Abstract
BACKGROUND/OBJECTIVE
The aim of this study was to examine the effect of high dietary methionine (Met) consumption on plasma and hepatic oxidative stress and dyslipidemia in chronic ethanol fed rats.
MATERIALS/METHODS
Male Wistar rats were fed control or ethanol-containing liquid diets supplemented without (E group) or with DL-Met at 0.6% (EM1 group) or 0.8% (EM2 group) for five weeks. Plasma aminothiols, lipids, malondialdehyde (MDA), alanine aminotransferase (ALT), and aspartate aminotransferase were measured. Hepatic folate, S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH) were measured.
RESULTS
DL-Met supplementation was found to increase plasma levels of homocysteine (Hcy), triglyceride (TG), total cholesterol (TC), and MDA compared to rats fed ethanol alone and decrease plasma ALT. However, DL-Met supplementation did not significantly change plasma levels of HDL-cholesterol, cysteine, cysteinylglycine, and glutathione. In addition, DL-Met supplementation increased hepatic levels of folate, SAM, SAH, and SAM:SAH ratio. Our data showed that DL-Met supplementation can increase plasma oxidative stress and atherogenic effects by elevating plasma Hcy, TG, and TC in ethanol-fed rats.
CONCLUSION
The present results demonstrate that Met supplementation increases plasma oxidative stress and atherogenic effects by inducing dyslipidemia and hyperhomocysteinemia in ethanol-fed rats.
Keywords: Ethanol, DL-Methionine supplementation, oxidative stress, plasma lipids, aminothiols
INTRODUCTION
Chronic ethanol exposure increases the risk of adverse health effects through simple hepatic steatosis to steatohepatitis, fibrosis, and cirrhosis. Chronic ethanol consumption perturbs methionine (Met) metabolism, which results in decreased methionine production and increased homocysteine (Hcy) production as a result of reduction in methionine synthase (MS) activity [1,2,3]. Hcy is a potentially toxic aminothiol compound to cells. Numerous clinical and epidemiological studies have demonstrated that increased cardiovascular disease and risk of stroke are strongly associated with enhanced plasma levels of Hcy [4,5,6].
Hcy is a sulfur amino acid intermediate in the transmethylation and transsulfuration pathways, and a precursor of S-adenosylmethionine (SAM) and cysteine (Cys) biosynthesis. Recent studies have shown a direct causal relationship between induction of hyperhomocysteinemia and accelerated atherosclerosis in apolipoprotein E-deficient mice with diet- and/or genetic-induced hyperhomocysteinemia [7]. Methionine is essential for the adequate growth and development of mammals and a precursor of SAM synthesis. SAM is also a precursor of reduced glutathione (GSH) through its conversion to Cys by means of the transsulfuration [8,9]. Chronic ethanol treatment resulted in a significant fall in hepatic SAM level, SAM/S-adenosylhomocysteine (SAH) ratio, and DNA methylation. Therefore, administration of SAM resulted in a partial correction of SAM depletion and a consequent restoration of glutathione levels and prevented chronic alcohol-induced mitochondrial dysfunction in the rat liver [8,10,11]. Hepatic SAM depletion and a decreased SAM:SAH ratio due to chronic ethanol exposure is associated with different degrees of liver injury in animals such as fatty liver, inflammation, and fibrosis [12,13].
In addition, the pathogenesis of ethanol-induced liver injury is associated with the adverse effects of ethanol metabolites and the ability of ethanol to induce oxidative stress. Acute or chronic ethanol ingestion increases reactive oxygen species (ROS) in the liver and plasma [14,15,16]. Oxidative stress, a state of imbalance between the generation of reactive species and the antioxidant level, represents an important pathogenic factor of acute or chronic liver disease, evidenced by decreased antioxidants such as GSH and increased lipid peroxidation products such as malondialdehyde (MDA).
Finkelstein and Martin [17] demonstrated that methionine supplementation of 1.5% increased hepatic SAH only and methionine supplementation of 3.0% caused hepatic accumulation of SAM and SAH. On the other hand, feeding rabbits a chow diet enriched with 0.3% methionine for 6 or 9 months caused hepatic injury including hepatitis and fatty liver [18] and rats received drinking water containing ethanol (20% v/v) and methionine supplemented diet (2% w/w) augmented hepatotoxicity and prooxidant status in chronically ethanol-fed rats [19]. The role of methionine supplementation, toxic and potentially beneficial, remains to be determined in ethanol-fed rats and the underlying mechanisms of its effect on liver or plasma are poorly understood.
In the present study, we examined the effects of dietary methionine supplementation on ethanol-induced changes in oxidative stress, plasma lipid profile, and liver toxicity induced by ethanol ingestion in rats. We evaluated hepatotoxicity and vascular atherogenic potency by analysing biochemical parameters of plasma and liver.
MATERIALS AND METHODS
Animals and diets
Five-week-old male Wistar rats weighing 175-185 g (Orient Bio, Seongnam, Korea) were initially fed a chow diet until they reached body weights of 200-220 g. After a 7 day adjustment, the rats were acclimated for 3 days to the liquid control diet. The animals were randomly assigned to one of four groups of 8 rats each: (1) a pair-fed control group (2) E group; essentially the same as the liquid ethanol diet described by Lieber and DeCarli [20] with the proportion of energy as follows: 11% carbohydrate, 35% fat, 18% protein, and 36% ethanol. Ethanol was introduced into the diets gradually over 7 days. Soybean oil was used as the fat source in the liquid diet. (3) EM1 group: these animals obtained ethanol diet containing 6 g DL-Met/L (4) EM2 group: these animals obtained ethanol diet containing 8 g DL-Met/L. Control and E group were received a liquid diet containing 0.3g DL-Met/L. All diets contained 0.5 g Cys/L and 10 g sulfathiazole (Sigma)/L to inhibit folate formation by gut bacteria. The diets were fed for 5 weeks.
The amount of diet consumed was monitored daily. We tried to pair-feed the animals in the control group by feeding the same amount of food as was consumed by the E group over the preceding 24 hrs.
Rats were housed individually in plastic cages at a temperature of 23 ± 1℃ and a humidity of 50 ± 5% with a daily light cycle from 0600 to 2000 hr. Body weight was measured weekly. The animal experiments followed protocols established by the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Hannam University (HNU 2012-1).
At the end of the 5-week feeding period, the rats were fasted overnight and anesthetised with carbon dioxide. Blood samples were collected by cardiac puncture into heparinised syringes. Blood was immediately centrifuged for 15 min at 1,500 × g and 4℃ to collect the plasma. Liver tissues were removed, washed in ice-cold saline, and frozen rapidly in liquid nitrogen. The samples were stored at -70℃ until analysis.
Plasma biochemical parameters
Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglyceride (TG) were measured using a photometric auto-analyser (Stanbio Lab, Texas, USA). Plasma levels of total cholesterol (TC) and the fraction of HDL-cholesterol (HDL-C) was estimated enzymatically with standard kits (Stanbio Lab, Texas, USA).
Plasma and liver aminothiol compounds
Plasma and hepatic levels of aminothiols including total Hcy, Cys, cysteinylglycine (CysGly), and GSH were determined by high performance liquid chromatography (HPLC) simultaneously with fluorometric detection (excitation at 385 nm and emission at 515 nm) according to Nolin et al. [21]. Aminothiol compounds were separated on a Hypersil Gold ODS analytical column (250 × 4.6 mm I.D., 5 µm particle size) (Thermo, Runcorn, UK). Hepatic levels of Hcy, Cys, and CysGly were below detection limits in this analytical method.
Liver and plasma thiobarbituric acid reactive substances (TBARS)
Plasma and liver lipoperoxides were analysed by HPLC separation of MDA-thiobarbituric acid (TBA) adduct [22]. MDA-TBA adducts were separated on a Zorbax Eclipse C8 column (150 ×4.6 mm I.D., 5 µm particle size) (Agilant, Santa Clara, CA, USA) and Security Guard C8 guard column (Phenomenex, Torrance, USA). MDA-TBA adducts were quantified with fluorometric detection (excitation at 515 nm and emission at 553 nm).
Liver SAM and SAH
To determine hepatic levels of SAM and SAH, portions of the frozen liver were homogenized with 0.4 M HClO4 and centrifuged at 12,000 × g at 4℃ for 30 min. SAM and SAH were analysed by HPLC equipped with 250 × 4.6 mm Ultrasphere 5-µm ODS Betasil analytical column (Thermo, Runcorn, UK) according to Wagner et al. [23].
Liver folate
Liver folate was measured by 96-well microplate microbial (L. rhamnosus) assay [24]. Portions of liver were homogenised and autolysed for hydrolysis of γ-glutamyl residues in the presence of sodium ascorbate at 37℃. Supernatants of liver homogenates were used for the folate assay.
Statistical analysis
The results were expressed as mean ± standard error. Differences among the experimental groups were examined by one-way ANOVA at a significance level of P < 0.05; if significant differences were found, a post-hoc analysis was performed using Duncan's multiple range test. All statistical analyses were performed using SPSS 20.0 for Window (SPSS Inc., Chicago, IL, USA).
RESULTS
Body weight and food intake
It was shown that food intake in rats fed ethanol (E group) was less than in rats fed the control diet (Table 1) because we failed to do complete pair-feeding during the first 3 days of experimental diet period. However, control rats were completely pair-fed with the E group for the consecutive 32 days of the experimental period. Food intake in rats fed ethanol with supplemental methionine (EM1 and EM2) was significantly less during 5 weeks feeding than in rats fed the ethanol alone, exhibiting toxicity of excessive methionine supplementation (Table 1). The final body weight in rats fed methionine supplemented diet was 11.8% (EM1), and 22.9% (EM2) lower than in rats fed ethanol alone. We found that voluntary food intake tended to be lower in rats fed methionine supplemented diet and methionine supplementation depressed food intake in a dose-dependent manner (EM1 and EM2). Ethanol treatment (E) significantly increased the liver:body weight ratios compared to those of rats fed control, whereas methionine supplementation (EM1) decreased the ratios compared to those of ethanol-fed rats (E).
Table 1. Effects of methionine supplementation on body and liver weights in chronic ethanol-treated rats.
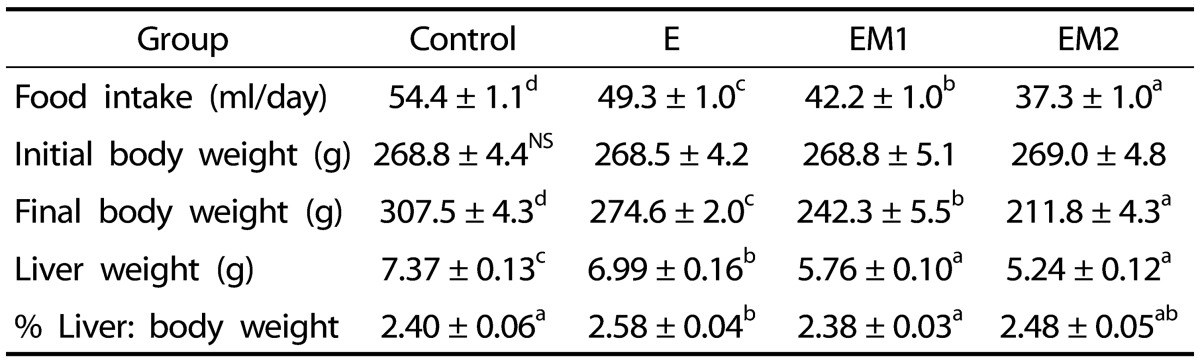
Data are presented as mean ± SE (n = 8). Statistical significance of means was determined using one-way ANOVA followed by Duncan's multiple-range post hoc test. Values not sharing a common superscript letter are significantly different at P < 0.05. NS, not significant. Control, no ethanol; E, ethanol; EM1, ethanol + 0.6% DL-Met; EM2, ethanol + 0.8% DL-Met
Plasma ALT and lipids
Ethanol treatment (E) caused significant increase in plasma ALT activity (P<0.05) and HDL-C (P<0.001) (Table 2). Methionine supplementation (EM1 and EM2) attenuated the rise in plasma ALT activity induced by ethanol treatment, but the change was not statistically significant. Ethanol treatment did not significantly alter plasma TG and TC (Table 2). Methionine supplementation with ethanol feeding (EM1 and EM2) caused significant increase in plasma TG and TC compared with the E group.
Table 2. Effects of methionine supplementation on plasma aminotransferases and lipids in chronic ethanol-treated rats.
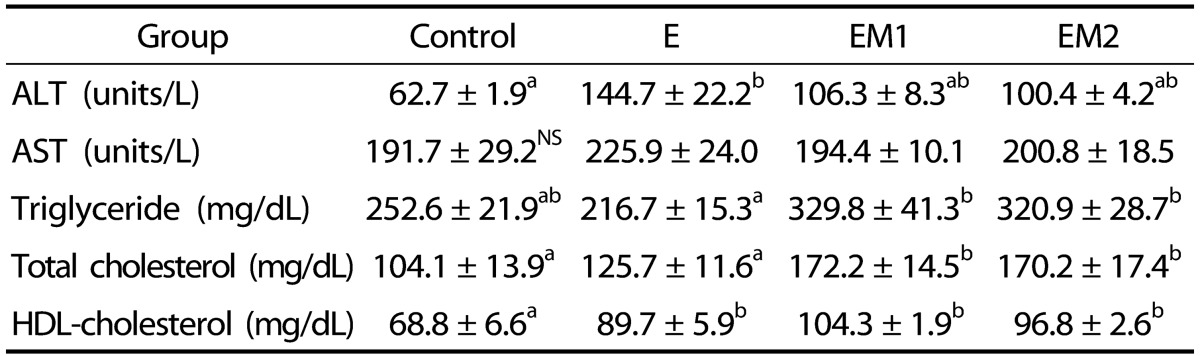
Data are presented as mean ± SE (n = 8). Statistical significance of means was determined using one-way ANOVA followed by Duncan's multiple-range post hoc test. Values not sharing a common superscript letter are significantly different at P < 0.05. NS, not significant. Control, no ethanol; E, ethanol; EM1, ethanol + 0.6% DL-Met; EM2, ethanol + 0.8% DL-Met
Plasma and liver aminothiols and MDA
We evaluated the effect of chronic ethanol treatment and methionine supplementation on plasma and hepatic levels of aminothiol compounds including Hcy, Cys, CysGly, and GSH. As shown in Table 3, ethanol treatment significantly increased plasma Hcy, but it did not affect the concentrations of plasma Cys, CysGly, and GSH. Plasma Hcy was significantly altered by ethanol consumption, and increased moderately by the EM1 diet and severely by the EM2 diet (P < 0.001). In contrast to plasma aminothiol levels, hepatic levels of aminothiol compounds, except for GSH, were very low and not detectable by the analytical method we used in this experiment. Approximate detection limits of aminothiol compounds under the conditions we used were estimated as 1µM for Hcy and CysGly and 15 µM for Cys. The mean hepatic GSH concentration was 3.22 ± 0.23 µmol/g in the control group, which was approximately sixtyfold-higher compared to plasma GSH (Table 3). Plasma GSH was not altered by the ethanol treatment and methionine supplementation. However, liver GSH was increased by ethanol treatment. Methionine supplemented diets with ethanol (EM1 and EM2) did not alter hepatic GSH in comparison to ethanol diet (E). Liver folate concentrations were not affected by ethanol treatment alone, but were elevated by 50% in rats fed the EM1 diet and by 33% in rats fed the EM2 diet (P < 0.05) (Table 3).
Table 3. Effects of methionine supplementation on plasma aminothiols and liver folate in chronic ethanol-treated rats.

Data are presented as mean ± SE (n = 8). Statistical significance of means was determined using one-way ANOVA followed by Duncan's multiple-range post hoc test. Values not sharing a common superscript letter are significantly different at P < 0.05. NS, not significant, Control, no ethanol; E, ethanol; EM1, ethanol + 0.6% DL-Met; EM2, ethanol + 0.8% DL-Met
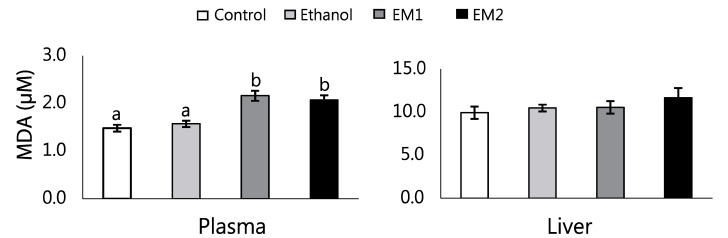
We found that the EM1 and EM2 diet significantly elevated plasma MDA, a marker of lipid peroxidation, compared to the E group (P < 0.01) (Fig. 1). However, liver MDA was not changed by the ethanol treatment and methionine supplementation.
Fig. 1. Effects of methionine supplementation on plasma and liver MDA in chronic ethanol-treated rats.
Data are presented as mean ± SE (n = 8). Statistical significance of means was determined using one-way ANOVA followed by Duncan's multiple-range post hoc test. Values not sharing a common superscript letter are significantly different at P < 0.05. Control, no ethanol; E, ethanol; EM1, ethanol + 0.6% DL-Met; EM2, ethanol + 0.8% DL-Met
Liver SAM, SAH, and SAM:SAH ratio
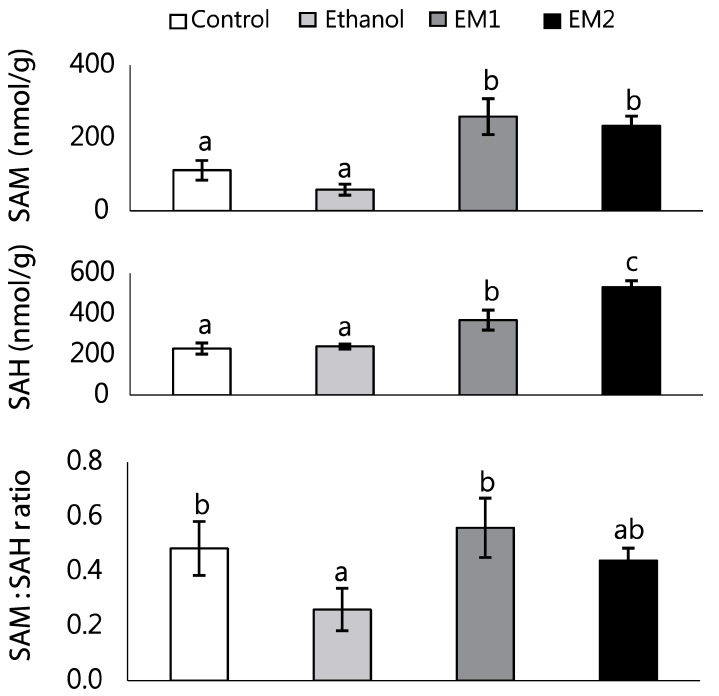
Ethanol treatment (E) significantly decreased hepatic SAM:SAH ratio, whereas hepatic SAM and SAH level was not affected by ethanol feeding (Fig. 2). Dietary methionine-supplementation (EM1 and EM2) resulted in a marked accumulation of hepatic SAM and SAH in rats fed ethanol (P < 0.001). We observed a small increase in hepatic SAH in rats fed the EM1 diet and a remarkable increase of it in rats fed the EM2 diet (P < 0.01). However, hepatic SAM:SAH ratios were restored to control levels by methionine supplementation (EM1 and EM2).
Fig. 2. Effects of methionine supplementation on liver SAM and SAH in chronic ethanol-treated rats.
Data are presented as mean ± SE (n = 8). Statistical significance of means was determined using one-way ANOVA followed by Duncan's multiple-range post hoc test. Values not sharing a common superscript letter are significantly different at P < 0.05. Control, no ethanol; E, ethanol; EM1, ethanol + 0.6% DL-Met; EM2, ethanol + 0.8% DL-Met
DISCUSSION
Ethanol-induced liver injury has been associated with abnormal methionine metabolism [1,25]. Methionine metabolism occurs predominantly in the liver and the liver is known to be the only tissue that responds to excessive dietary methionine by increasing the rate of methionine metabolism [10,13,17]. Chronic ethanol treatment resulted in a significant fall in hepatic SAM level and SAM:SAH ratio and led to DNA hypomethylation. SAM is the principle biological methyl donor in numerous transmethylation reactions and a precursor of reduced GSH through its conversion to Cys by the transsulfuration pathway. GSH is a major endogenous antioxidant that protects cells against injury by scavenging free radicals that play a pathogenic role in alcoholic liver disease.
In the present study, we examined the effect of high dietary methionine supplementation on plasma lipid profile and hepatic and plasma oxidative stress induced by chronic ethanol treatment in rats. Chronic ethanol consumption retarded general growth, elevated plasma aminotransferase activity and Hcy level, and disturbed overall methionine metabolism in the liver. Excessive addition of DL-methionine at a dosage 0.6% (EM1) and 0.8% (EM2) in liquid ethanol diet reduced general growth further and increased plasma TG, cholesterol, and MDA levels in rats, although it tended to decrease plasma ALT. These results show that excess methionine supplementation may exert atherosclerotic effect in rats by inducing dyslipidemia and oxidative stress.
In plasma, excessive intake of methionine (EM1 and EM2) elevated Hcy only among the thiol compounds produced in the transmethylation and transsulfuration pathway of methionine metabolism (Table 3). Change in plasma Hcy was not linear to the applied methionine amount. EM1 diet elevated plasma Hcy slightly whereas EM2 diet elevated it remarkably. This result may be arising from overloading Hcy metabolic capacity with exogenous methionine in the EM2 rat liver. A number of studies have demonstrated that excessive intake of dietary methionine (2.0%, 2.4%, or 3% methionine diet) elevated plasma Hcy concentrations [26,27,28]. The highly reactive thiol group of Hcy is readily oxidized in plasma to form ROS. Thus, high plasma Hcy levels may exert its atherogenic effect on cardiovascular tissue through a mechanism involving oxidative damage.
GSH is synthesized by the γ-glutamyl cycle as a by-product of the transsulfuration pathway and Cys levels are rate-limiting for the synthesis of GSH, the major antioxidant. GSH is the most prevalent cellular thiol compound and it both protects cells from the toxic effects of reactive oxygen compounds and serves for storage and transport form of Cys moieties. Our data demonstrated that the hepatic level of GSH was significantly higher in the E group compared to the control group. Numerous other studies have shown that the hepatic concentrations of GSH are either unchanged or elevated by chronic ethanol consumption [29,30]. Ethanol oxidation generates ROS and induces a state of oxidative stress which contributes to the pathogenesis of alcoholic liver injury. An increase in hepatic GSH may be due to increased synthesis of GSH in respond to oxidative stress by ethanol consumption, reflecting an adaptive change against ethanol-induced lipid peroxide toxicity [29,30]. The hepatic accumulation of GSH is shown to be markedly dependent on the methionine supply in rats [31]. However, plasma and hepatic GSH levels were not affected by methionine supplementation in ethanol-fed rats in our study (Table 3). Since oxidative stress alters GSH, further studies are necessary to clarify the effects of the increased SAM and SAM:SAH ratio on hepatic oxidative stress.
SAM is the principle biological methyl donor to various acceptor substances, including nucleic acids, histones, proteins, phospholipids, and biological amines. Most SAM is used in transmethylation reactions and converted to SAH, which, conversely, is the principal inhibitor of all methylation reactions. Because the Km of many methylation reactions is similar to their inhibitory Ki, the ratio of SAM to SAH may be considered an index of methylation capacity [8,10]. We observed that chronic ethanol consumption decreased the hepatic SAM:SAH ratio significantly, although the change in hepatic SAM and SAH was not statistically significant (Fig. 2). Previous studies have shown that chronic ethanol consumption caused a decrease in SAM concentration and SAM:SAH ratio largely because of decrease in methionine availability due to decreased hepatic activities of methionine synthase [1,13]. Hepatic SAM, SAH, and SAM:SAH ratios were significantly elevated by the supplementation of excess methionine (EM1 and EM2) compared to rats fed ethanol alone (P < 0.01). The effects of ethanol feeding on SAM concentrations have known to be somewhat variable [13]. In rats fed the Lieber-DeCarli ethanol diet for 4 wks, the SAM level was unchanged and actually decreased with more prolonged feeding (for 8wk) [32]. It was proposed that the increase in SAM is caused by both increased synthesis and impaired utilization of SAM because SAH is a potent competitive inhibitor of transmethylation reaction. Both a decrease in the SAM:SAH ratio and increase in the SAH level are known to inhibit transmethylation reaction.
SAM is also a precursor of GSH through its conversion to Cys and particularly important for attenuating the toxicity of free radicals generated by various toxins, including alcohol [32,33]. In patients with liver cirrhosis, hepatic SAM concentration is greatly decreased as a result of reduction in the methionine adenosyltransferase reaction [10,34]. SAM has recently been shown to attenuate ethanol-induced liver injury by restoring hepatic concentrations of GSH depleted by ethanol as well as by up-regulating the transsulfuration pathway [25,35,36]. SAM has been shown to be a noncompetitive inhibitor of cytochrome P450 2E1 (CYP2E1) activity, and depletion of SAM sensitizes hepatocytes to CYP2E1 toxicity in vitro while exogenous SAM protects against CYP2E1-dependent hepatotoxicity in vivo [34]. Depletion of SAM correlates with increased lipid peroxidation and mitochondrial damage. Thus, chronic depletion of SAM can predispose the liver to injury. Changes in hepatic SAM levels vary depending on the levels of supplemental methionine. Excess dietary methionine caused a transient increase in methionine, SAM, and Hcy [17].
GSH have important antioxidative properties [37,38]. GSH is a major endogenous antioxidant that protects cells against injury by scavenging free radicals, which play a pathogenic role in alcoholic liver disease. SAM is a precursor of reduced glutathione through its conversion to Cys by means of the transsulfuration pathway [8,34]. In the transsulfuration pathway, SAM regulates the synthesis of GSH by its facilitation of the cystationine β synthase reaction. Therefore reduced SAM leads to reduced production of GSH. In this study, the hepatic content of SAM was increased by methionine load, and increased SAM levels may stimulate methionine adenosyltransferase further. We found that excess methionine increased plasma MDA level but not hepatic MDA in ethanol-fed rats (Fig. 1). These results may show that methionine supplementation can attenuate ethanol-induced hepatic oxidative stress by increasing SAM and SAM:SAH ratio.
In summary, excess methionine supplementation appeared to increase the atherogenic effects on plasma, as indicated by increased plasma levels of Hcy, TG, cholesterol, and MDA. On the other hand, excess methionine supplementation increased hepatic levels of SAM, SAH, and the SAM:SAH ratio indicating a possible protective role against alcohol-induced hepatic oxidative stress by activation of the transsulfuration pathway producing GSH. Further studies are needed to clarify the effects of methionine supplementation with ethanol consumption on oxidative stress of plasma and liver using various indicators of oxidative stress.
Footnotes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (2010-0024002) and by Hannam University Research Funds in 2013.
References
- 1.Barak AJ, Beckenhauer HC, Tuma DJ. Methionine synthase. a possible prime site of the ethanolic lesion in liver. Alcohol. 2002;26:65–67. doi: 10.1016/s0741-8329(01)00201-4. [DOI] [PubMed] [Google Scholar]
- 2.Halsted CH, Villanueva J, Chandler CJ, Stabler SP, Allen RH, Muskhelishvili L, James SJ, Poirier L. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology. 1996;23:497–505. doi: 10.1002/hep.510230314. [DOI] [PubMed] [Google Scholar]
- 3.Waly MI, Kharbanda KK, Deth RC. Ethanol lowers glutathione in rat liver and brain and inhibits methionine synthase in a cobalamin-dependent manner. Alcohol Clin Exp Res. 2011;35:277–283. doi: 10.1111/j.1530-0277.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 5.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Jiang X, Yang F, Gaubatz JW, Ma L, Magera MJ, Yang X, Berger PB, Durante W, Pownall HJ, Schafer AI. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood. 2003;101:3901–3907. doi: 10.1182/blood-2002-08-2606. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 9.Lieber CS, Casini A, DeCarli LM, Kim CI, Lowe N, Sasaki R, Leo MA. S-adenosyl-L-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology. 1990;11:165–172. doi: 10.1002/hep.1840110203. [DOI] [PubMed] [Google Scholar]
- 10.Mato JM, Alvarez L, Ortiz P, Mingorance J, Durán C, Pajares MA. S-adenosyl-L-methionine synthetase and methionine metabolism deficiencies in cirrhosis. Adv Exp Med Biol. 1994;368:113–117. doi: 10.1007/978-1-4615-1989-8_11. [DOI] [PubMed] [Google Scholar]
- 11.Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, Page GP, Chhieng D, Jhala N, Landar A, Kharbanda KK, Ballinger S, Darley-Usmar V. S-adenosylmethionine prevents chronic alcoholinduced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;291:G857–G867. doi: 10.1152/ajpgi.00044.2006. [DOI] [PubMed] [Google Scholar]
- 12.Lieber CS. S-adenosyl-L-methionine: its role in the treatment of liver disorders. Am J Clin Nutr. 2002;76:1183S–1187S. doi: 10.1093/ajcn/76/5.1183S. [DOI] [PubMed] [Google Scholar]
- 13.Lu SC, Huang ZZ, Yang H, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G178–G185. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- 14.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278–290. doi: 10.1079/pns2006496. [DOI] [PubMed] [Google Scholar]
- 15.Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151–180. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- 16.Munday R. Toxicity of thiols and disulphides: involvement of free-radical species. Free Radic Biol Med. 1989;7:659–673. doi: 10.1016/0891-5849(89)90147-0. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Adaptation to methionine excess. J Biol Chem. 1986;261:1582–1587. [PubMed] [Google Scholar]
- 18.Toborek M, Kopieczna-Grzebieniak E, Drózdz M, Wieczorek M. Increased lipid peroxidation and antioxidant activity in methionine-induced hepatitis in rabbits. Nutrition. 1996;12:534–537. doi: 10.1016/s0899-9007(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 19.Yalçinkaya S, Unlüçerçi Y, Uysal M. Methionine-supplemented diet augments hepatotoxicity and prooxidant status in chronically ethanol-treated rats. Exp Toxicol Pathol. 2007;58:455–459. doi: 10.1016/j.etp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Lieber CS, DeCarli LM. Animal models of chronic ethanol toxicity. Methods Enzymol. 1994;233:585–594. doi: 10.1016/s0076-6879(94)33061-1. [DOI] [PubMed] [Google Scholar]
- 21.Nolin TD, McMenamin ME, Himmelfarb J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:554–561. doi: 10.1016/j.jchromb.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong SH, Knight JA, Hopfer SM, Zaharia O, Leach CN, Jr, Sunderman FW., Jr Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem. 1987;33:214–220. [PubMed] [Google Scholar]
- 23.Wagner J, Claverie N, Danzin C. A rapid high-performance liquid chromatographic procedure for the simultaneous determination of methionine, ethionine, S-adenosylmethionine, S-adenosylethionine, and the natural polyamines in rat tissues. Anal Biochem. 1984;140:108–116. doi: 10.1016/0003-2697(84)90140-4. [DOI] [PubMed] [Google Scholar]
- 24.Tamura T. Microbiological assay of folates. In: Picciano MF, Stokstad EL, Gregory JF III, editors. Folic Acid Metabolism in Health and Disease. New York (NY): Wiley-Liss; 1990. pp. 121–137. [Google Scholar]
- 25.Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C. S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J Nutr Biochem. 2003;14:591–597. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 26.Toue S, Kodama R, Amao M, Kawamata Y, Kimura T, Sakai R. Screening of toxicity biomarkers for methionine excess in rats. J Nutr. 2006;136:1716S–1721S. doi: 10.1093/jn/136.6.1716S. [DOI] [PubMed] [Google Scholar]
- 27.Benevenga NJ, Harper AE. Alleviation of methionine and homocystine toxicity in the rat. J Nutr. 1967;93:44–52. doi: 10.1093/jn/93.1.44. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Ma J, Xia M, Zhu H, Ling W. Mild hyperhomocysteinemia induced by feeding rats diets rich in methionine or deficient in folate promotes early atherosclerotic inflammatory processes. J Nutr. 2004;134:825–830. doi: 10.1093/jn/134.4.825. [DOI] [PubMed] [Google Scholar]
- 29.Yang CM, Carlson GP. Effects of ethanol on glutathione conjugation in rat liver and lung. Biochem Pharmacol. 1991;41:923–929. doi: 10.1016/0006-2952(91)90197-d. [DOI] [PubMed] [Google Scholar]
- 30.Aykaç G, Uysal M, Yalçin AS, Koçak-Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985;36:71–76. doi: 10.1016/0300-483x(85)90008-3. [DOI] [PubMed] [Google Scholar]
- 31.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 32.Barak AJ, Beckenhauer HC, Tuma DJ. S-adenosylmethionine generation and prevention of alcoholic fatty liver by betaine. Alcohol. 1994;11:501–503. doi: 10.1016/0741-8329(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 33.Avila MA, García-Trevijano ER, Martínez-Chantar ML, Latasa MU, Pérez-Mato I, Martínez-Cruz LA, del Pino MM, Corrales FJ, Mato JM. S-Adenosylmethionine revisited: its essential role in the regulation of liver function. Alcohol. 2002;27:163–167. doi: 10.1016/s0741-8329(02)00228-8. [DOI] [PubMed] [Google Scholar]
- 34.Cederbaum AI. Hepatoprotective effects of S-adenosyl-L-methionine against alcohol- and cytochrome P450 2E1-induced liver injury. World J Gastroenterol. 2010;16:1366–1376. doi: 10.3748/wjg.v16.i11.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Ruiz C, Morales A, Colell A, Ballesta A, Rodés J, Kaplowitz N, Fernández-Checa JC. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- 36.Colell A, García-Ruiz C, Morales A, Ballesta A, Ookhtens M, Rodés J, Kaplowitz N, Fernández-Checa JC. Transport of reduced glutathione in hepatic mitochondria and mitoplasts from ethanol-treated rats: effect of membrane physical properties and S-adenosyl-L-methionine. Hepatology. 1997;26:699–708. doi: 10.1002/hep.510260323. [DOI] [PubMed] [Google Scholar]
- 37.Lapenna D, de Gioia S, Ciofani G, Mezzetti A, Ucchino S, Calafiore AM, Napolitano AM, Di Ilio C, Cuccurullo F. Glutathione-related antioxidant defenses in human atherosclerotic plaques. Circulation. 1998;97:1930–1934. doi: 10.1161/01.cir.97.19.1930. [DOI] [PubMed] [Google Scholar]
- 38.Kugiyama K, Ohgushi M, Motoyama T, Hirashima O, Soejima H, Misumi K, Yoshimura M, Ogawa H, Sugiyama S, Yasue H. Intracoronary infusion of reduced glutathione improves endothelial vasomotor response to acetylcholine in human coronary circulation. Circulation. 1998;97:2299–2301. doi: 10.1161/01.cir.97.23.2299. [DOI] [PubMed] [Google Scholar]