Abstract
Data in literature seem to show that, in patients with contact allergic dermatitis, dietary nickel might be a cause of systemic dermatitis, but little information exists in literature about the role of nickel sensitization and dietary nickel in patients with allergic-like chronic dermatitis syndromes. The prevalence of nickel sensitization in patients with chronic allergic-like, non-IgE-mediated skin diseases, and the possible impact of dietary nickel on symptom provocation and persistence has been assessed in the present retrospective study on a case series of 1726 patients referred to our allergy unit for chronic allergic-like skin diseases. IgE-mediated pathogenesis and other differential diagnoses excluded, patients were patch tested. Nickel-positive patients underwent an elimination diet and double-blind placebo-controlled nickel challenge (DBPCNC) test. A total of 339 (20%) tested nickel-positive. Fifty-two patients (15%) recovered by avoiding sources of nickel contact and 29 (10%) dropped out. Out of the remaining nickel-sensitized patients, 277 (80%) achieved complete or near complete recovery with low-nickel content diet, and 185 of them (89%) were positive to DBPCNC. We conclude that nickel sensitization and dietary nickel seem to be the chief trigger for provocation and persistence of symptoms in an important part (∼11%) of patients with chronic allergic-like dermatitis syndromes.
Keywords: Nickel allergy, nickel sensitization, allergic-like chronic dermatitis, chronic urticarial, adult atopic eczema, chronic pruritus, systemic contact dermatitis, dietary nickel, nickel diet, nickel challenge test
Atopic eczema, chronic urticaria, generalized chronic pruritus, and other chronic allergic-like dermatitis syndromes very often are defined as “idiopathic,” because in most cases it is not possible to find a clear-cut aetiopathogenetic cause. The term “allergic-like” disease is often used to classify and put together different diseases quite similar to the allergic ones, with which they can share the same underlying immunologic mechanisms, but for which an allergic sensitization and a true allergic (IgE-mediated) pathogenesis cannot be proved.
A small number of clinical and experimental studies suggest that dietary nickel could be the possible cause for the provocation and persistence of symptoms in some patients with concomitant nickel sensitization.1–11
Nickel-sensitized patients, when exposed to the hapten given per os, can develop a flare-up reaction of previous contact dermatitis. Systemic contact dermatitis (SCD) is the term normally used to describe clinical manifestations of the systemic provocation by nickel and some other haptens.12–15 The flare-up of healing or quiescent contact dermatitis is the most frequent reaction, but pompholix, dyshidrosic or vescicular eczema, toxicoderma-like rash, chronic pruritus, maculo-papular rash, vasculitis-like lesions, flexural dermatitis, papuloerythroderma-like eruptions, chronic urticaria, pseudo-atopic dermatitis, generalized rash, and baboon syndrome are several clinical variants within the spectrum of systemically induced allergic contact dermatitis (ACD).1–21 SCD should be considered as an “umbrella term,” which includes a broad spectrum of clinical manifestations that can occur when a person sensitized to a contact allergen is exposed to the same allergen through a systemic route.
Data in medical literature about systemically induced nickel dermatitis present some inconsistencies related to diagnostic procedures, diet, and doses of challenge and do not allow to draw definitive conclusions about the true significance of the syndrome in clinical practice. As a matter of fact, most data have been obtained by experimental procedures of challenge in patients with ACD, and little information exists about the role of dietary nickel in patients with allergic-like dermatitis syndromes.
With the present retrospective study, we have tried to ascertain the clinical and epidemiologic relevance of nickel sensitization in patients with allergic-like skin diseases and the possible impact of dietary nickel on symptoms provocation and persistence.
METHODS
Selection and Inclusion Criteria
We performed a retrospective study on a case series of patients referred to our allergy unit from January 1, 2000 to December 31, 2010 because of chronic skin diseases, compatible with allergic skin disorders for their clinical and morphologic features. These include:
- chronic urticarious skin eruptions
- skin lesions suggesting adult atopic eczema (localized variants excluded)
- generalized chronic pruritus with or without lesions resulting from scratching
- scattered, pruriginous eczemas compatible with allergic skin disorders because of their clinical features (erythematous or erythematous-squamous, blurred-edged patches with more or less evident lichenification)
All the cases considered for the present study have been reviewed based on the allergy unit and (as far as the nickel challenge procedure concerns) the day-hospital clinical records. The diagnostic procedures below described have not changed during the whole period of the study.
For the purpose of the present study, urticarious skin manifestations were described as chronic if symptoms persisted for at least two months. In all the other cases, the patients were selected if the symptoms persisted for at least four months.
The allergic, IgE-mediated pathogenesis of skin disorders was excluded based on the next criteria:
- no personal case history of atopy
- negative skin tests with commercial extracts of more common pollen and inhalant allergens and a large array of food (including but not limited to those listed below)
- negative IgE against the next food allergens: milk, egg, codfish, wheat, natural yeast, tomato, peanuts, soybean, walnuts, and peach and for any other allergens suspected of a possible etiologic role based on case history data
When required, specific elimination diet and open challenge was used to exclude foods reported as suspected. Positive results of the open provocation test were further checked by double-blind placebo-controlled food challenge.
A similar procedure has been followed for drugs potentially related to clinical manifestations.
The search for parasites in the feces, special hematochemical, cultural, and/or instrumental tests were performed in a number of cases (notably cases of chronic urticaria and generalized pruritus) to exclude diseases (for instance autoimmune disorders) possibly related to skin manifestations.
Patch tests were performed in all patients using a standard series of haptens, listed by the Italian Group for the Research for Contact Allergic Dermatitis. Patch test results were given according to grading patch test criteria, established by International Contact Dermatitis Research Group guidelines. The subjects with nickel-positive test were given suitable instructions to avoid any possible sources of allergic contact.
The role of contact allergens other than nickel to which the patients could be sensitized was preliminarily ruled out. Patients were instructed to avoid contact exposure, and for haptens that could be cause of SCDs, appropriate avoidance diet was prescribed. Cobalt avoidance diet has not been considered, because cobalt is in general seen as mere nickel cosensitization and dietary suggestions overlap nickel by a large extent.13,15,22
Low-Nickel Diet
When any possible role of a contact exposure, either professional or extraprofessional, was excluded with certainty, they were prescribed a low-nickel content diet23–28 for a period of two to four weeks (Table 1).
Table 1.
Low-nickel diet and instructions for patients
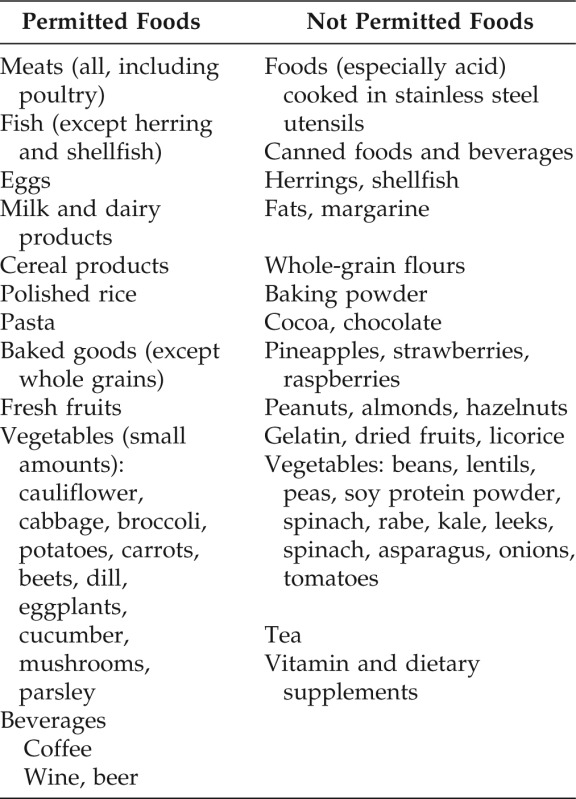
Eat with caution and in small amount foods not listed. Not listed food can have high nickel content by the soil components or nickel content is not established. Do not keep acidic food, or containing vinegar and other acidic seasonings, in contact with metal containers. To cook food or drink, remove the first few liters of water from taps not used for a long time (for example in the morning). It is recommended to carefully observe instructions to avoid contact with nickel and with any other hapten for which sensitization was found.
The regular use of antihistamines and steroids was suspended at the beginning of the diet, allowing the use of antihistamines on demand only for the first days. In particular, if the subject was obliged to resort to antihistaminic or other antiallergic drugs after the first 10–12 days of dieting, the diet was regarded as ineffective and the role of dietary nickel in the provocation of symptoms was definitively excluded.
Symptoms Score
The trend of subjective symptoms was obtained with a diary based on arbitrary score filled by the patient according to the severity and duration of the manifestations (0: none; 1: mild, tolerable also without regular use of drugs; 2: moderate, acceptable control by therapy; and 3: severe, poor controlled, interfering with ordinary activities and sleep).
The evolution and the extension of skin lesions were controlled based on the score derived from the skin maps, based on Rule of Nine's, Scoring Atopic Dermatitis or Atopic Dermatitis Area and Severity Index system, filled at the first and the last week of the diet.29–31
Nickel Challenge Test
The patients who showed healing or evident improvement of cutaneous lesions, no less than 60% of skin map initial score, and the patients with chronic pruritus without cutaneous manifestations with stable lowering of at least one step of the subjective symptom score, always adhering to the prescribed diet, underwent an oral, double-blind placebo-controlled nickel challenge (DBPCNC).
In previous studies, the nickel challenge test (up to a dose of 20 mg) proved highly specific, because it resulted ineffective in healthy subjects and in patients with hand eczema or allergic-like dermatitis syndromes not sensitized to nickel.8,32,33
The DBPCNC test was performed in day-hospital regimen with the next, noncumulative doses of 0.5, 1, 5, 10, and 20 mg of nickel sulfate hexahydrate, respectively, equal to 0.11, 0.22, 1.11, 2.23, and 4.47 mg of elemental nickel. Three capsules of p were interpolated at random into the sequence of the active test dose maintaining the progressive increase from lowest to maximum dose (example of blind sequence: 0.5 mg, p, 1 mg, 5 mg, p, 10 mg, p, and 20 mg). The capsules were administered in the morning on an empty stomach, one challenge dose per day (challenge test maximum duration, eight days).
The patients were asked to record the symptoms triggered by each dose, including the time of onset, their intensity, and duration. Patients with positive challenge underwent a medical check-up. The challenge test was regarded as positive when it proved it had caused a relapse or a sudden aggravation of the residual clinical manifestations, with widespread itching and an increase of at least 60% of the skin map score, after the intake of one dose of nickel but not after the administration of the p.
The study was approved by the Local Ethical Committee (approval number 946, 05.10.95), the challenge tests were performed in day-hospital regimen, and written informed consent was obtained from all patients.
RESULTS
Patient Population
According to the criteria listed in the method section, we screened a population of 1726 adult patients, aged 16–65 years, mean age 36 ± 14 years, 1415 (82%) woman. Study results are summarized in the flowchart shown in Fig. 1.
Figure 1.
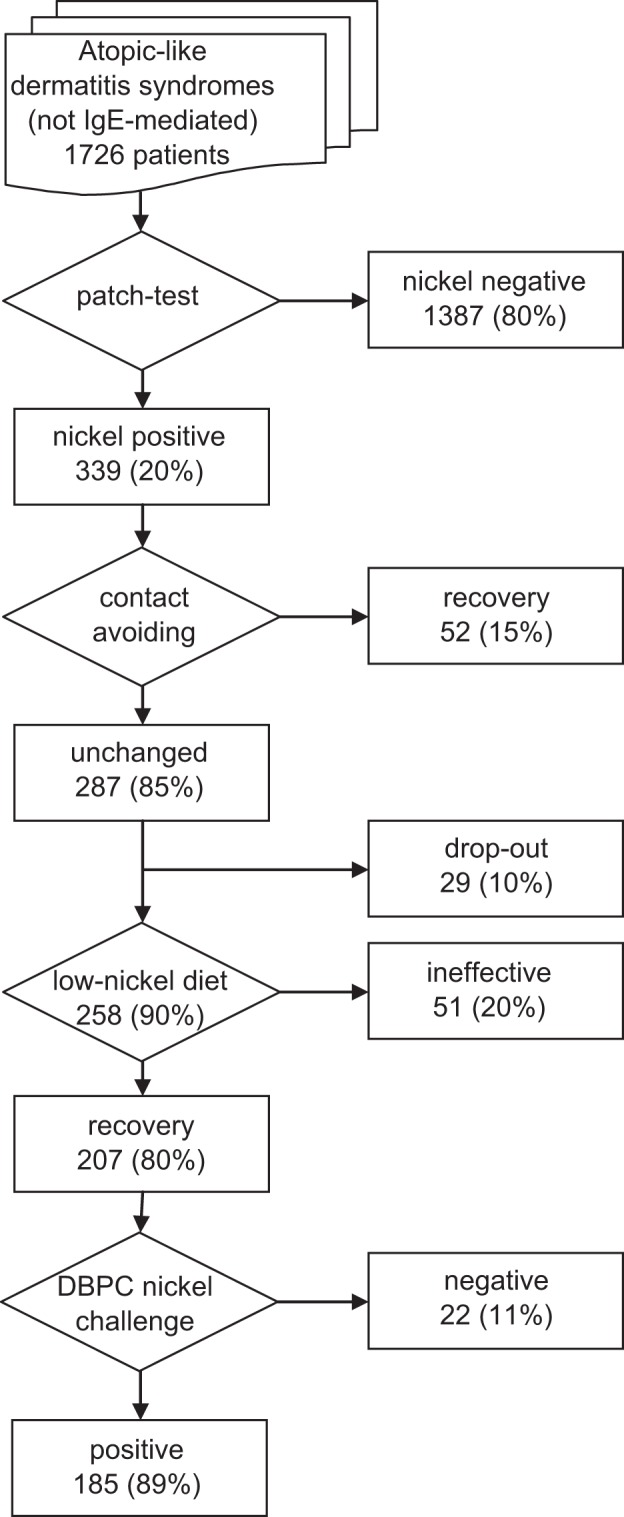
Flowchart of the study and summary of the results.
Nickel patch test positivity was found in 339 patients, a percentage (19.64%) not different from that found in general population in Europe.34,35
A total of 118 of these patients (34.80%) were suffering from chronic urticarious skin manifestations, 96 (28.32%) from scattered chronic pruriginous eczema, 91 (26.84%) from adult atopic-like eczema lesions, and 34 (10.03%) from chronic pruritus.
Cosensitization was seen in 96 cases. Thirty-four patients (33%) to cobalt and 68 to fragrance mix (65%). Eight patient showed cosensitization to balsam of Peru (8%); 41 patents (41%) showed other contact sensitizations, but none potentially related to SCDs. For the patients sensitized to balsam and/or fragrance, avoidance diet36,37 was prescribed for four weeks without significant improvement.
Most patients showed no lesions consistent with ACD. More than half of them (183 = 53.98%) neither had nor had ever had a history of contact dermatitis. Of the remaining patients, 84 (24.78%) referred some problems with costume jewelry or had suffered from a mild form of ACD in the past, and only 72 (21.24%) showed an association with some skin lesions possibly related to a contact dermatitis.
None of the patients considered in the present study suffered from relevant extracutaneous symptoms. They should not be included in the so-called Systemic Nickel Allergy Syndrome, which might be a different phenotypic expression of nickel allergy. This definition has been recently coined to describe a clinical condition that links together cutaneous manifestations (such as ACD, pompholyx, erythema, urticaria, angioedema, and dysydrosis), gastrointestinal disorders with mucosa histopathologic changes, and a number of systemic clinical manifestations, including, but not limited to, headache, chronic fatigue, cystitis and/or vulvovaginitis, and iron-deficiency anemia, all related to the intake of nickel-containing foods38–40
Low-Nickel Diet and Challenge Test Results
Fifty-two patients (15.33%) recovered by avoiding the contact with any possible sources of nickel.
Of the patients whose clinical symptoms did not improve by exclusion of nickel contact, 29 (10.10%) dropped out, most of them because of a poor compliance with prescribed diet. The remaining 258 patients (84.66%) accomplished a nickel elimination diet.
Low-nickel diet resulted in a complete or near complete recovery of skin manifestations in 207 patients (80.23%) and proved ineffective only for 51 patients (19.77%).
The DBPCNC proved positive in 185 (89.37%), a number corresponding to 10.72% of the entire population included in the study. Seventy patients (38%) suffered from adult atopic eczema; 54 (29%) from chronic urticarious skin eruptions; 43 (23%) from scattered eczema; and 18 (10%) from generalized pruritus. The distribution of the patients with positive nickel challenge was not significantly different from the starting population by skin clinical manifestations (p = 0.071, not significant).
Negative DBPCNC was observed in 22 patients (10.63%), half of them suffering from chronic pruritus, nine from urticarious skin eruptions, and two from scattered eczema.
Challenge dose ranged from 0.5 to 20 mg, with an average dose of 6.19 ± 5.86 mg. A total of 15 patients (8.11%) reacted to the minimum dose of 0.5 mg and 21 (11.35%) to the maximum dose of 20 mg.
The time of onset of cutaneous manifestations after the challenge ranged about from 3 to12 hours, but it was not always precisely reported because of a gradual beginning and a slow progression of symptoms. Only in the cases in which the effects of the challenge test showed a very rapid evolution of clinical manifestations, the patients were able to report the trigger time more precisely. Anyway, the average time of provocation has been estimated of 5.30 ± 2.45 hours.
No precise correlation was found between dose and severity degree of clinical manifestations induced by the challenge. Obviously, in the cases of weak or dubious result of the previous test dose, the prosecution of the challenge procedure and, as a consequence, the progressive increase of the doses was required to achieve stronger and clearer response. Nevertheless, we were able to observe that, as general criterion, the stronger and prompter the response, the smaller the challenge dose was.
In a number of patients (26/185 = 14.05%), the provocation test induced particularly marked effects, with intense itching, symmetrically spread erythematous or maculo-papular rash, generalized urticarious rash, or baboon syndrome. In all these cases, a severe flare-up of the previous positive nickel patch test was observed.
However, a recall of the previous positive nickel patch test was regularly seen in all patients with a strong reaction to challenge test; seventy-nine patients altogether, with a cumulative frequency of approximately 43% of all positive challenge tests.
No patient showed signs of systemic anaphylaxis, shock, or any other serious or life-threatening symptoms, neither relevant provocation of extracutaneous symptoms was seen. During the medical check-up, all cases of positive challenge were treated with betamethasone (4 or 1.5 mg, intramuscular or intravenous, based on severity of skin reactions) and second generation antihistamine per os. A clear improvement of skin manifestation was usually seen within a few hours (two to four hours) and complete disappearance generally not later than 24 hours. However, they were requested to continue therapy orally at home, with antihistamine and corresponding reduced doses of steroid (1 or 2 mg) for the next two days.
DISCUSSION
The results of the present retrospective study confirm that dietary nickel can be the chief trigger for provocation and persistence of symptoms in a subpopulation of patients with chronic allergic-like idiopathic dermatitis syndromes and nickel sensitization.7–11
Previous experimental studies in patients with ACD had demonstrated that systemically absorbed nickel can be the real cause for the onset of both secondary eruptions in those where a simple contact exposure cannot account for the distribution or the aggravation of skin lesions and, in some cases, of widespread dermatitis syndromes, which include a variety of clinical manifestations ranged from maculo-papular rash to flexural dermatitis, toxicoderma, and chronic urticaria.3–6,12–14,16–20
But still, the relationship between dietary nickel and SCD remains controversial. The studies concerning systemically induced nickel dermatitis have been brought into questions because of some inconsistencies related to diagnostic procedures, diet, and doses of challenge. 41,42
Indeed, a standardized or shared study protocol does not exist, and the various studies are not comparable because of substantial differences on typology of patients, criteria for selection and inclusion, methods, doses, and challenge procedures.
There is no doubt that, as for any other food allergy or intolerance, the gold standard of a correct diagnosis is based on the results of the elimination diet and of the corresponding, double blind, p-controlled provocation test. Unfortunately, major criticisms concern just the diet and the doses of the challenge.
Nickel appears to be a common contaminant of foods, and its content in the diet can considerably vary according to food habits and the concentration of the metal in the subsoil (from 5 to 500 μg/g), which in turn influences the nickel content in specific food, in drinking water (5–100 μg/L), and from stainless steel cook utensils. Without going into details, measuring the daily intake by diet is extremely difficult, but an average intake of 0.2–0.6 mg appears to be consistent with results of different sources.27,28,43–45
Of course, different estimates of nickel content in food can influence the prescription of a low-nickel diet, but there is general agreement that some food (i.e., peanuts, legumes and soybeans, oats, cocoa and chocolate, nuts, grains, whole wheat, and wholemeal flours) are of high-nickel content and constitute the core of a low-nickel content diet.27,28 Moreover, great differences in individual sensitivity to nickel and in individual nickel absorption and excretion have been demonstrated.5,46–48
In our opinion, all this could be a diagnostic problem only as a cause of false inefficacy of the prescribed diet and, as a consequence, a cause of underestimation of the epidemiologic relevance of the problem.
For practice purposes, when we can verify a good control of the symptoms and the objective recovery or near complete healing of the skin manifestations, variations on nickel content cannot change the clinical judgment on efficacy of the diet, but clearly they can influence the value of the threshold dose on the following DBPCNC.
A pivotal criticism concerns the doses needed to elicit a positive response by challenge test, because in most studies, they exceed up to 10 times the nickel amount deemed present in a normal diet.32–49 Also in our experience, the average challenge dose was higher than in normal diet but just of a light increase (from 0.78 to 1.38 mg above the maximum and the minimum estimated intake by diet), and at least for a certain number of cases, mainly those with stronger and prompter response to the challenge, the dose proved very small (less than or equal to 0.2 mg).
To explain the difference between challenge dose and average intake by diet, we can suppose that the intake on an empty stomach, in form of nickel-salt (not by food), could influence the absorption and the metabolism of the hapten.33
In any case, we must consider that the nickel challenge test is very specific, because it proved ineffective in healthy subjects and patient with hand eczema or allergic-like dermatitis syndromes not sensitized to nickel.8,32,33 Moreover, the flare-up reaction of previously positive nickel patch test is an identifying mark of a systemic provocation and is generally accepted as conclusive, firm evidence of the specificity of nickel provocation.32,49–51
A recall of previous nickel-positive patch test can occur in varying measure, depending on challenge dose, on the time of previous patch test, and the intensity of cutaneous reaction.3,5,18,50
In agreement with published data, also in the present study, a strong correlation between the intensity of the cutaneous response and the flare-up of the previous positive nickel patch test was found.
The pathogenesis of the nickel-induced SCD is not clearly defined.
A number of studies show that, after oral nickel challenge, tissue lymphocyte alterations and cytokine profiles are consistent with a T cell-mediated, delayed type IV hypersensitivity reaction.38,52–54
However, in all cases of generalized skin response (i.e., excluding some cases of mere reactivation of localized ACD), there are no reports in literature of positive oral nickel challenge later than 12 hours. In the present study, the trigger times after oral intake almost always ranged between three and seven hours.
Both the times of symptoms provocation after nickel challenge, which are shorter than expected, and the provocation of widespread rash with urticaria are considered little consistent with a classic cell-mediated reaction.
A combined type III and IV immune-reaction has been hypothesized. Old studies had shown in the blood antibodies against hapten-albumin complexes.55,56 These experimental studies have not been repeated by other authors, and unfortunately Ig complexes have been demonstrated only in a single case of SCD to mitomycin.57
Some studies have shown that skin symptoms like urticaria and angioedema could be directly mediated by T cells in some patients for whom the involvement of IgE antibodies had been excluded.58
In nickel SCD, an IgE-mediated reaction appears highly unlikely. Skin-prick tests resulted negative, and specific IgE against nickel cannot been found in our previous experience on this topic.8
However, as in other cases of allergic-like reactions, a nonspecific or at least non-IgE-mediated release of inflammation mediators from mast cells and basophiles could be a possible, alternative mechanism.59
The possible role of mast cells in the pathogenesis of flare-up reactions by nickel challenge has been held by some histologic examinations.48,51 And more recent studies on the hapten-induced contact hypersensitivity, which represent the classic model of a T cell-mediated hypersensitivity reaction, show that a strong inflammation response on the skin is elicited well before the activation of nickel-specific T cells.60,61 A broad spectrum of chemokines is released, including Regulated on Activation, Normal T cell Expressed and Secreted (RANTES), which can play a fundamental role in histamine and serotonin generation and trigger human mast cell degranulation.62–64
Here, we must emphasize the concept that the results of this retrospective study have been derived exclusively from a case series of patients suffering from widespread allergic-like dermatitis, not from ACD. More than half of them neither had nor had ever had a history of contact dermatitis. Most of the remaining patients reported some problems with costume jewelry or had suffered from a mild form of ACD in the past, but only a very small minority of patients showed in association some skin lesions consistent with a contact dermatitis.
We think that the results of the present study, which confirms our previous research on the topic,8 highlight, in clinical practice, the real importance of nickel sensitization and of dietary nickel for the provocation and persistence of skin lesions in a subpopulation of patient with allergic-like dermatitis syndromes. Limited (our unpublished) data inferred from the follow-up of some patients considered for the present study seem to demonstrate that a prolonged diet period, for about one year, could induce a stable healing. However, in literature there is neither information on the “natural” course of nickel SCDs nor clinical studies on long-term effects of low-nickel diet.
The results of the present study are based on retrospective clinical observations, so in theory they might have some bias. Prospective studies should corroborate and add evidence to these observations.
In conclusion, we believe that in practical, routine clinical work, patients with idiopathic urticaria and other allergic-like, non-IgE-mediated dermatitis syndromes should be patch tested and, if nickel-positive, should go on a low-nickel content diet and if necessary a confirmatory DBPCNC.
ACKNOWLEDGMENTS
We thank our nurse, Mrs. Rossana Baioni, for her valuable aid of clinical cases tracing in archives.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Shannon J. Pseudo atopic dermatitis. Dermatologica 131:176–190, 1965. [PubMed] [Google Scholar]
- 2. Andersen KE, Hjorth N, Menné T. The baboon syndrome: Systematically induced allergic contact dermatitis. Contact Dermatitis 10:97–100, 1984. [DOI] [PubMed] [Google Scholar]
- 3. Christensen OB, Moller H. External and internal exposure to the antigen in the hand eczema of nickel allergy. Contact Dermatitis 1:136–141, 1975. [DOI] [PubMed] [Google Scholar]
- 4. Kaaber K, Veien NK, Tjiell JC. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br J Dermatol 98:197–201, 1978. [DOI] [PubMed] [Google Scholar]
- 5. Gawkrodger DJ, Cook SW, Fell GS, et al. Nickel dermatitis: The reaction to oral nickel challenge. Br J Dermatol 115:33–38, 1986. [DOI] [PubMed] [Google Scholar]
- 6. Boscolo P, Andreassi M, Sabbioni E, et al. Systemic effects of ingested nickel on the immune system of nickel sensitised women. Life Sci 64:1485–1491, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Abeck D, Treanckner I, Steinkraus V, et al. Chronic urticaria due to nickel intake. Acta Dermatol Venereol 73:438–439, 1993. [DOI] [PubMed] [Google Scholar]
- 8. Antico A, Soana R. Chronic allergic-like dermopathies in nickel-sensitive patients. Results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc 20:235–242, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Antoszczyk G, Obtulowicz K, Wojas-Pelc A. Nickel allergy in contact and atopic dermatitis. Pzegl Lek 60:334–337, 2003. [PubMed] [Google Scholar]
- 10. Schiavino D, Nucera E, Buonomo A, et al. Systemic nickel allergy. Int J Immunopathol Pharmacol 18(4 suppl):7–9, 2005. [PubMed] [Google Scholar]
- 11. Guerra L, Rogkakou A, Massacane P, et al. Role of contact sensitization in chronic urticaria. J Am Acad Dermatol 56:88–90, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Menné T, Hjorth N. Reactions from systemic exposure to contact allergens. Semin Dermatol 1:15–24, 1982. [Google Scholar]
- 13. Nijhawan RI, Molenda M, Zirwas MJ, Jacob SE. Systemic contact dermatitis. Dermatol Clin 27:355–364, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Veien NK. Systemic contact dermatitis. Int J Dermatol 50:1445–1456, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Yoshihisa Y, Shimizu T. Metal allergy and systemic contact dermatitis: An overview. Dermatol Res Pract 2012:749561, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chronin E. Contact Dermatitis XVII. Quarterly review. Reactions to contact allergens given orally or systemically. Br J Dermatol 86:104–107, 1972. [DOI] [PubMed] [Google Scholar]
- 17. Veien NK, Hattel T, Justesen O, et al. Oral challenge with nickel and cobalt in patients with positive patch tests to nickel and/or cobalt. Acta Derm Venereol 67:321–325, 1987. [PubMed] [Google Scholar]
- 18. Moller H, Ohlsson K, Linder C, et al. The Flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis 40:200–204, 1999. [DOI] [PubMed] [Google Scholar]
- 19. Menné T, Veien NK, Maibach HL. Systemic contact dermatitis. Am J Contact Dermatitis 5:1–12, 1994. [Google Scholar]
- 20. Hausermann P, Harr Th, Bircher AJ. Baboon syndrome resulting from systemic drugs: Is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis 51:297–310, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Kato K, Satoh T, Tanaka T, et al. Systemic nickel allergy presenting as papuloerythroderma-like eruptions. Acta Derm Venereol. 90:655–6, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Veien NK, Hattel T, Justesen O, Nørholm A. Oral challenge with metal salts. (I). Vesicular patch-test-negative hand eczema. Contact Dermatitis 9:402–406, 1983. [DOI] [PubMed] [Google Scholar]
- 23. Veien NK, Hattel T, Laurberg G. Low nickel diet: An open, prospective trial. J Am Acad Dermatol 29:1002–1007, 1993. [DOI] [PubMed] [Google Scholar]
- 24. Brun R. Nickel dans les aliments et eczéma de contact. Dermatologica 159:365–370, 1979. [PubMed] [Google Scholar]
- 25. Flyvholm MA, Nielsen GD, Andersen A. Nickel content of food and estimation of dietary intake. Lebensm Unters Forsch 179:427–431, 1984. [DOI] [PubMed] [Google Scholar]
- 26. Veien NK, Menné T. Nickel contact allergy and a nickel-restricted diet. Semin Dermatol 9:197–205, 1990. [PubMed] [Google Scholar]
- 27. Sharma AD. Relationship between nickel allergy and diet. Indian J Dermatol Venereol Leprol 73:307–312, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Melisa Medica Foundation. Metals & Disease. Nickel. www.melisa.org/metals-disease/metals/nickel/ Accessed October 13, 2013 Updated 2014.
- 29. Metcalfe DD, Sampson HA. Workshop on experimental methods for clinical studies of adverse reactions to foods and food additives. J Allergy Clin Immunol 86:421–442, 1990. [DOI] [PubMed] [Google Scholar]
- 30. Bahmer FA, Shafer J, Schubert HJ. Quantification of the extend and the severity of atopic dermatitis: The ADASI score. Arch Dermatol 127:1239–1240, 1991. [PubMed] [Google Scholar]
- 31. European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: The SCORAD index. Dermatology, 186:23–31, 1993. [DOI] [PubMed] [Google Scholar]
- 32. Jensen CS, Menné T, Lisby S, et al. Experimental systemic contact dermatitis from nickel: A dose-response study. Contact Dermatitis 49:124–132, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Nielsen GD, Soderberg U, Jorgensen PJ, et al. Absorption and retention of nickel from drinking water in relation to food intake and nickel sensitivity. Toxicol Appl Pharmacol 154:67–75, 1999. [DOI] [PubMed] [Google Scholar]
- 34. Uter W, Ramsch C, Aberer W, et al. The European baseline series in 10 European Countries, 2005/2006. Results of the European surveillance system on contact allergies (ESSCA). Contact Dermatitis 61:31–38, 2009. [DOI] [PubMed] [Google Scholar]
- 35. Thyssen JP, Menné T. Metal allergy-a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol 15:309–318, 2010. [DOI] [PubMed] [Google Scholar]
- 36. Veien NK, Hattel T, Justesen O, Nørholm A. Reduction of intake of balsams in patients sensitive to balsam of Peru. Contact Dermatitis. 12:270–273, 1985. [DOI] [PubMed] [Google Scholar]
- 37. Scheinman PL. Allergic contact dermatitis to fragrance: A review. Am J Contact Dermat 7:65–76, 1996. [PubMed] [Google Scholar]
- 38. Di Gioacchino M, Boscolo P, Cavallucci E, et al. Lymphocyte subset changes in blood and gastrointestinal mucosa after oral nickel challenge in nickel-sensitized women. Contact Dermatitis 43:206–211, 2000. [DOI] [PubMed] [Google Scholar]
- 39. Tammaro A, Narcisi A, Persecnino S, et al. Topical and systemic therapy for nickel allergy. Dermatitis 22:251–255, 2011. [DOI] [PubMed] [Google Scholar]
- 40. Ricciardi L, Arena A, Arena E, et al. Systemic nickel allergy syndrome: Epidemiological data from four Italian allergy units. Int J Immunopathol Pharmacol 27:131–136, 2014. [DOI] [PubMed] [Google Scholar]
- 41. Burrows D. Is systemic nickel important? J Am Acad Dermatol 26:632–635, 1992. [DOI] [PubMed] [Google Scholar]
- 42. Pizzutelli S. Systemic nickel hypersensitivity and diet: Myth or reality? Eur Ann Allergy Clin Immunol 43:5–18, 2011. [PubMed] [Google Scholar]
- 43. Christensen OB, Moller H. Release of nickel from cooking utensils. Contact Dermatitis 4:343–346, 1978. [DOI] [PubMed] [Google Scholar]
- 44. Kuligowski J, Halperin KM. Stainless steel cookware as a significant source of nickel, chromium, and iron. Arch Environ Contam Toxicol 23:211–215, 1992. [DOI] [PubMed] [Google Scholar]
- 45. Andersen KE, Nielsen GD, Flyvholm MA, et al. Nickel in tap water. Contact Dermatitis 9:140–143, 1983. [DOI] [PubMed] [Google Scholar]
- 46. Menné T, Thorboe A. Nickel dermatitis - nickel excretion. Contact Dermatitis 2:353–354, 1976. [DOI] [PubMed] [Google Scholar]
- 47. Christensen OB, Lagesson V. Nickel concentration in blood and urine after oral administration. Ann Clin Lab Sci 11:119–125, 1981. [PubMed] [Google Scholar]
- 48. Hindsén M, Christensen OB, Moller H. Nickel levels in serum and urine in five different groups of eczema patients following oral ingestion of nickel. Acta Derm Venereol 74:176–178, 1994. [DOI] [PubMed] [Google Scholar]
- 49. Jensen CS, Menné T, Johansen JD. Systemic contact dermatitis after oral exposure to nickel: A review with a modified meta-analysis. Contact Dermatitis 54:79–86, 2006. [DOI] [PubMed] [Google Scholar]
- 50. Hindensen M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reaction. J Am Acad Dermatol 44:616–623, 2001. [DOI] [PubMed] [Google Scholar]
- 51. Christensen OB, Lindstrom C, Lofberg H, et al. Micromorphology and specificity of orally induced flare-up reactions in nickel-sensitive patients. Acta Derm Venereol 61:505–510, 1981. [PubMed] [Google Scholar]
- 52. Gawkrodger DJ, McVittie E, Hunter JA. Immunophenotyping of the eczematous flare-up reaction in a nickel-sensitive subject. Dermatologica 175:171–177, 1987. [DOI] [PubMed] [Google Scholar]
- 53. Veien NK, Sviejgaard E, Menné T. In vitro lymphocyte transformation to nickel: A study of nickel-sensitive patients before and after epicutaneous and oral challenge with nickel. Acta Derm Venereol 59:447–451, 1979. [PubMed] [Google Scholar]
- 54. Jensen CS, Lisby S, Larsen JK, et al. Characterization of lymphocyte subpopulations and cytokine profiles in peripheral blood of nickel-sensitive individuals with systemic contact dermatitis after oral nickel exposure. Contact Dermatitis 50:31–38, 2004. [DOI] [PubMed] [Google Scholar]
- 55. Polak L, Turk JL. Studies on the effect of systemic administration of sensitizers in guinea-pigs with contact sensitivity to inorganic metal compounds. II. The flare-up of previous test sites of contact sensitivity and the development of a generalized rash. Clin Exp Immunol 3:253–262, 1968. [PMC free article] [PubMed] [Google Scholar]
- 56. Veien NK, Christiansen AH, Svejgaard E, Kaaber K. Antibodies against nickel-albumin in rabbits and man. Contact Dermatitis. 5:378–382, 1979. [DOI] [PubMed] [Google Scholar]
- 57. Kunkeler L, Nieboer C, Bruynzeel DP. Type III and type IV hypersensitivity reactions due to mitomycin C. Contact Dermatitis 42:74–76, 2000. [DOI] [PubMed] [Google Scholar]
- 58. Orasch CE, Helbling A, Zanni MP, et al. T-cell reaction to local anaesthetics: Relationship to angioedema and urticaria after subcutaneous application, patch testing and LTT in patients with adverse reaction to local anaesthetics. Clin Exp Allergy 29:1549–54, 1999. [DOI] [PubMed] [Google Scholar]
- 59. Bossi F, Frossi B, Radillo O, et al. Mast cells are critically involved in serum-mediated vascular leakage in chronic urticaria beyond high-affinity IgE receptor stimulation. Allergy 66:1538–1546, 2011. [DOI] [PubMed] [Google Scholar]
- 60. Goebeler M, Trautmann A, Voss A, et al. Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am J Phatol 158:431–440, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 64:1699–1714, 2009. [DOI] [PubMed] [Google Scholar]
- 62. Zhao ZZ, Sugerman PB, Zhou XJ, et al. Mast cell degranulation and the role of T cell RANTES in oral lichen planus. Oral Dis 7:246–251, 2001. [PubMed] [Google Scholar]
- 63. Castellani ML, De Lutiis MA, Toniato E, et al. Impact of RANTES, MCP-1 and IL-8 in mast cells. J Biol Regul Homeost Agents 24:1–6, 2010. [PubMed] [Google Scholar]
- 64. Sharma R, Sircar K, Singh S, Rastogi V. Role of mast cells in pathogenesis of oral lichen planus. J Oral Maxillofac Pathol 15:267–271, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


