Abstract
Using an alternative sigma factor ecf3 as target, we successfully established the first markerless mutagenesis system in the Veillonella genus. This system will be a valuable tool for mutagenesis of multiple genes for gene function analysis as well as for gene regulation studies in Veillonella.
Keywords: Markerless, Veillonellae, Mutagenesis, PheS
Veillonellae are one of the most prevalent and numerically dominant bacteria in the oral microbiota (Dzink, et al., 1989, Kamma, et al., 1995, Palmer, et al., 2006, Zaura, et al., 2009, Periasamy & Kolenbrander, 2010). Two characteristics of the Veillonella genus make them one of the bridging species in the development of the oral biofilm. One is their utilization of lactate as a preferred carbon and energy source (Rogosa, 1964); the other is their prolific coaggregation with many initial, middle, and late colonizers (Hughes, et al., 1988, Palmer, et al., 2006, Chalmers, et al., 2008). In addition to the human oral cavity, veillonellae are also dominant colonizers of the human gastrointestinal and respiratory tracts (Madan, et al., 2012).
The genus Veillonella consists of 13 species (Aujoulat, et al., 2014). Despite their prevalence in the human microbiome, little is known about their biology and pathogenic potential, partially due to our inability to genetically manipulate this group of bacteria until recently. Our group successfully established the first tractable genetic transformation system in a clinical strain of Veillonella atypica (Liu, et al., 2012). Using this system, we have made insertional (single-crossover) mutations of several genes important for cell-cell coaggregation (Zhou, et al., 2014). However, the single-crossover mutagenesis system could not create mutations in a multigene operon without posing polar effects on downstream genes. As our attempts to develop a double-crossover mutagenesis system failed, we sought to develop a markerless mutagenesis system using a single-crossover strategy.
To develop this system, we chose a mutant pheS gene as the counter-selectable marker and an alternative sigma factor ecf3 as the target gene. The pheS gene encodes the highly conserved phenylalanyl-tRNA synthetase alpha subunit (Plumbridge & Springer, 1980). A point mutation in pheS generating an A294G substitution in E. coli confers sensitivity to the phenylalanine analog p-chloro-phenylalanine (p-Cl-Phe), and has been used as a counter-selectable marker to create markerless mutations in several bacterial species (Kast & Hennecke, 1991, Kristich, et al., 2007, Barrett, et al., 2008, Xie, et al., 2011, Carr, et al., 2015).
To create the mutant pheS in Veillonella, a GCC → GGT mutation was created from the OK5 pheS gene, generating an A308G (equivalent to A294G in E. coli) substitution. First, the wild-type pheS was amplified by PCR using primers pheS-F and pheS-R-EcoRI (Table 1). Next, the highly expressed OK5 mdh (malate dehydrogenase) promoter was amplified by PCR using primers Pmdh-F-XhoI and Pmdh-R, and fused with pheS by overlapping PCR. The PCR amplicon was double digested with EcoRI and XhoI and inserted into the suicide vector pBST containing the tetracycline resistance gene tetM (Liu, et al., 2012). The recombinant plasmid pBST-Pmdh-pheS was then used as template for inverse PCR using the phosphorylated primers pheSm-F and pheSm-R, which contained the site-specific mutation (GCC → GGT), to create pheS*. The PCR product was ligated and transformed into E. coli DH5α. Plasmid pBST-Pmdh-pheS* containing the expected GCC → GGT mutation was confirmed by sequencing. This plasmid was later used as a carrier for the markerless deletion of the target gene.
Table 1.
Bacterial strains, plasmids and primers used in this study
| Characteristics | Reference | |
|---|---|---|
| Strains | ||
| E. coli DH5α | Cloning strain | |
| V. atypica OK5 | Wild type | (Liu et al., 2012) |
| Δecf3 | OK5 ecf3 deletion mutant | This work |
| Plasmids | ||
| pBST | Suicide vector of V. atypica, the beta-lactamase gene in pBluescript II KS (+) was replaced by tetM | (Liu et al., 2012) |
| pBST-Pmdh-pheS* | Carrier plasmid for markerless deletion of any gene | This work |
| pBST-Pmdh-pheS*-ecf3 | Carrier plasmid for ecf3 markerless deletion | This work |
| Primers | Sequence (5′ to 3′) | Purpose |
| pheS-F | ATGGAACAAGAATTACAACGCATA | pheS amplification |
| pheS-R-EcoRI | CGGAATTCCTAAAATTGTTCCAAGAAACGGATATCA | pheS amplification |
| Pmdh-F-XhoI | CCGCTCGAGATACATACATCACTATATCTGTAACA | mdh promoter amplification |
| Pmdh-R | TATGCGTTGTAATTCTTGTTCCATTGTTAAAACCTCTTTTCAGAAAATATGTA | mdh promoter amplification |
| pheSm-F | CCAAAACCTTTCACCTTATTAGGATCA | Site-directed mutation of pheS |
| pheSm-R | TTTTGGTATGGGCGTAGAACGTA | Site-directed mutation of pheS |
| ecf3-KO-up-F | CGGGATCCGAAAAGAGTTTTTTGTGTGA | ecf3 deletion |
| ecf3-KO-up-R | TAAAAAATATTTTAGATTTTTAAAAGATTCGTTCCTTTCTGCCTA | ecf3 deletion |
| ecf3-KO-down-F | TAGGCAGAAAGGAACGAATCTTTTAAAAATCTAAAATATTTTTTA | ecf3 deletion |
| ecf3-KO-down-R | GCTCTAGAGTATGCCGATATTATAGGCTGCA | ecf3 deletion |
To delete complete ecf3 ORF, the upstream and downstream regions were amplified by PCR using primer pairs ecf3-KO-up-F/ ecf3-KO-up-R and ecf3-KO-down-F/ ecf3-KO-down-R, respectively (Table 1). The two PCR amplicons were then ligated by overlapping PCR. The PCR product was double digested with XbaI and BamHI and ligated with plasmid pBST-Pmdh-pheS*. The recombinant plasmid pBST-Pmdh-pheS*-ecf3 was confirmed by PCR and sequencing.
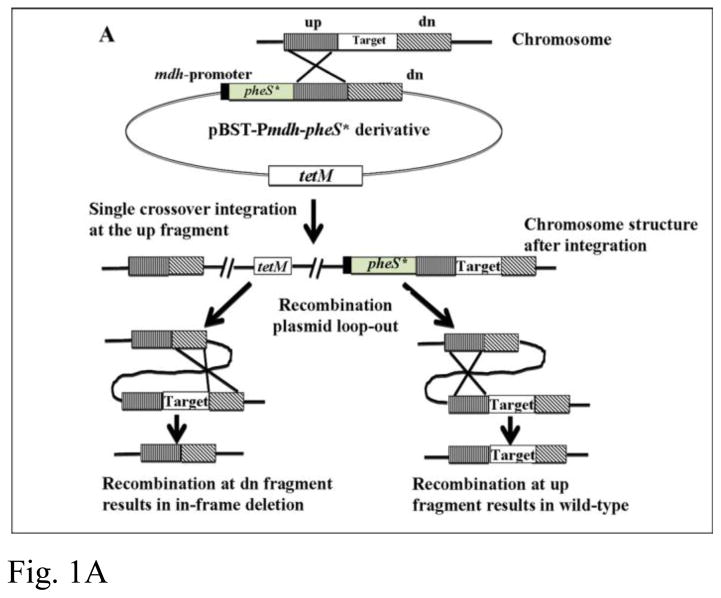
The ecf3 markerless deletion strain Δecf3 was constructed by a two-step process: single-crossover integration, and recombinative excision. First, plasmid pBST-Pmdh-pheS*-ecf3 was transformed into V. atypica OK5 via electroporation as previously described (Liu, et al., 2012). The transformation mixture was plated on brain-heart-infusion plus 0.6% lactate (BHIL) plates containing 2.5 μg ml−1 tetracycline (Tet). Tet resistant colonies all contain the transforming plasmid integrated either at the upstream or downstream regions of ecf3 via single-crossover recombination (Fig. 1A). Positive colonies were further purified and confirmed by PCR (data not shown).
Fig. 1.
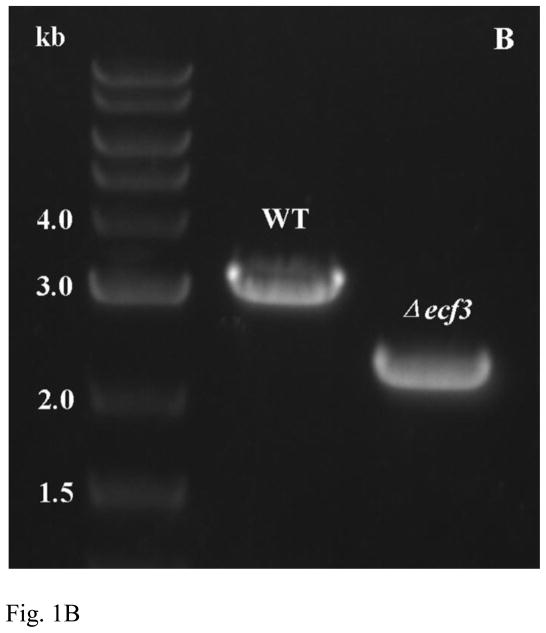
Construction of a pheS-based markerless mutagenesis system in V. atypica. (A), a schematic presentation of the strategy for constructing the markerless deletion system. Here only integration at the upstream region is illustrated. Integration can also happen at an equal chance in the downstream region. When this happens, the result from the second step is opposite to what illustrated here; i. e. recombination excision at the downstream region would recreate the wild-type genotype, while recombination at the upstream region would generate the deletion. (B), confirmation of Δecf3 deletion by PCR. The expected wild-type amplicon is approximately 3.0 kb, while the Δecf3 deletion mutant is expected to be approximately 2.2 kb. Only one clone is shown here.
For the second step, a randomly selected positive clone was grown overnight in liquid BHIL without antibiotics to allow recombinative excision of the plasmid. The overnight culture was then serially diluted and plated on BHIL plates containing 15 mM p-Cl-Phe for counter-selection. Cells growing on this plate all lost the plasmid via recombinative excision (Fig. 1A). These cells also automatically lost resistance to tetracycline. As recombinative excision can occur with equal chances at the upstream or downstream regions of ecf3, theoretically ~50% of p-Cl-Phe resistant, Tet-sensitive colonies should contain the deletion while the other 50% should recreate a wild-type genotype (Fig. 1A).
To determine which colony contained the ecf3 deletion, chromosomal DNA was isolated from randomly selected colonies, and the primer pair ecf3-KO-up-F/ecf3-KO-down-R (Table 1) was used to amplify the ecf3 surrounding regions by PCR. The wild-type genomic DNA was used as a control. As demonstrated in Fig. 1B, a 3-kb PCR band was obtained from the wild-type, while a 2.2 kb fragment was generated from one of the mutants. To confirm the 2.2 kb PCR product indeed contained the expected deletion, the DNA band was sequenced and showed the correct deletion (data not shown).
Among 20 colonies thus tested, 7 have the expected deletion, while 13 have the wild-type genome type (data not shown). While this difference may not be statistically significant due to the small sample size, this slightly lower percentage of deletion mutant could be due to the longer upstream region (1300 bp) vs the downstream region (1000 bp) used in constructing the ecf3 deletion. As illustrated in Fig. 1A, a longer upstream fragment could increase the chance of recombination at this region over the shorter downstream region.
In summary, we have successfully created the first markerless mutagenesis system in V. atypica. Combined with the single-crossover mutagenesis and the shuttle plasmid system constructed previously (Liu, et al., 2012), we now have a versatile genetic tool box, which can be used not only for gene deletion studies, but also for insertion of reporters, because the same principle illustrated in Fig. 1A also works for insertions. This work was supported by an NIH/NIDCR grant 2R15DE019940 to FQ.
Highlights.
The First markerless system in Veillonella
A Versatile mutagenesis system
Counter-selection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aujoulat F, Bouvet P, Jumas-Bilak E, Jean-Pierre H, Marchandin H. Veillonella seminalis sp. nov., a novel anaerobic Gram-stain-negative coccus from human clinical samples, and emended description of the genus Veillonella. Int J Syst Evol Microbiol. 2014;64:3526–3531. doi: 10.1099/ijs.0.064451-0. [DOI] [PubMed] [Google Scholar]
- Barrett AR, Kang Y, Inamasu KS, Son MS, Vukovich JM, Hoang TT. Genetic tools for allelic replacement in Burkholderia species. Appl Environ Microbiol. 2008;74:4498–4508. doi: 10.1128/AEM.00531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JF, Danziger ME, Huang AL, Dahlberg AE, Gregory ST. Engineering the genome of Thermus thermophilus using a counter-selectable marker. J Bacteriol. 2015 doi: 10.1128/JB.02384-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers NI, Palmer RJ, Jr, Cisar JO, Kolenbrander PE. Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque. J Bacteriol. 2008;190:8145–8154. doi: 10.1128/JB.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzink JL, Gibbons RJ, Childs WC, 3rd, Socransky SS. The predominant cultivable microbiota of crevicular epithelial cells. Oral Microbiol Immunol. 1989;4:1–5. doi: 10.1111/j.1399-302x.1989.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Hughes CV, Kolenbrander PE, Andersen RN, Moore LV. Coaggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Appl Environ Microbiol. 1988;54:1957–1963. doi: 10.1128/aem.54.8.1957-1963.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamma JJ, Nakou M, Manti FA. Predominant microflora of severe, moderate and minimal periodontal lesions in young adults with rapidly progressive periodontitis. J Periodontal Res. 1995;30:66–72. doi: 10.1111/j.1600-0765.1995.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Kast P, Hennecke H. Amino acid substrate specificity of Escherichia coli phenylalanyl-tRNA synthetase altered by distinct mutations. J Mol Biol. 1991;222:99–124. doi: 10.1016/0022-2836(91)90740-w. [DOI] [PubMed] [Google Scholar]
- Kristich CJ, Chandler JR, Dunny GM. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid. 2007;57:131–144. doi: 10.1016/j.plasmid.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xie Z, Merritt J, Qi F. Establishment of a tractable genetic transformation system in Veillonella spp. Appl Environ Microbiol. 2012;78:3488–3491. doi: 10.1128/AEM.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan JC, Koestler DC, Stanton BA, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012:3. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RJ, Jr, Diaz PI, Kolenbrander PE. Rapid succession within the Veillonella population of a developing human oral biofilm in situ. J Bacteriol. 2006;188:4117–4124. doi: 10.1128/JB.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S, Kolenbrander PE. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J Bacteriol. 2010;192:2965–2972. doi: 10.1128/JB.01631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge JA, Springer M. Genes for the two subunits of phenylalanyl-tRNA synthesis of Escherichia coli are transcribed from the same promoter. J Mol Biol. 1980;144:595–600. doi: 10.1016/0022-2836(80)90341-1. [DOI] [PubMed] [Google Scholar]
- Rogosa M. The Genus Veillonella. I. General Cultural, Ecological, and Biochemical Considerations. J Bacteriol. 1964;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Okinaga T, Qi F, Zhang Z, Merritt J. Cloning-independent and counterselectable markerless mutagenesis system in Streptococcus mutans. Appl Environ Microbiol. 2011;77:8025–8033. doi: 10.1128/AEM.06362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Liu J, Merritt J, Qi F. A YadA-like autotransporter, Hag1 in Veillonella atypica is a multivalent hemagglutinin involved in adherence to oral streptococci, Porphyromonas gingivalis, and human oral buccal cells. Mol Oral Microbiol. 2014 doi: 10.1111/omi.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]