Abstract
Peanut (PN) and tree nut (TN) allergies are among the leading causes of fatal food-induced anaphylaxis and are increasing in prevalence, especially in children. Their cosensitization and concurrent clinical allergy have been understudied. This retrospective study investigated the correlation between PN and TN allergy, both in terms of in vitro sensitization (IVS) and clinical allergic manifestations. We conducted a retrospective medical record review at the Allergy Clinic at University Hospital of Brooklyn. Fourteen hundred six charts were reviewed, of which 76 (5.4%) had documented relevant clinical allergy: PN allergy but not TN allergy (n = 29) or TN allergy but not PN allergy (n = 11) or both (n = 30). Six patients with PN allergy but no TN exposure history were not included in the analysis. The majority of patients (67/76, 88.1%) had a concurrent history of asthma, rhinoconjunctivitis, or AD. Sensitivity of TN IVS predicting PN IVS was 38/39 (97%). Similarly, sensitivity of PN IVS predicting TN IVS was 38/42 (91%). Sensitivity of TN clinical allergy predicting PN allergy was 30/59 (51%). Sensitivity of PN clinical allergy predicting TN allergy was 30/41 (73%). The total number of organ systems involved in reported clinical reactions correlated with IVS to TN (p = 0.004) but not IVS to PN (p = 0.983). In summary, we found PN sensitization predicts TN sensitization in vitro, with lower predictability for clinical reactions.
Keywords: Allergy, clinical reaction, cosensitization, cross-reactivity, peanut, sensitization, tree nut
Peanut (PN) and tree nut (TN) allergies can be severe and account for a relatively high proportion of fatal food-induced anaphylaxis.1,2 These allergies can be lifelong3 and appear to be increasing in prevalence, especially in children.4,5 The current prevalence of nut allergy (2%) has doubled in the past 10 years.5 PN and TN allergy are a major health concern.6 PN is a legume, the same family as beans such as soy and peas,7 and is different from TNs. Studies have shown cross-reactive allergen epitopes between PNs and TNs8,9 but whether this results in clinical cross-reactivity remains unclear. This cohort study investigated the correlation between PN and TN allergy, in terms of clinical allergic manifestations and in vitro sensitization (IVS).
METHODS
We conducted a retrospective medical chart review (2003–2012) at the Allergy Clinic at University Hospital of Brooklyn. Cases of children (>3 years old) and adults who had PN and/or TN allergy (include cashew, walnut, almond, hazelnut, pistachio, or pecan) were reviewed. Presence of concurrent allergic disease including allergic rhinoconjunctivitis, asthma, and atopic dermatitis (AD) was also included in the database. This study was approved by the Institutional Review Board from State University of New York Downstate Medical Center. The data were collected without identifiers, and all of the information was kept confidential.
The total number of organ systems involved in clinical reactivity was determined, including dermatologic (urticaria, pruritus, angioedema, swelling, and worsening of AD), respiratory (wheezing, throat tightness, shortness of breath, cough, and tight chest), gastrointestinal (nausea, vomiting, diarrhea, and abdominal pain), and neurological (dizziness and syncope). Degree of IVS was quantified as follows: 0, not reactive (IgE, <0.35 kU/L); 1, weakly reactive (IgE, 0.35–0.7 kU/L); 2, moderately reactive (IgE, 0.7–3.5 kU/L); 3, highly reactive (HR; IgE, 3.5–17.5 kU/L); 4, very highly reactive (VHR; IgE, 17.6–100kU/L; Quest Diagnostics, Madison, NJ). For patients with multiple TN sensitivities by serum IgE, the greatest level of sensitivity was used in statistical analysis. Spearman correlation coefficients were generated to determine the relationship between total allergic reaction organ involvement and level of IVS.
Statistical data analysis was performed using SAS Version 9.3 software (SAS, Cary, NC).
RESULTS
Of 1406 charts were reviewed; 76 (5.4% of 1406) patients were identified with self-reported PN or TN allergy.
Demographic Profile
As shown in Table 1, the majority of this population was African–American (89.4%) and had concurrent allergic disease (88.1%) including allergic rhinoconjunctivitis (57.9%), asthma (25%), and AD (22.3%).
Table 1.
Demographic profile of PN- and/or TN-allergic subjects (n = 76)
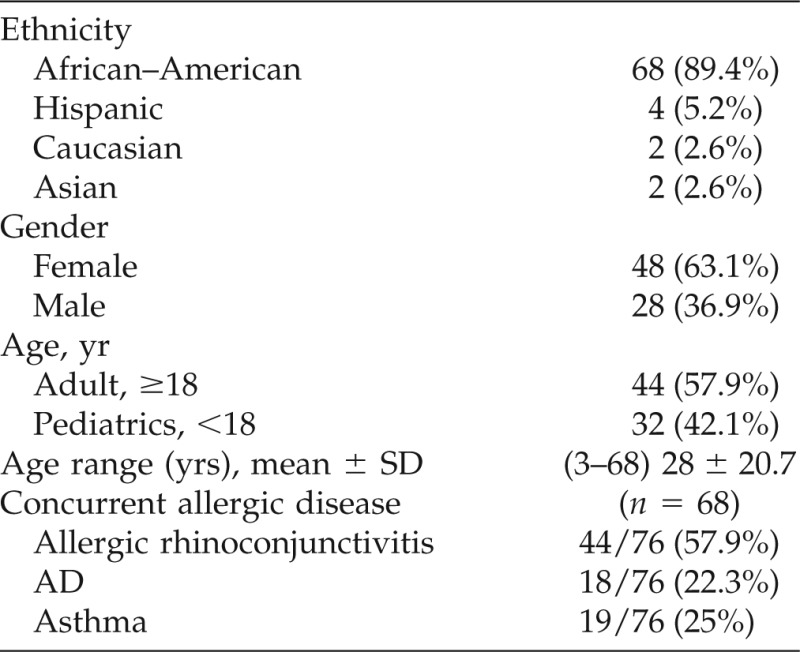
AD = atopic dermatitis; PN = peanut; TN = tree nut.
Of the 76 identified subjects with PN or TN allergy, 8 (10.5%) had no associated second allergic disease. Of these 8 subjects, 6 had PN allergy only, 1 had both PN allergy and hazelnut allergy, and 1 had TN allergy only.
The Most Common Clinical Reaction Was Angioedema
As shown in Table 2, angioedema involving lip, tongue, throat, eye, or whole face was reported as the most common reaction in both PN allergy (55.9%) and TN allergy (46.3%) patients, followed by skin manifestations including urticaria and pruritus in PN (52.5%) and TN (41.4%) allergic patients. Respiratory issues including wheezing, dyspnea, and laryngeal edema were reported in 25.4% of PN allergic patients and in 17% of TN allergy patients. There were no reports of hypotensive symptoms including dizziness or syncope. Anaphylaxis (involving two or more than two systems)10 was reported in 23.7% of PN allergy patients and in 21.9% of TN allergy patients.
Table 2.
Clinical reactions to PN and TN
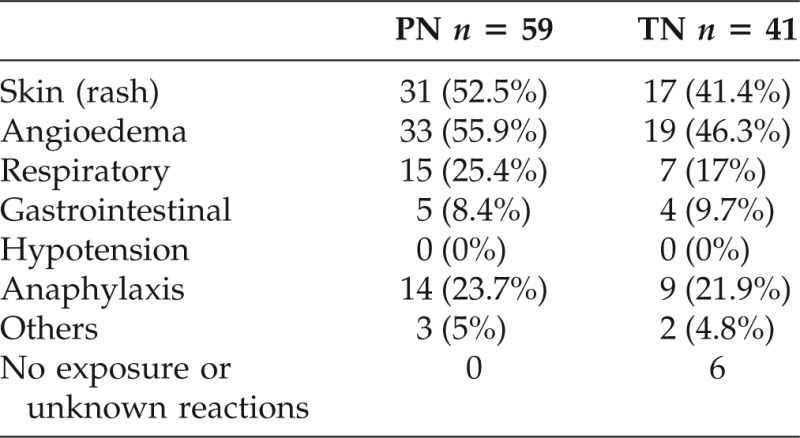
PN = peanut; TN = tree nut.
One-Half of Patients with Clinical PN Allergy Had Clinical Allergy to TN
Of those reporting allergic reactions, 38.1% (29/76) of patients reported PN allergy only, 14.5% (11/76) of patients reported TN allergy only, and 39.5% (30/76) of patients reported both PN and TN allergies. In total, 59/76 (77.6%) patients reported PN allergy and 41/76 (53.9%) patients reported TN allergy.
Of all patients with PN allergy, 30/59 (51%) had documented clinical allergy to TN. Sensitivity of TN clinical allergy predicting PN allergy was 30/59 (51%). Sensitivity of PN clinical allergy predicting TN allergy was 30/41 (73%), (Table 3). Patients with no exposure history or unknown reactions to TN were considered frequency missing and were not included in data analysis.
Table 3.
Frequency of concurrence of PN and TN reported clinical allergy
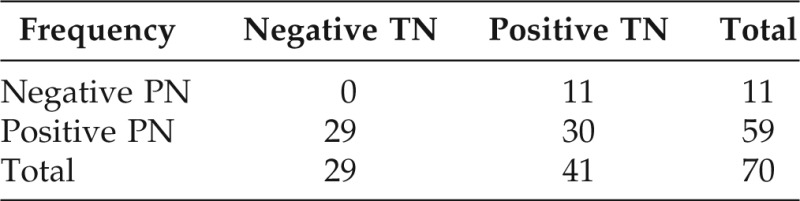
Self-reported clinical allergy reaction to PNs and TNs. Reviewed 1330 cases that can not be characterized—no reaction or do not know.
Frequency missing = 6.
PN = peanut; TN = tree nut.
IVS to PN and TN
The distribution of IVS to PN and TN is shown in Table 4. Sensitivity of TN IVS predicting PN IVS was 38/39 (97%). Similarly, sensitivity of PN IVS predicting TN IVS was 38/42 (91%; Table 4). There was a significant correlation between PN IVS and each type of TN sensitization: hazelnut, p < 0.001; cashew, p = 0.001; walnut, p < 0.001; pecan. p < 0.001; pistachio, p < 0.001; and almond, p = 0.003.
Table 4.
Frequency of concurrence of positive IVS (IgE) to PNs and TNs of patients reporting PN and/or TN allergy (n = 76)
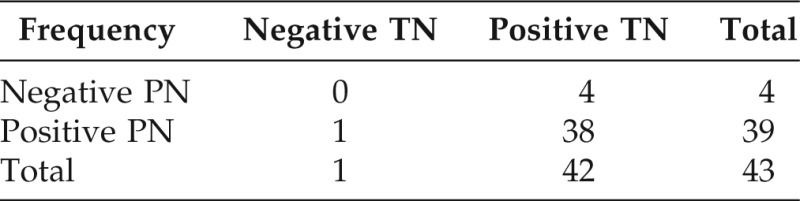
Frequency missing = 21. Reviewed 1330 cases that can not be characterize—no reaction or do not know.
IVS = in vitro (sensitization IgE of TNs or PNs, >0.35 kU/L); PN = peanut; TN = tree nut.
High PN or TN In Vitro Value Was Not Predictive of TN or PN Clinical Reactivity, Respectively
As shown in Table 5, we created a model of PN-specific IgE level of VHR (IgE, 17.6–100 kU/L) to predict TN clinical reaction, but the relation was not statistically significant (p = 0.50). TN-specific IgE level of VHR (IgE, 17.6–100 kU/L) predicting PN nut clinical reaction was also not statistically significant (p = 0.21).
Table 5.
Specific IgE level to PNs and TNs
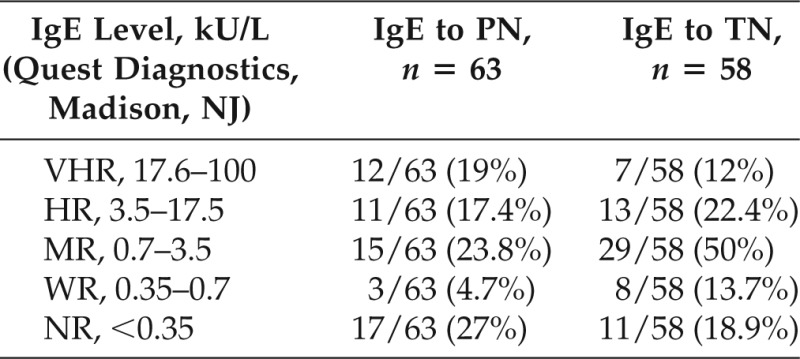
Data include multiple sensitizations to the following nuts: hazelnut, cashew, walnut, pecan, pistachio, and almond.
HR = highly reactive (IgE, 3.5–17.5 kU/L); MR = moderately reactive (IgE, 0.7–3.5 kU/L); NR = not reactive (IgE, <0.35 kU/L [Quest Diagnostics, Madison, NJ]); PN = peanut; TN = tree nut; VHR = very highly reactive (IgE, 17.6–100 kU/L); WR = weakly reactive (IgE, 0.35–0.7 kU/L).
Total Number of Organ Systems Affected during Allergic Reactions Correlates with Serum-Specific IgE Level for TNs, But Not for PNs
Table 6 shows the simple statistics for the number of organ systems reported affected during allergic reactions to TN and PN as well as the level of sensitization to TN and PN. Number of affected organ systems affected in allergic reactions to TNs correlates with level of specific IgE sensitization to TNs (p = 0.0043). Number of affected organ systems affected in allergic reactions to PN does not correlate with level of specific IgE sensitization to PN (p = 0.983).
Table 6.
Simple statistics of total number of organ systems in allergic reactions to PNs or TNs and greatest degree of IVS per subject
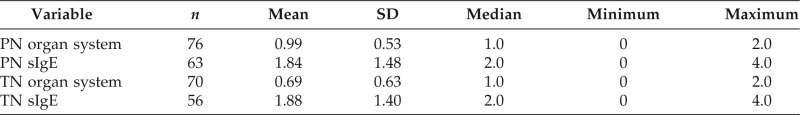
Each organ system symptom reported received a score of 1. Organ system symptoms included dermatologic (urticaria, pruritus, angioedema, swelling, and worsening of atopic dermatitis), respiratory (wheezing, throat tightness, shortness of breath, cough, and tight chest), gastrointestinal (nausea, vomiting, diarrhea, and abdominal pain), neurological (dizziness and syncope). Degree of IVS was quantified as follows: 0, not reactive (IgE, <0.35 kU/L); 1, WR (IgE, 0.35–0.7 kU/L); 2, MR (IgE, 0.7–3.5 kU/L); 3, HR (IgE, 3.5–17.5 kU/L); 4, VHR (IgE, 17.6–100 kU/L; Quest Diagnostics, Madison, NJ).
HR = highly reactive; IVS = in vitro sensitization; MR = moderately reactive; PN = peanut; TN = tree nut; VHR = very highly reactive; WR = weakly reactive.
DISCUSSION
This is the first study including both pediatric and adult patients to show the clinical reactivity and IVS between patients with PN and TN allergies. We found that PN sensitization highly correlates with TN sensitization in vitro. This concurrence of sensitization did not extend to clinical cross-reactivity between PN and TN. A retrospective study of TN allergy among PN-allergic children found increasing sensitization to TNs up to age 5 years.11
The concurrence of IVS between PN and TNs may be caused by, in part, molecular allergen cross-reactivity between PN and TN allergens.8,9 PN major allergens (Ara h 1, Ara h 2, and Ara h 3) are seed storage proteins as are most TN allergens.12–15 Seed storage proteins from PNs and TNs are considered the major allergens contributing to the anaphylactic reactions of patients with nut allergy.15 PN Ara h 2 is recognized as functionally more potent and considered the most clinically important PN allergen.16–19 Serum IgE to Ara h 2 is an important predictor of clinical reactivity to PN. De Leon and colleagues have indicated that PN Ara h 2 shares common IgE-binding epitopes with almond and brazil nut allergens and contributes to allergenic cross-reactivity with TN proteins.8,20 Maleki find similar IgE-binding epitopes of between walnut allergens and PN Ara h 2, contributing to cross-reactivity between nut allergen.21 Whether Ara h 2 is present in almonds and contributes to their allergenicity has not been confirmed.22
Barre and colleagues report PN Ara h1 allergen and TNs including walnut (Jug r 2), hazelnut (Cor a 11), and cashew nut (Ana o 1) share structurally related IgE-binding epitopes.9 Sharing IgE-binding epitopes between PN (Ara h 1, Ara h 2) and TN (almond, Brazil nut, walnut, etc.) may provide an explanation that the common cosensitization or concurrent clinical reactions might be caused by the molecular cross-reaction among nuts. Whether this cosensitization or cross-reaction is associated with significant clinical reactions or with the severity of the reactions remains unknown.
Sampson reports predictability of nut clinical reaction by using food-specific IgE concentrations: PN-specific IgE concentrations of ≥14 kU/L has positive predictive value of 100% of PN clinical allergy and TN-specific IgE concentrations of ≥15 kU/L has positive predictive value of 95% of TN clinical allergy.23 We did not find that high PN or TN in vitro value was predictive of TN or PN clinical reactivity, respectively.
Our retrospective study discovered that the extent of clinical organ system symptoms reportedly involved in food-allergic reactions to TNs correlates with serum levels of specific IgE to TNs. This correlation was not significant for allergic symptoms associated with PN allergy and serum levels of PN-specific IgE.
The limitation of the current study is that the clinical allergy diagnosis is based on self-reported allergy reaction and is not based on oral food challenge, which is the gold standard for diagnosis of food allergy.24,25 This lack of confirmation may impair the ability of predicting the relationships between PN allergy and TN allergy. Additionally, the cohort size of patients allergic to each of the nuts is relatively small. More patients will be needed to confirm the predictability of this cosensitization or concurrent allergy between PN and the TNs.
In conclusion, PN sensitization correlates with TN sensitization in vitro, with lower sensitivity for coexistent clinical reactivity. The higher sensitivity of nut sensitization may be caused by primary cross-reactivity between PN and TN allergens as there are shared epitopes in nuts and possible cross-reactivity via different allergen components between PN and TNs, along with possible PN and TN cross-contact during the food manufacturing and processing.26
ACKNOWLEDGMENTS
The authors acknowledge Jeremy Weedon, Ph.D., from the Scientific/Academic Computing Center at SUNY Downstate Medical Center, who provided assistance with statistical analysis.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Sampson HA, Mendelson LM, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med 327:380–384, 1992. [DOI] [PubMed] [Google Scholar]
- 2. Yunginger JW, Sweeney KG, Sturner WQ, et al. Fatal food-induced anaphylaxis. JAMA 260:1450–1452, 1988. [PubMed] [Google Scholar]
- 3. Bock SA, Atkins FM. The natural history of peanut allergy. J Allergy Clin Immunol 83:900–904, 1989. [DOI] [PubMed] [Google Scholar]
- 4. Sicherer SH, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. J Allergy Clin Immunol 112:1203–1207, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-Year follow-up. J Allergy Clin Immunol 125:1322–1326, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Warner JO. Peanut allergy: A major public health issue. Pediatric Allergy and Immunology 10:14–20, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Kartha KK, Pahl K, Leung NL, Mroginski LA. Plant regeneration from meristems of grain legumes: Soybean, cowpea, peanut, chickpea, and bean. Can J Bot 59:1671–1679, 1981. [Google Scholar]
- 8. de Leon MP, Drew AC, Glaspole IN, et al. IgE cross-reactivity between the major peanut allergen Ara h 2 and tree nut allergens. Mol Immunol 44:463–471, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Barre A, Sordet C, Culerrier R, et al. Vicilin allergens of peanut and tree nuts (walnut, hazelnut and cashew nut) share structurally related IgE-binding epitopes. Mol Immunol 45:1231–1240, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis network symposium. J Allergy Clin Immunol 117:391–397, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Burks KD, Bock SA, Atkins FM, Fleisher DM. Natural history of peanut and tree nut allergy: Development of tree nut allergy/sensitization. Abstract 907 J Allergy Clin Immunol 121:S235, 2008. [Google Scholar]
- 12. Burks W, Sampson HA, Bannon GA. Peanut allergens. Allergy 53:725–730, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Radauer C, Bublin M, Wagner S, et al. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol 121:847–852.e7, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Kleber-Janke T, Crameri R, Appenzeller U, et al. Selective cloning of peanut allergens, including profilin and 2S albumins, by phage display technology. Int Arch Allergy Immunol 119:265–274, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Roux KH, Teuber SS, Sathe SK. Tree nut allergens. Int Arch Allergy Immunol 131:234–244, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Bernard H, Paty E, Mondoulet L, et al. Serological characteristics of peanut allergy in children. Allergy 58:1285–1292, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Astier C, Morisset M, Roitel O, et al. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol 118:250–256, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Flinterman AE, van Hoffen E, den Hartog Jager CF, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy 37:1221–1228, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Koppelman SJ, Wensing M, Ertmann M, et al. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy 34:583–590, 2004. [DOI] [PubMed] [Google Scholar]
- 20. de Leon MP, Glaspole IN, eDrew AC, et al. Immunological analysis of allergenic cross-reactivity between peanut and tree nuts. Clin Exp Allergy 33:1273–1280, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Maleki SJ, Teuber SS, Cheng H, et al. Computationally predicted IgE epitopes of walnut allergens contribute to cross-reactivity with peanuts. Allergy 66:1522–1529, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poltronieri P, Cappello MS, Dohmae N, et al. Identification and characterization of the IgE-binding proteins 2S albumin and conglutin gamma in almond (Prunus dulcis) seeds. Int Arch Allergy Immunol 128:97–104, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Sampson HA. Food allergy. J Allergy Clin Immunol 111:S540–S547, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Bock SA, Sampson HA, Atkins FM, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an official procedure: A manual. J Allergy Clin Immunol 82:986–997, 1988. [DOI] [PubMed] [Google Scholar]
- 25. Niggemann B, Beyer KJ. Diagnosis of food allergy in children: Toward a standardization of food challenge. Pediatr Gastroenterol Nutr 45:399–404, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Sicherer SH, Sampson HA. Peanut and tree nut allergy. Curr Opin Pediatr 12:567–573, 2000. [DOI] [PubMed] [Google Scholar]


