Abstract
Objective
To investigate the association between the presence of sperm in the vasal fluid during vasectomy reversal (VR) and postoperative patency.
Methods
We performed a systematic review and meta-analysis of the English-language literature reporting on the association between the presence of sperm in the intraoperative vasal fluid (i.e., whole/parts versus none) and patency (i.e., patent or not) after microsurgical vasovasostomy (VV) for men with obstructive azoospermia due to vasectomy. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to quantify the strength of the association reported by each study. Meta-analysis was performed using a random effects model.
Results
Four case series and two retrospective cohort studies of a total of 1,293 eligible patients were identified. The mean age at VR was 37.8 years and the mean obstructive interval was 7.1 years. The unadjusted odds of postoperative patency were 4.1 (95% CI: 2.3 to 7.3) times higher given the presence of intravasal sperm or sperm parts as opposed to their absence at the time of VR (Q = 3.4; df = 5; p = 0.6; I2 = 22%). The pooled OR should be interpreted with caution as only the two retrospective cohort studies reported meaningful data on this association. Because of inconsistent reporting, analysis of other vasal fluid characteristics (e.g., consistency) and outcomes (e.g., pregnancy) was not possible.
Conclusion
The presence of whole sperm or sperm parts in the vasal fluid during VR is positively associated with postoperative patency. Our review highlights the poor methodological quality of existing evidence and underscores the need for more thorough follow up and higher standards of reporting in future studies.
Keywords: Azoospermia, vasovasostomy, microsurgery
Introduction
About 175,000 to 354,000 vasectomies are performed in the United States each year 1, and up to 6% of patients who undergo this procedure later choose to undergo vasectomy reversal (VR) 2. A landmark multi-center study on the outcomes of 1,469 patients who underwent VR was published by the Vasovasostomy Study Group in 1991 3. They demonstrated that a longer obstructive interval and an absence of sperm granuloma on physical exam were associated with decreased patency following VR. Other factors that appeared to influence success of VR were the character of the vasal fluid and the presence of sperm or sperm parts at the time of reversal.
During surgery, the physician's decision to proceed with vasovasostomy (VV) or epididymovasostomy (EV) depends on the gross quality of fluid expressed from the testicular end of the vas deferens and on the microscopic examination of the fluid for sperm. Findings may include motile or non-motile whole sperm, sperm parts (i.e., sperm heads or tails alone), or no sperm. VV is routinely performed if whole sperm are identified in the vasal fluid or if the fluid appears clear and copious, even in the absence of sperm 4. In contrast, when the fluid quality is poor (i.e., paste-like) and sperm are absent, EV is generally required. Modern series indicate that patency after VV approaches 99.5% when whole sperm are identified 5. Even in cases of bilateral intravasal azoospermia, patency in some series approaches 80% and achievement of pregnancy approaches 38% when the obstructive interval is less than 11 years 6. Unfortunately, studies that have carefully evaluated and reported data on vasal fluid quality have generally been small, retrospective, and based on data from single institutions.
A meta-analysis evaluating the outcomes of VV did not assess intraoperative vasal fluid characteristics 7. Although one other group has reviewed this topic 8, the data were not synthesized using a meta-analytic framework. Therefore, we performed a systematic review and meta-analysis of the published literature to evaluate the association between the presence of sperm or sperm parts in the vasal fluid and patency following VV.
Materials and Methods
Study Design
This study was a systematic review and meta-analysis. An a priori protocol was written and agreed to by the authors to include study design, search strategy, inclusion and exclusion criteria, primary outcomes, statistical methods, and bias assessment. We followed the PRISMA guidelines for performing and reporting a meta-analysis.
Literature Search
English-language studies reporting on outcomes of microscopic VV for VR between November 1977 (the first report of microsurgical VV 9) and March 2014 were sought by electronic search of MEDLINE, scanning the reference lists of identified articles, and correspondence with study investigators. The computer-based search included variations of the terms “vasectomy reversal” and “vasovasostomy.”
Study Selection
Studies were eligible for inclusion if they employed a microscopic VV approach and reported on outcomes for ≥ 10 patients. Studies of patients undergoing VR for reasons other than a desire for fertility such as a history of epididymitis, hernia repair, idiopathic obstruction, or trauma were excluded. If multiple publications reporting on the same patient population were identified, only the latest study was included.
Data Collection
The following information was independently extracted by two reviewers from each article using a standardized form: study population (including population source, sampling method employed, sample size, and patient demographic characteristics); geographic location; publication year; mean patient age and obstructive interval at the time of VR; number of patients with sperm or sperm parts in intraoperative vasal fluid; definition of postoperative patency; and number of patients achieving patency.
Data Synthesis
All analyses were performed using only within-study comparisons to limit possible biases. The mean age and obstructive interval at VR reported by each study were combined and summarized using an arithmetic mean weighted by study sample size. An odds ratio (OR) and corresponding 95% confidence interval (CI) for the association between the presence of intravasal sperm or sperm parts and postoperative patency were calculated for each study. In order to include the results of case series reporting incomplete data, 0.5 was added to each count in two-by-two contingency tables that contained a value of zero in any cell 10. Meta-analysis was performed using a random effects model. The consistency of findings across studies was assessed using Cochrane's Q test11 and the I2 statistic 12. Publication bias was assessed by funnel plot and Egger et al. regression asymmetry analysis. Statistical significance was defined as a two-tailed p value < 0.05. Analyses were performed using R version 3.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Studies Included for Systematic Review and Meta-analysis
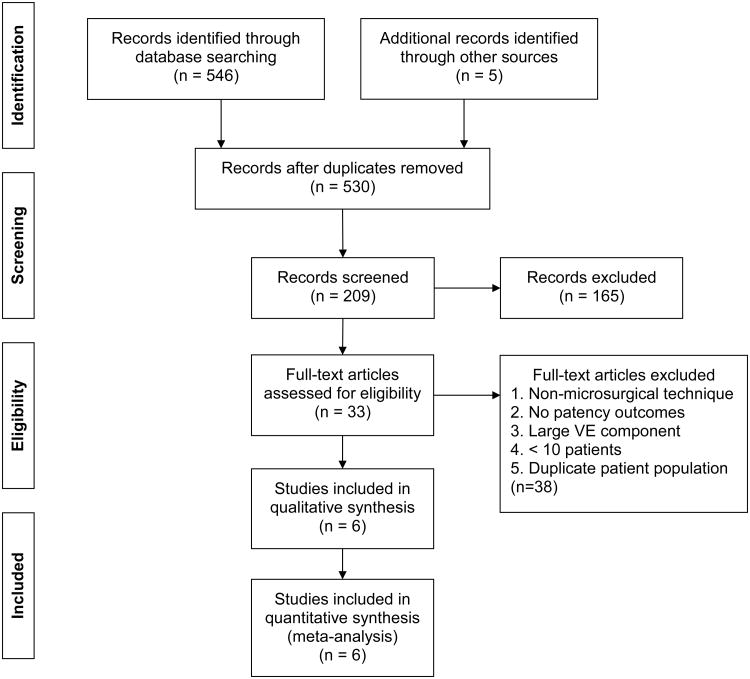
Four case series 6,13-15 and two retrospective cohort studies 3,16 of 1,293 eligible patients were identified (Figure 1). The studies were published between 1977 and 2014 and took place in Asia, Australia, and the United States (Table 1). Reported sample sizes ranged from 11 to 605 patients undergoing microsurgical VV. The weighted mean patient age was 37.8 years and obstructive interval was 7.1 years at the time of VR. The studies used wet-mount light microscopy to assess for the presence of sperm in the vasal fluid.
Figure 1. PRISMA flow diagram of study selection.
Table 1. Characteristics of studies reporting outcomes on microsurgical vasovasostomy.
| First Author | Year | Sperm present in vasal fluid | Sperm absent in vasal fluid | Mean age (y) | Mean obstructive interval (mo) | ||
|---|---|---|---|---|---|---|---|
| Patent (n) | Not Patent (n) | Patent (n) | Not Patent (n) | ||||
| Belker(3) | 1991 | 437 | 49 | 50 | 33 | 36.0 | 84.0 |
| Sheynkin(13) | 2000 | 0 | 0 | 7 | 8 | 40.1 | 108.0 |
| Sigman(14) | 2004 | 51 | 1 | 6 | 0 | 39.0 | 118.8 |
| Kolettis(6) | 2005 | 26 | 8 | 0 | 0 | 42.0 | 120.0 |
| Bolduc(16) | 2007 | 468 | 83 | 36 | 19 | 37.2 | 82.0 |
| Smith(15) | 2014 | 10 | 1 | 0 | 0 | 40.2 | 82.8 |
The definition of patency used by the studies varied markedly. The Vasovasostomy Study Group 3 and Sigman et al. 14 defined patency as the presence of whole sperm or sperm parts in the postoperative semen analysis; Sheynkin et al.13 defined it as the presence of whole sperm but not sperm parts; and Bolduc et al. 16, Kolettis et al. 6, and Smith et al. 15 defined it as the presence of motile sperm. Three of the six studies 3,15,16 defined whole sperm as being “mostly normal and motile” or “mostly normal and non-motile.” In these three studies, sperm parts were defined as “mostly heads without tails” or “only heads without tails” on intraoperative examination. Sigman et al. defined sperm parts as “short tails” or “sperm heads” 14. Kolettis et al. and Sheynkin et al. did not differentiate between whole sperm and sperm parts 6,13.
Meta-analysis
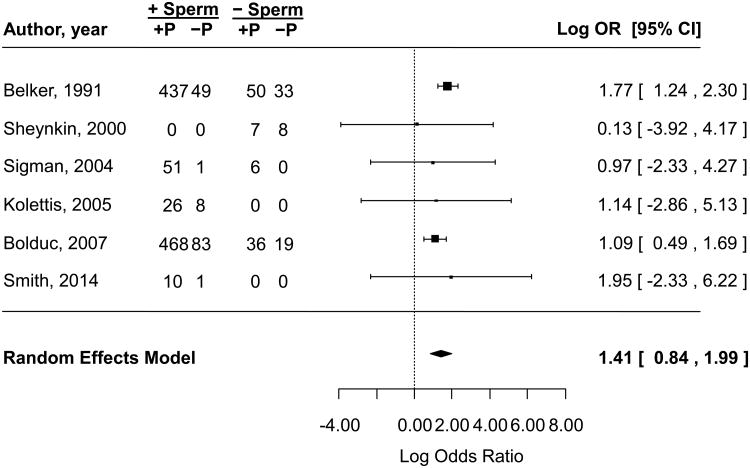
The unadjusted odds of postoperative patency were 4.1 (95% CI: 2.3 to 7.3) times higher given the presence of intravasal sperm or sperm parts as opposed to their absence at the time of VR (Figure 2). The pooled OR should be interpreted with caution as only the two retrospective cohort studies reported meaningful data on this association. Although surrogate statistical techniques were used to include case series in this meta-analysis 10, their data did not contribute significantly to the pooled OR. There was no evidence of statistically significant heterogeneity among the six studies (Q = 3.4, df = 5, p = 0.6; I2 = 22%), nor was there obvious publication bias by funnel plot (Figure 3) or Egger et al. regression analysis (z = −0.4, p = 0.7).
Figure 2.
Association between the presence of sperm in the vasal fluid during vasectomy reversal and postoperative patency in identified retrospective studies. The summary estimate was calculated using a random effects model. Exponentiation of the pooled log(OR) plotted above yielded an unadjusted OR of 4.1 (95% CI: 2.3 to 7.3). The pooled log(OR) calculated using a fixed effect model was 1.5 (95% CI: 1.1 to 1.9) with a corresponding OR of 4.3 (95% CI: 2.9 to 6.4). Assessment of heterogeneity: Q = 3.4, df = 5, p = 0.6, I2 = 22%. CI = confidence interval, OR = odds ratio, P = patent.
Figure 3.
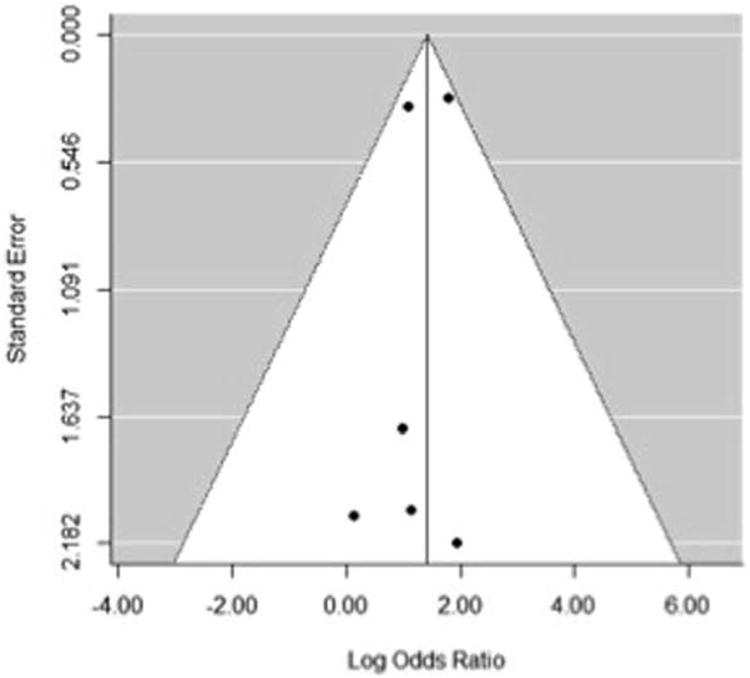
Funnel plot assessment of publication bias. Egger et al. regression analysis yielded: z = −0.4, p = 0.7.
Sensitivity Analysis
A sensitivity analysis in which case series were excluded yielded an unadjusted OR of 4.3 (95% CI: 2.2 to 8.3) and revealed some inconsistency among the findings of the two remaining studies by I2 criteria (Q = 2.8, df = 1, p = 0.1; I2 = 64%).
Discussion
More than 20 years have passed since the landmark study of the Vasovasostomy Study Group 3, yet these results are still often cited to patients desiring VR. A review of the literature revealed post-reversal patency percentages ranging from approximately 69 to 98% 17,18 with achievement of pregnancy in 37 to 93% of couples 19,20. The precise extent to which the presence of sperm in the intraoperative vasal fluid is associated with postoperative patency remains uncertain. To help clarify and quantify the available evidence to better counsel patients desiring VR, we performed a focused systematic review and meta-analysis of 1,239 patients undergoing VV in six retrospective studies. Our findings suggest that the presence of whole sperm or sperm parts in the vasal fluid during VR is positively associated with postoperative patency. This conclusion relies on indirect comparisons of retrospective studies with a majority of data coming from two cohorts, importantly influencing the meta-analytic outcome.
A finding of intravasal azoospermia during VR creates a difficult decision for the surgeon who must determine whether VV or VE is the best choice for vasal reconstruction. Since microscopic examination of vasal fluid is not universally performed, certainly some of the failures with VV may have occurred in patients who actually required a VE because of epididymal obstruction. Of course, performing VE rather than VV is an intraoperative decision, and it remains difficult to determine prior to surgery which patients will have epididymal obstruction and require VE. For our review, we sought a homogenous population of patients who underwent VV only, which is the more widely available and commonly performed technique. Given the wide variations in technique for VE, the availability of VE (either due to training or practice pattern), and the lower chance of postoperative patency and pregnancy, we did not include VE patients in this analysis. We also excluded papers reporting reconstruction for reasons other than prior vasectomy.
At our institution we perform intraoperative vasal fluid analysis in the operating room. Vasal fluid is examined using an overhead camera at × 400 magnification. We classify gross quality of vasal fluid as creamy vs. cloudy vs. clear. We classify microscopic examination as whole motile sperm vs. whole non-motile sperm vs. sperm heads / short tails vs. azoospermia. Based on the review of the literature, we perform VE only in cases of intravasal azoospermia. Gross quality of fluid may be used to prognosticate success with vasectomy reversal but has minimal influence of intraoperative decision making between performing a vasovasostomy or a vasoepididymostomy. During the operation we use a standardized form to record intraoperative findings (Supplemental Figure 1).
There were several major weaknesses in almost all of the studies analyzed. The marked heterogeneity in the definitions of the presence of sperm at microscopy and patency made comparisons across studies difficult. Furthermore, some studies did not describe the technique used to evaluate sperm intraoperatively. Few studies reported on the quality of vasal fluid and even fewer provided data on the microscopic examination of the vasal fluid. Patency after VV may also depend on multiple variables, including surgeon experience, obstructive interval, age of the female partner, whether the female partner is the same individual before and after VR, and female factor infertility 3,21-24. Unfortunately, there was a lack of consistent reporting of these factors. In fact, there was no report of female factor among studies included in the final analysis. Because of this inconsistent reporting, meta-analysis of other vasal fluid characteristics (e.g., fluid consistency) and outcomes (e.g., pregnancy) was not possible, and we were unable to statistically adjust our primary analysis of vasal sperm to better determine whether it provides independently meaningful information in the operating room.
An additional weakness of our meta-analysis was that it depended on the published literature. Because of the relatively small number of studies available, we made the decision to include case series, primarily to illustrate the deficiencies in the available data. This decision did not significantly affect the pooled OR as evidenced by our sensitivity analysis in which these studies were excluded. Although the current literature-based meta-analysis has provided the most comprehensive assessment yet of vasal sperm and postoperative patency, it has relied on aggregated published data rather than on individual patient data, which would be preferred. Undoubtedly, there is also publication bias in reporting only good outcomes after VR, despite the fact that this bias was not obviously detected in our statistical analyses. We were also unable to control for surgical technique, although this may also be construed as a strength, as an analysis using a large number of surgeons may imply reproducibility. However, it must be noted that the number of men who had VV performed when sperm were absent in the vasal fluid was much smaller than the comparison group of men who had sperm present. Many men with intravasal azoospermia likely would have undergone EV, which would bias our analysis. Nonetheless, despite the limitations of the data, we believe our study is robust in critically evaluating the published literature on the presence of sperm in vasal fluid during VV.
Our meta-analysis emphasizes the need for more prospective studies of VR outcomes with standardized reporting measures if we are to truly define measures of surgical success. We recommend that future studies include data on age (both patient and partner), length of obstructive interval, gross intravasal fluid appearance (i.e., clear, cloudy, creamy, or pasty), presence of intravasal whole sperm and/or sperm parts (i.e., sperm heads or tails alone), length of testicular vas remnant, presence of granuloma, and achievement of patency and pregnancy. Larger prospective studies involving concomitant measurement and reporting of these variables are needed in particular to address the important question of whether the presence of vasal sperm constitutes a useful independent clinical factor for intraoperative decision making.
Conclusions
We have performed a systematic review and meta-analysis of VV outcomes in the current era. The odds of postoperative patency were approximately four times higher given the presence of intravasal sperm or sperm parts as opposed to their absence at the time of VR. There was marked variability in outcomes and factors reported. Our review highlights the poor methodological quality of existing evidence and underscores the need for more thorough follow up and higher standards of reporting in the future.
Supplementary Material
Supplemental Figure 1. Standardized intraoperative data reporting sheet.
Acknowledgments
Source of Funding: None
Footnotes
Conflicts of Interest: J.M.S., D.A.M., R.R., L.A.H, W.H., and L.I.L. have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisenberg ML, Lipshultz LI. Estimating the number of vasectomies performed annually in the United States: data from the National Survey of Family Growth. J Urol. 2010;184:2068–2072. doi: 10.1016/j.juro.2010.06.117. [DOI] [PubMed] [Google Scholar]
- 2.Sandlow JI, Nagler HM. Vasectomy and vasectomy reversal: important issues. Preface. Urol Clin North Am. 2009;36:xiii–xiv. doi: 10.1016/j.ucl.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Belker AM, Thomas AJ, Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991;145:505–511. doi: 10.1016/s0022-5347(17)38381-7. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz LI, Rumohr JA, Bennett RC. Techniques for vasectomy reversal. Urol Clin North Am. 2009;36:375–382. doi: 10.1016/j.ucl.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998;159:188–190. doi: 10.1016/s0022-5347(01)64053-9. [DOI] [PubMed] [Google Scholar]
- 6.Kolettis PN, Burns JR, Nangia AK, et al. Outcomes for vasovasostomy performed when only sperm parts are present in the vasal fluid. J Androl. 2006;27:565–567. doi: 10.2164/jandrol.05190. [DOI] [PubMed] [Google Scholar]
- 7.Herrel L, Hsiao W. Microsurgical vasovasostomy. Asian journal of andrology. 2013;15:44–48. doi: 10.1038/aja.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elzanaty S, Dohle GR. Vasovasostomy and predictors of vasal patency: a systematic review. Scandinavian journal of urology and nephrology. 2012;46:241–246. doi: 10.3109/00365599.2012.669790. [DOI] [PubMed] [Google Scholar]
- 9.Silber SJ, Galle J, Friend D. Microscopic vasovasostomy and spermatogenesis. J Urol. 1977;117:299–302. doi: 10.1016/s0022-5347(17)58440-2. [DOI] [PubMed] [Google Scholar]
- 10.Haldane JB. The estimation and significance of the logarithm of a ratio of frequencies. Annals of human genetics. 1956;20:309–311. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 11.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Sheynkin YR, Chen ME, Goldstein M. Intravasal azoospermia: a surgical dilemma. BJU international. 2000;85:1089–1092. doi: 10.1046/j.1464-410x.2000.00665.x. [DOI] [PubMed] [Google Scholar]
- 14.Sigman M. The relationship between intravasal sperm quality and patency rates after vasovasostomy. J Urol. 2004;171:307–309. doi: 10.1097/01.ju.0000102322.90257.8b. [DOI] [PubMed] [Google Scholar]
- 15.Smith RP, Kovac JR, Badhiwala N, et al. The significance of sperm heads and tails within the vasal fluid during vasectomy reversal. Indian J Urol. 2014;30:164–168. doi: 10.4103/0970-1591.126898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolduc S, Fischer MA, Deceuninck G, et al. Factors predicting overall success: a review of 747 microsurgical vasovasostomies. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2007;1:388–394. doi: 10.5489/cuaj.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratana-olarn K, Gojaseni P, Muangman V, et al. Vasectomy reversal: experience in Ramathibodi Hospital, Thailand. J Med Assoc Thai. 1982;65:240–245. [PubMed] [Google Scholar]
- 18.Patel SR, Sigman M. Comparison of outcomes of vasovasostomy performed in the convoluted and straight vas deferens. J Urol. 2008;179:256–259. doi: 10.1016/j.juro.2007.08.169. [DOI] [PubMed] [Google Scholar]
- 19.Chiang HS. Clinical study of vasectomy reversal: results of 60 single-surgeon cases in Taiwan. J Formos Med Assoc. 1996;95:866–869. [PubMed] [Google Scholar]
- 20.Silber SJ, Grotjan HE. Microscopic vasectomy reversal 30 years later: a summary of 4010 cases by the same surgeon. J Androl. 2004;25:845–859. doi: 10.1002/j.1939-4640.2004.tb03150.x. [DOI] [PubMed] [Google Scholar]
- 21.Kolettis PN, D'Amico AM, Box L, et al. Outcomes for vasovasostomy with bilateral intravasal azoospermia. J Androl. 2003;24:22–24. [PubMed] [Google Scholar]
- 22.Kolettis PN, Sabanegh ES, D'Amico AM, et al. Outcomes for vasectomy reversal performed after obstructive intervals of at least 10 years. Urology. 2002;60:885–888. doi: 10.1016/s0090-4295(02)01888-5. [DOI] [PubMed] [Google Scholar]
- 23.Kolettis PN, Woo L, Sandlow JI. Outcomes of vasectomy reversal performed for men with the same female partners. Urology. 2003;61:1221–1223. doi: 10.1016/s0090-4295(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 24.Kolettis PN, Sabanegh ES, Nalesnik JG, et al. Pregnancy outcomes after vasectomy reversal for female partners 35 years old or older. J Urol. 2003;169:2250–2252. doi: 10.1097/01.ju.0000063780.74931.d6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Standardized intraoperative data reporting sheet.