 activation and is required for chemokine release
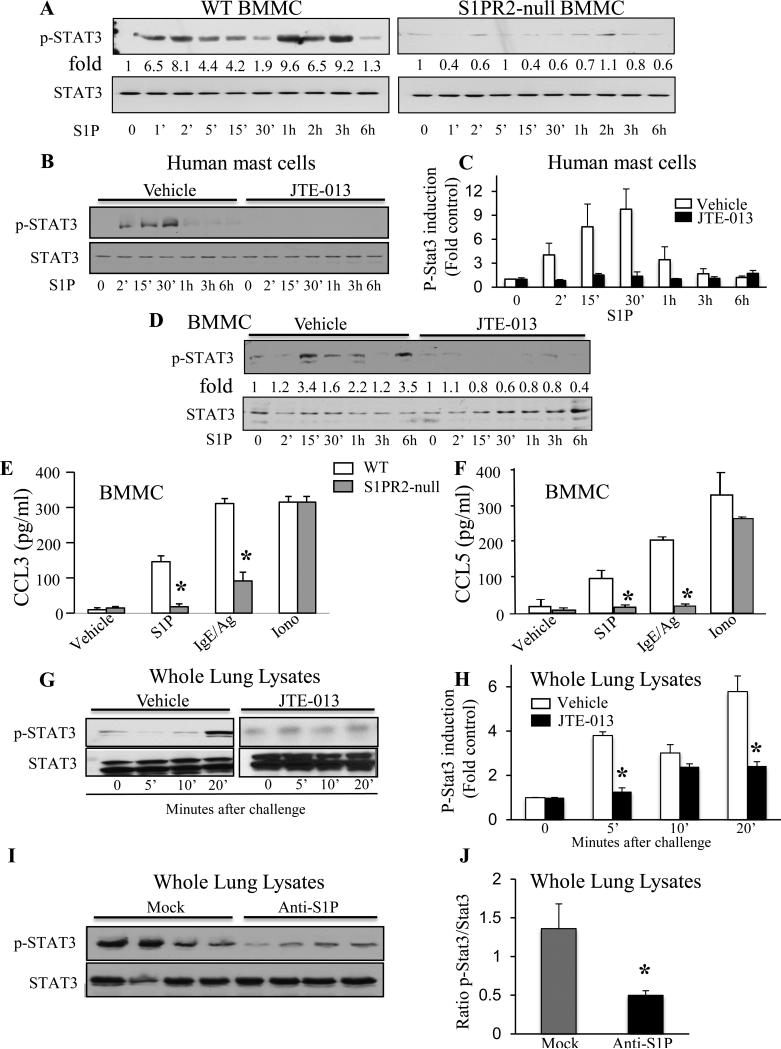
We next sought to determine the signaling pathways downstream of S1PR2 that are involved in S1PR2-driven chemokine secretion from mast cells utilizing BMMC prepared from WT and S1PR2-null mice. Phosphorylation of ERK1/2 and Akt by S1P were not affected by S1PR2 deletion (data not shown). Remarkably, S1P exposure triggered Stat3 activation in WT BMMC, as demonstrated by increased phosphorylation on Tyr705, that was almost completely absent in S1PR2-null BMMC (Fig 7A). Moreover, S1P-mediated Stat3 activation in both human MC (Fig 7B-C) and mouse BMMC (Fig 7D) was eliminated by JTE-013 treatment, further supporting the role of MC S1PR2 in Stat3 activation. Consistent with these results, S1P- or IgE/Ag-induced secretion of the chemokines CCL3 (Fig 7E) and CCL5 Fig 7F) were greatly attenuated in S1PR2-null BMMC compared to WT, while ionomycin-induced chemokine secretion was unaffected.
Fig. 7.
Activation of Stat3 by the S1P/S1PR2 axis in MC in vitro and in lungs after Ag challenge. (A) WT or S1PR2-null BMMC or (B-C) vehicle- or JTE-013-pretreated human MC or (D) WT BMMC were stimulated with S1P (100 nM) for the indicated times. Cell lysates were immunoblotted with the indicated Abs and p-Stat3 was quantified. Activated-BMMC supernatants were assayed for (E) CCL3 and (F) CCL5. (G-H) Lung lysates from mice treated with vehicle or JTE-013 prior to IgE/Ag challenge were immunoblotted as above. (I) Immunoblots of lung lysates from mice treated with anti-S1P or mock mAb prior to IgE/Ag challenge and (J) quantification of pStat3/Stat3 ratios. (*p<0.04, Student's t-test).
Importantly, in vivo Stat3 activation was observed 20 min after Ag challenge in the lungs of sensitized mice (Fig 7G-H). Pretreatment with JTE-013, which reduced chemokine secretion (Fig 6O and 12) and prevented early T cell recruitment (Fig 6), also suppressed Stat3 activation (Fig 7G-H). Moreover, increased Stat3 phosphorylation after Ag challenge was greatly reduced in mice treated with anti-S1P mAb but not affected by treatment with a nonspecific mock mAb (Fig 7I-J). Therefore, MC S1PR2 stimulation by S1P is important for activation of Stat3, a master transcription factor that regulates expression of many chemokines 29, 30 and contributes to T cell recruitment in MC-dependent allergic responses.
DISCUSSION
The distinctive ability of MC to produce a wide variety of preformed and de novo synthesized bioactive products makes these tissue-dwelling cells first-line regulators of many immune functions 1. S1P is produced by MC and secreted upon IgE/Ag activation. It can then activate its S1PR1 and S1PR2 receptors in an autocrine or paracrine manner to regulate degranulation, cytokine and chemokine production, and chemotaxis towards Ag 6. Investigations of S1P functions during a systemic allergic reaction uncovered an important role for S1PR2 on MC and also on vascular endothelial cells, exerting seemingly contrasting effects at early versus late stages of anaphylaxis 12, 15. We found that activation of S1PR2 on tissue-resident MC was involved in the early development of pulmonary edema and labored breathing in sensitized mice after systemic Ag challenge 12. However, at later stages, high plasma S1P levels aid recovery from shock by counteracting hypotension and promoting plasma histamine clearance, an effect attributed to activation of S1PR2 in the vasculature 15. A recent publication by Olivera et al. explains these apparent discrepancies 31. First, lack of S1PR2 reduced IgE/Ag-induced degranulation only in certain types of MC in vitro. Second, IgE from different sources differed in their ability to elicit MC signaling and effector responses (termed cytokinergic ability) and induced differential S1PR2-dependent anaphylactic responses. In contrast to the protective effects induced by moderately cytokinergic IgE, a strongly cytokinergic IgE induced a more acute response with increased lethality in WT mice compared to S1PR2-null mice. Using a mouse model of active anaphylaxis, a study by Cui et al. also identified a new signaling pathway comprising endothelial S1PR2-driven Akt and nitric oxide synthase inhibition in vascular integrity 32. Of note, these previous studies focused on S1PR2 functions in vascular tone and histamine clearance impairment whereas our current study investigates the role of S1PR2-dependent MC-mediated cytokine/chemokine production and T cell recruitment.
The importance of S1P production in murine asthma models is well established 7, 11, 33, 34. In agreement, we found that a highly specific anti-S1P neutralizing mAb that is now in Phase 2 clinical trials 35 significantly attenuated MC-dependent systemic allergic responses and decreased T cell lung infiltration likely due to the distinct ability of this Ab to predominantly block local tissue S1P 36.
Homeostatic S1P tissue levels are low compared to circulating concentrations, thus augmented local S1P levels in inflamed tissues are likely to perturb the existing S1P gradient between tissues and the circulation, which controls lymphocyte trafficking 37. We recently reported increased levels of lung S1P in a MC-dependent mouse model of asthma, which were normalized after administration of a specific SphK1 inhibitor 11. Resident MC could be a source of peripheral S1P at sites of inflammation. As Sphingomab inhibits in vitro MC activation, systemic S1P neutralization in vivo likely suppresses MC-derived pro-inflammatory mediator release.
The relationship between airway dysfunction and inflammation is not completely understood. Our data underpin the occurrence of an early onset of T cell recruitment, leading to massive infiltration within 1 h after Ag challenge. Interestingly, few macrophages were also recruited and almost no eosinophils or neutrophils were detected. Our results suggest that MC-derived mediators are involved in early T cell recruitment into inflamed lungs as this is greatly diminished in KitW-sh/W-sh mice devoid of MC (but restored in MC-reconstituted KitW-sh/W-sh mice), consistent with reduced chronic inflammation in an asthma model 38. Reconstitutions of KitW-sh/W-sh mice were conducted employing intraperitoneal rather than intravenous injections of WTBMMC, as it was reported that intravenous injection of BMMC resulted in artificially increased number of MC in the lung parenchyma of BMMC-engrafted Kit W-sh/W-sh mice, compared to Kit+/+ mice 17. Specifically, the absence of MC correlated with significantly reduced levels of circulating histamine, CCL2, CCL3 and CCL5 after IgE/Ag triggering. It is striking that early T cell recruitment to lung perivascular localization and MC-derived T cell chemokine production were strongly mitigated upon S1P neutralization, as well as S1PR2 antagonism or deficiency, making it likely that the S1P/S1PR2 axis serves as a dominant link between activated MC and T cell recruitment. These data are consistent with previous work showing that a humanized version of the anti-S1P mAb substantially reduced macrophage infiltration in a mouse model of choroidal neovascularization 39.
Several MC-derived mediators could contribute to lymphocyte recruitment 1, 40. Many studies have highlighted the critical role of locally activated MC in mediating T cell recruitment 41, 42 and chemokine production 27, 43. It is noteworthy that allergy-promoting Th2 cell recruitment is mediated by CCR4 ligands, such as CCL2, CCL3, CCL5 and CCL17. Importantly, we found that the rapid increase of all four chemokines in serum following Ag challenge was attenuated by neutralizing extracellular S1P, and was dependent on the S1P/S1PR2 axis and the presence of MC. Our results point to a unique signaling pathway in MC linking S1PR2 to chemokine formation mediated by Stat3 activation. In this regard, Stat3 is a critical regulator of CCL2 and CCL3 expression 29, 44. Moreover, RANTES/CCL5 transcription is dependent on the binding of a p65/Stat3 complex to NF-kappaB-binding sites within its promoter 30. Although the S1P/S1PR1 axis is involved in Stat3 activation in cancer cells 45-47, in MC, as in muscle satellite cells 48 and in cardiomyocytes 49, S1P/S1PR2 signaling plays a critical function as S1PR2 deficiency abrogated Stat3 phosphorylation. Although activation of S1PR2 directly can activate Stat3 in a Rho-dependent manner 48, the possibility cannot be excluded that IL-6 released from MC could also contribute to Stat3 activation 50. Interestingly, we previously demonstrated that secretion of IL-6 from MC is also dependent on the S1P/S1PR2 axis 12. Consistent with our findings, a recent study revealed compromised allergic reactions and MC degranulation in patients harboring Stat3 mutations, as well as in human MC transduced with shRNAs against Stat3 and in a corresponding mouse model of Stat3 mutation 51. Taken together, our data suggest that S1P/S1PR2 signaling in MC leads to activation of Stat3 and chemokine production which in turn plays an important role in early T cell mobilization in acute pulmonary allergic responses.
Our study furthers understanding of MC as early inflammatory orchestrators and offers a new paradigm for the initiation of allergic Th2 immunity that supports the importance of MC functions in inflammatory disease etiology.
Supplementary Material
KEY MESSAGES.
Kinetic analyses of acute mast cell-dependent allergic inflammatory events triggered after a single dose of antigen administered systemically revealed early T cell recruitment in the lungs of sensitized mice, an event originally associated with the late-phase response.
T cell recruitment was driven by mast cell activation via S1PR2 signaling, leading to Stat3 activation and release of T cell chemokines.
Targeting early T cell recruitment to inflamed lungs via a novel pathway linking mast cell S1PR2/Stat3 signaling to chemokine secretion may offer a relevant approach for treating patients with inflammatory diseases to prevent chronic inflammation.
CAPSULE SUMMARY.
Early T cell recruitment occurs in an acute mast cell-dependent preclinical model of allergic reaction via S1PR2/Stat3 signaling and chemokine production. S1P neutralization with Sphingomab™ or S1PR2 inactivation/deficiency suppresses local and systemic inflammation.
ACKNOWLEDGMENTS
We are grateful to Dr. Daniel Conrad for kindly providing IgE. We thank Dr. Christine Tkaczyk, Medimmune LLC, Infectious Diseases Department, Gaithersburg, MD, for generously sharing the mouse lung digestion protocol.
This work was supported by National Institutes of Health (NIH) grants K01AR053186 and R01 AI095494 to C.A.O., R01 AI50094 to S.S. and U19 AI077435 to S.S. and J.J.R.
Abbreviations used
- BMMC
Bone marrow-derived mast cell
- HR
hyperresponsiveness
- Sk-MC
human skin mast cells
- MC
mast cell
- MIP-1α
macrophage inflammatory protein 1 alpha
- RANTES
regulated on activation, normal T cell expressed and secreted
- S1P
sphingosine-1-phosphate
- S1PR2
S1P receptor 2
- Stat3
signal transduced and activator of transcription 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflicts of interest: S. Spiegel and J.J. Ryan have received a grant from the Asthma and Allergic Diseases Cooperative Research Centers. S. Spiegel, J.J. Ryan and C.A. Oskeritzian have received grants from the National Institutes of Health. Roger Sabbadini is the founder and a shareholder at Lpath Inc. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–70. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–63. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–9. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roviezzo F, D'Agostino B, Brancaleone V, De Gruttola L, Bucci M, De Dominicis G, et al. Systemic administration of sphingosine-1-phosphate increases bronchial hyperresponsiveness in the mouse. Am J Respir Cell Mol Biol. 2010;42:572–7. doi: 10.1165/rcmb.2009-0108OC. [DOI] [PubMed] [Google Scholar]
- 8.Trifilieff A, Fozard JR. Sphingosine-1-phosphate-induced airway hyper-reactivity in rodents is mediated by the sphingosine-1-phosphate type 3 receptor. J Pharmacol Exp Ther. 2012;342:399–406. doi: 10.1124/jpet.112.191585. [DOI] [PubMed] [Google Scholar]
- 9.Marsolais D, Yagi S, Kago T, Leaf N, Rosen H. Modulation of chemokines and allergic airway inflammation by selective local sphingosine-1-phosphate receptor 1 agonism in lungs. Mol Pharmacol. 2011;79:61–8. doi: 10.1124/mol.110.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karmouty-Quintana H, Siddiqui S, Hassan M, Tsuchiya K, Risse PA, Xicota-Vila L, et al. Treatment with a sphingosine-1-phosphate analog inhibits airway remodeling following repeated allergen exposure. Am J Physiol Lung Cell Mol Physiol. 2012;302:L736–45. doi: 10.1152/ajplung.00050.2011. [DOI] [PubMed] [Google Scholar]
- 11.Price MM, Oskeritzian CA, Falanga YT, Harikumar KB, Allegood JC, Alvarez SE, et al. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. J Allergy Clin Immunol. 2013;131:501–11. e1. doi: 10.1016/j.jaci.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–74. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol. 2012;188:1809–18. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 15.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–240. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–52. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 17.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai WQ, Goh HH, Bao Z, Wong WS, Melendez AJ, Leung BP. The role of sphingosine kinase in a murine model of allergic asthma. J Immunol. 2008;180:4323–9. doi: 10.4049/jimmunol.180.6.4323. [DOI] [PubMed] [Google Scholar]
- 19.Speiran K, Bailey DP, Fernando J, Macey M, Barnstein B, Kolawole M, et al. Endogenous suppression of mast cell development and survival by IL-4 and IL-10. J Leukoc Biol. 2009;85:836–36. doi: 10.1189/jlb.0708448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–97. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien N, Jones ST, Williams DG, Cunningham HB, Moreno K, Visentin B, et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res. 2009;50:2245–57. doi: 10.1194/jlr.M900048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242:31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 24.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 25.Koya T, Takeda K, Kodama T, Miyahara N, Matsubara S, Balhorn A, et al. RANTES (CCL5) regulates airway responsiveness after repeated allergen challenge. Am J Respir Cell Mol Biol. 2006;35:147–54. doi: 10.1165/rcmb.2005-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V, et al. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat Immunol. 2011;13:67–76. doi: 10.1038/ni.2173. [DOI] [PubMed] [Google Scholar]
- 27.McAlpine SM, Issekutz TB, Marshall JS. Virus stimulation of human mast cells results in the recruitment of CD56(+) T cells by a mechanism dependent on CCR5 ligands. FASEB J. 2012;26:1280–9. doi: 10.1096/fj.11-188979. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J, et al. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180:2039–43. doi: 10.4049/jimmunol.180.4.2039. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2013;288:1489–99. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacic JC, Gupta R, Lee AC, Ma M, Fang F, Tolbert CN, et al. Stat3-dependent acute Rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice. J Clin Invest. 2010;120:303–14. doi: 10.1172/JCI40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivera A, Dillahunt SE, Rivera J. Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE and IgG-mediated anaphylaxis with minimal effect on its onset. Immunol Lett. 2013;150:89–96. doi: 10.1016/j.imlet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui H, Okamoto Y, Yoshioka K, Du W, Takuwa N, Zhang W, et al. Sphingosine-1-phosphate receptor 2 protects against anaphylactic shock through suppression of endothelial nitric oxide synthase in mice. J Allergy Clin Immunol. 2013;132:1205–14. doi: 10.1016/j.jaci.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, et al. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1085–L93. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 34.Chiba Y, Suzuki K, Kurihara E, Uechi M, Sakai H, Misawa M. Sphingosine-1-phosphate aggravates antigen-induced airway inflammation in mice. Open Respir Med J. 2010;4:82–5. doi: 10.2174/1874306401004010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol. 2011;162:1225–38. doi: 10.1111/j.1476-5381.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sensken SC, Nagarajan M, Bode C, Graler MH. Local inactivation of sphingosine 1-phosphate in lymph nodes induces lymphopenia. J Immunol. 2011;186:3432–40. doi: 10.4049/jimmunol.1002169. [DOI] [PubMed] [Google Scholar]
- 37.Olivera A, Allende ML, Proia RL. Shaping the landscape: Metabolic regulation of S1P gradients. Biochim Biophys Acta. 2013;1831:193–202. doi: 10.1016/j.bbalip.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–59. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 39.Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro PA. Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J Cell Physiol. 2009;218:192–8. doi: 10.1002/jcp.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyahara N, Ohnishi H, Miyahara S, Takeda K, Matsubara S, Matsuda H, et al. Leukotriene B4 release from mast cells in IgE-mediated airway hyperresponsiveness and inflammation. Am J Respir Cell Mol Biol. 2009;40:672–82. doi: 10.1165/rcmb.2008-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalo JA, Qiu Y, Lora JM, Al-Garawi A, Villeval JL, Boyce JA, et al. Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol. 2007;179:1740–50. doi: 10.4049/jimmunol.179.3.1740. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Macneil AJ, Junkins R, Li B, Berman JN, Lin TJ. Mast cell FcεRI-induced early growth response 2 regulates CC chemokine ligand 1-dependent CD4+ T cell migration. J Immunol. 2013;190:4500–7. doi: 10.4049/jimmunol.1203158. [DOI] [PubMed] [Google Scholar]
- 43.Ohsawa Y, Hirasawa N. The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via anti-pruritic and anti-inflammatory effects in NC/Nga mice. Allergy. 2012;67:1014–22. doi: 10.1111/j.1398-9995.2012.02854.x. [DOI] [PubMed] [Google Scholar]
- 44.Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768–79. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–8. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng J, Liu Y, Lee H, Herrmann A, Zhang W, Zhang C, et al. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642–54. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang W-C, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–20. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh KC, Leong WI, Carlson ME, Oskouian B, Kumar A, Fyrst H, et al. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS One. 2012;7:e37218. doi: 10.1371/journal.pone.0037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frias MA, James RW, Gerber-Wicht C, Lang U. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1-phosphate. Cardiovasc Res. 2009;82:313–23. doi: 10.1093/cvr/cvp024. [DOI] [PubMed] [Google Scholar]
- 50.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 51.Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung MY, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132:1388–96. doi: 10.1016/j.jaci.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.