Abstract
Background
Enterococci have assumed great clinical importance because of their increasing resistance to various antimicrobial agents. Thus, knowledge about the antibiogram of these multidrug resistant isolates is of utmost importance in formulating an effective antibiotic policy to treat these infections and reducing the morbidity and mortality. Aim of this study was to assess the antimicrobial resistance pattern of enterococci and determine the prevalence of multidrug resistance among them.
Methods
This cross sectional study was carried out from August 2011 to February 2014, in which 200 non-repetitive clinical isolates of enterococci were included. Antimicrobial susceptibility testing was done by disc diffusion method. Minimum inhibitory concentration (MIC) of gentamicin, streptomycin, vancomycin, teicoplanin and linezolid was determined by E-test method.
Results
The prevalence of multidrug resistance among enterococcal isolates was found to be 63%. Varying levels of resistance was seen to various antibiotics. Most of the isolates were resistant to penicillin (95%), ampicillin (95%) and cotrimoxazole (90%). High level aminoglycoside resistance (HLAR) and glycopeptide resistance was seen in 39% and 14% isolates respectively. Only 4 isolates (2%) were found to be resistant to linezolid.
Conclusion
The prevalence of multidrug resistance among enterococci was found to be 63%, the resistance being more common in Enterococcus faecium as compared to Enterococcus faecalis. The study highlights the emergence and increased prevalence of multidrug resistant enterococci which pose a serious therapeutic challenge.
Keywords: Multidrug resistant enterococci, Antimicrobial susceptibility testing, Minimum inhibitory concentration, High-level aminoglycoside resistance, Glycopeptide resistance
Introduction
Enterococci are a part of the normal human fecal flora. Over the past few decades, they have evolved from being an intestinal commensal organism of little clinical significance to becoming one of the most common nosocomial pathogens associated with significant morbidity and mortality.1,2 The enterococci are now receiving increased attention because of its resistance to multiple antimicrobial drugs, which probably explains a large part of its prominence in nosocomial infections. The most common nosocomial infections caused by enterococci are urinary tract infections followed by surgical site infections.3–5 Development of antimicrobial resistance in enterococci has posed enormous challenges for clinicians in recent years. Prolonged stay in hospital, empirical use of antibiotics and lack of sufficient information and programs to control rapid spread of enterococci has led to increased mortality caused by enterococcal infections.6
The antimicrobial therapy of enterococcal infections is problematic because of the inherent resistance shown by enterococci to several commonly used antibiotics such as cephalosporins, low-level aminoglycosides, low-level clindamycin, trimethoprim-sulfamethoxazole. This problem is amplified because of their acquired resistance to all currently available antibiotics that leaves the clinicians with very limited treatment options and results in the selection and spreading of multidrug-resistant (MDR) strains in hospitals.6–8
Complete antimicrobial susceptibility testing and knowledge of the antibiogram is extremely essential to formulate therapeutic approach for the treatment of enterococcal infections in order to control the spread of such multidrug resistant bacteria.
This study was conducted with an aim to determine the antimicrobial resistance pattern and the prevalence of multidrug resistance among enterococcal isolates at a tertiary care centre.
Materials and methods
Isolation and identification of enterococci: A cross sectional study was carried out from August 2011 to February 2014. A total of 200 non-repetitive clinical isolates of enterococci from various clinical specimens received in a microbiology laboratory of a tertiary care centre were obtained. These isolates were identified at the species level with the help of conventional phenotypic methods which included Gram's stain, colony morphology, catalase test, bile esculin test, growth in 6.5% NaCl, mannitol fermentation, arginine dihydrolase test, motility test, arabinose fermentation, lactose fermentation and sucrose fermentation.
Antimicrobial susceptibility testing: All enterococcal isolates were tested for their susceptibility to various antibiotics by Kirby Bauer disc diffusion method. For high level aminoglycoside resistance testing, high-level gentamicin disc of 120 μg and high-level streptomycin disc of 300 μg was used. Minimum inhibitory concentration (MIC) of gentamicin, streptomycin, vancomycin, teicoplanin and linezolid was also determined by using E-test method (Figs. 1–3). All the results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Fig. 1.

E-test showing high-level gentamicin and high-level aminoglycoside resistance.
Fig. 2.

E-test showing vancomycin and teicoplanin resistant enterococcal isolate.
Fig. 3.

E-test showing linezolid resistant isolate of Enterococcus faecium.
Results
In the present study, the age of the patients from whom enterococci were obtained varied from 19 years to 78 years of age. Maximum number of cases was from age group more than 60 years comprising 30.5% of total cases followed by 21–30 years (25%) and age group 31–40 yrs (23.5%). It was noticed that amongst the 200 isolates, 108 samples were from female patients and 92 samples were from male patients. Male to female ratio was 0.85:1. Table 1 shows the age and sex wise breakup of the various isolates of enterococci. In this study, 54 patients from whom enterococci were isolated were from ICU, 80 from various wards and 66 were from OPD (Fig 4).
Table 1.
Age and sex wise distribution of patients from whom enterococci were isolated.
| Age | Male | Female | Total | % |
|---|---|---|---|---|
| Upto 10 yrs | – | – | 0 | |
| 11-20 yrs | 05 | 04 | 09 | 4.5 |
| 21–30 yrs | 24 | 26 | 50 | 25 |
| 31–40 yrs | 14 | 33 | 47 | 23.5 |
| 41–50 yrs | 05 | 08 | 13 | 6.5 |
| 51–60 yrs | 08 | 12 | 20 | 10 |
| Above 60 yrs | 36 | 25 | 61 | 30.5 |
| Total | 92 | 108 | 200 | 100 |
Fig. 4.
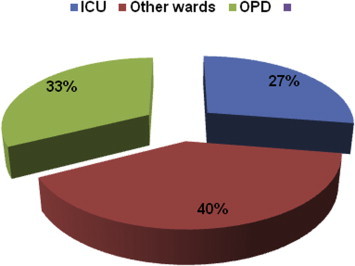
Ward-wise distribution of enterococcal isolates.
Out of 200 isolates, 150 were Enterococcus faecalis and 50 were Enterococcus faecium. No other enterococcal species were isolated. The most common clinical sample from which enterococci were isolated was urine (114), followed by pus (32), blood (28) and others (Fig 5). The urinary isolates which fulfilled the criteria for significant bacteriuria were only included in the study and processed further and other isolates which did not fulfill the criteria for significant bacteriuria were regarded as colonizers and discarded.
Fig. 5.
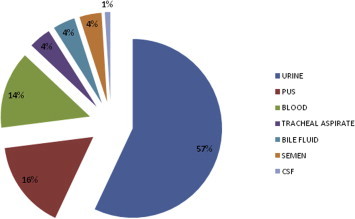
Sample-wise distribution of enterococcal isolates.
Out of these 200 isolates, 195 isolates (97.5%) were resistant to both penicillin and ampicillin, 190 (95%) were resistant to cotrimoxazole, 124 (62%) were resistant to ciprofloxacin and 102 (51%) were resistant to Levofloxacin (Fig 6).
Fig. 6.
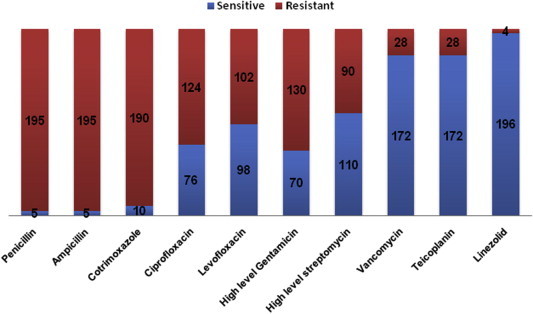
Antibiogram of enterococcal isolates.
A total of 106 (53%) isolates were resistant to high-level gentamicin and 76 (38%) were resistant to high-level streptomycin by disc diffusion method and 64 isolates were resistant to both. However, by E-test method, a total of 130 isolates (65%) were high-level gentamicin resistant (HLGR) with MIC ≥ 500 μg/ml and 90 isolates (45%) were high-level streptomycin resistant (HLSR) with MIC ≥ 1000 μg/ml. Out of these isolates, 78 isolates were found to be high-level aminoglycoside resistant (HLAR) as they showed high-level resistance to both gentamicin and streptomycin.
28 isolates (14%) were found to be resistant to both glycopeptides, i.e. teicoplanin and vancomycin by the disc diffusion method as well as E-test method. Only 4 out of 200 isolates (2%) were found to be linezolid resistant by both disc diffusion and E-test method.
It is seen that the resistance to all the antimicrobials is more common in E. faecium as compared to E. faecalis (Table 2).
Table 2.
Species-wise distribution of enterococcal isolates showing antimicrobial resistance pattern.
| Antibiotics | E. faecalis (n = 150) | E. faecium (n = 50) | Total (n = 200) |
|---|---|---|---|
| Penicillin | 146 (97.3%) | 49 (98%) | 195 (97.5%) |
| Ampicillin | 146 (97.3%) | 49 (98%) | 195 (97.5%) |
| Cotrimoxazole | 142 (94.7%) | 48 (96%) | 190 (95%) |
| Ciprofloxacin | 87 (58%) | 37 (74%) | 124 (62%) |
| Levofloxacin | 70 (46.7%) | 32 (64%) | 102 (51%) |
| High-level gentamicin | 92 (61.3%) | 38 (76%) | 130 (65%) |
| High-level streptomycin | 59 (39.3%) | 31 (62%) | 90 (45%) |
| Vancomycin | 06 (4%) | 22 (44%) | 28 (14%) |
| Teicoplanin | 06 (4%) | 22 (44%) | 28 (14%) |
| Linezolid | 01 (0.7%) | 03 (6%) | 4 (2%) |
Discussion
Enterococci colonize the bowels of more than 90% of healthy humans. E. faecalis accounts for more than 80% of the enterococcal isolates in clinical samples, but in recent years E. faecium has become more common, probably because of its greater antibiotic resistance to multiple antibiotics.3,4,9 For the last two decades, enterococci have been the 3rdmost common cause of hospital-acquired infections (HAI) after E.coli and Staphylococcus aureus and ahead of Pseudomonas aeruginosa.5,10 The most common nosocomial infections caused by enterococci are urinary tract infections (associated with instrumentation and antimicrobial administration) followed by intra-abdominal and pelvic infections. They also cause surgical site infections, bacteremia, endocarditis, neonatal sepsis and rarely meningitis.3–5,9–12
As shown in Fig 5, the most common clinical sample from which enterococci were isolated was urine (57%) followed by pus (16%), blood (14%), tracheal aspirate (4%), semen (4%), bile fluid (4%) and CSF (1%). In other studies also, urine was the most common sample yielding enterococci, such as, Mathur et al13 obtained 49%, Karmarkar et al14 obtained 50% and Udo et al15 obtained 36.6% of enterococci from urine samples.
In various studies, E. faecalis has been the predominant enterococcal species accounting for 80–85% of clinical isolates, followed by E. faecium which accounts for about 10–15% of clinical isolates.12 In the present study, various phenotypic tests were used to identify the species of the enterococcal isolates. Correct speciation is important since there is variation in antibiotic resistance by different species. In the present study, out of 200 isolates, 150 (75%) were E. faecalis and 50 (25%) were E. faecium.
In the present study, 195 (97.5%) isolates were resistant to penicillin and ampicillin. However, Mathur et al13 reported 66% isolates to be resistance to ampicillin. Not much difference was seen between the resistance of E. faecalis and E. faecium to both penicillin and ampicillin. Flouroquinolone resistance, in the present study, was found to be 51–62%. Similarly, Kapoor et al16 reported 72% strains resistant to ciprofloxacin by disk diffusion method. Resistance to fluoroquinolones was more common in E. faecium as compared to E. faecalis (Table 2).
Enterococci show intrinsic low-level cross resistance to all aminoglycosides due to decreased uptake of antibiotics. Therefore, there is no meaning in testing susceptibility of clinical isolates of enterococci to low-level aminoglycosides. Acquired resistance to high level of aminoglycosides can also be present in enterococci due to genes encoding aminoglycoside modifying enzymes (AMEs).
High-level resistance to aminoglycosides (HLAR) is of great clinical concern, since it eliminates synergy with cell wall active antibiotics, which renders treatment of serious enterococcal infections difficult.6 Out of 200 enterococcal isolates, 65% were found to be HLGR and 45% were HLSR. Various other studies have also indicated HLGR to be more common than HLSR in all species of enterococci.17 In the present study, 32% isolates were found to be HLAR by Kirby Bauer disc diffusion method as compared to 39% isolates by E-test method. This indicates that E-test method is a better method to confirm HLAR among enterococci because it is possible that disc diffusion method may not detect borderline resistance. Moreover, both HLGR and HLSR were seen to be more common in E. faecium (62%–76%) as compared to E. faecalis (39.3%–61.3%). These results are in concurrence with the results of other studies.13,18,19
In the present study, 28 (14%) isolates were found to be vancomycin and teicoplanin resistant by disk diffusion method. MICs of vancomycin and teicoplanin for all isolates were determined by the E-test method. All the 28 isolates showed high level resistance to both vancomycin and teicoplanin (MIC > 256 μg/ml) by E-test. 22 out of 50 isolates (44%) of E. faecium and 6 out of 150 isolates (4%) of E. faecalis were found to be resistant to vancomycin and teicoplanin. It has also been found in various studies that E. faecium accounts for far fewer clinical enterococcal isolates than E. faecalis, but it is far more resistant to glycopeptides. In a study conducted by Deshpande et al,6 less than 2% of E. faecalis were found to be resistant to vancomycin, whereas 52% of the E. faecium isolates were resistant to vancomycin. The frequency and extent of glycopeptide resistance in this study were much higher compared to those of previous reports from India.20–22
Linezolid has demonstrated good anti-enterococcal activity. However, the emergence of linezolid resistance in enterococci is an alarming problem in the treatment of VRE infections.12,23 In the present study, 4 out of the 200 (2%) isolates were found to be resistant to linezolid by disk diffusion method and by E-test method.
Multidrug resistance is defined as resistance to at least one agent of the three antimicrobial classes.24 In the present study, the prevalence of multidrug resistant (MDR) enterococci was found to be 63% (126/200), which is higher as compared to study by Deshpande et al.6 The multidrug resistance was more widespread in E. faecium (36/50) as compared to E. faecalis (90/150). Four isolates, which were found to be linezolid resistant, exhibited resistance to all tested antibiotics. This finding is of particular concern since the high prevalence of colonization and/or infection with MDR enterococci has left the clinician with no alternative treatment options.
In the present study, the average duration of hospitalization of 134 hospitalized patients was 8.11 days whereas that of patients infected with MDR enterococci was 15.65 days. Forty eight out of 54 isolates (88.9%) from ICU, 56/80 isolates (70%) from various wards and 22/66 isolates (33.3%) from OPD were found to be multidrug resistant. A total of 65 patients, from whom enterococci were isolated, were suffering from one or the other malignancy and were on immunosuppressive drugs or were neutropenic. Out of these 65 isolates, 52 (80%) were found to be multidrug resistant. All the 126 patients, from whom multidrug resistant enterococci were isolated, were exposed to multiple antibiotics, such as β-lactams, fluoroquinolones and glycopeptides, during their hospital stay.
The present study corroborates with the fact that the risk factors for infection with multidrug resistant enterococcal infection are elderly patients, prolonged hospitalization, prior exposure to antibiotics, severity of disease condition and immunosuppression.
Conclusion
The prevalence of multidrug resistance among enterococcal isolates was found to be 63%, resistance being more common in E. faecium (72%) than that in E. faecalis (45%). This study demonstrates the increased prevalence of multidrug resistant enterococci with few isolates being resistant to all the antibiotics tested, thus posing a serious therapeutic challenge. This situation warrants the implementation of an efficient infection control program and regular surveillance of antimicrobial resistance of enterococci in order to establish a rational antibiotic policy for the better management of enterococcal infections.
Conflicts of interest
All authors have none to declare.
References
- 1.Giridhara Upadhayay P.M., Ravikumar K.L., Umapathy B.L. Review of virulence factor of Enterococcus: an emerging nosocomial pathogen. Indian J Med Microbiol. 2009;27:301–305. doi: 10.4103/0255-0857.55437. [DOI] [PubMed] [Google Scholar]
- 2.Schouten M.A., Hoogkamp-Korstanje J.A., Meis J.F., Voss A. Prevalence of vancomycin resistant enterococci in Europe. Eur J Clin Microbiol Infect Dis. 2000;19:816–822. doi: 10.1007/s100960000390. [DOI] [PubMed] [Google Scholar]
- 3.Murray B.E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moellering R.C., Jr. Emergence of enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–1178. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 5.Schaberg D.R., Culver D.H., Gaynes R.P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91(suppl 3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande V.R., Karmarkar M.G., Mehta P.R. Prevalence of multidrug resistant enterococci in a tertiary care hospital in Mumbai, India. J Infect Dev Ctries. 2013;7(2):155–158. doi: 10.3855/jidc.3018. [DOI] [PubMed] [Google Scholar]
- 7.Cetinkaya Y., Falk P., Mayhall C.G. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000;13:686–707. doi: 10.1128/cmr.13.4.686-707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray B.E. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 9.The gram positive cocci Part II: Streptococci, Enterococci and the “Streptococcus-like” bacteria [chapter 12]. In: Koneman EW, Allen SD, Janda WM, Schrekenberger PC, Winn WC, eds. Color Atlas and Textbook of Diagnostic Microbiology, 5th ed. New York: JB Lipincott; 1997:577–649.
- 10.Centers for Disease Control. Nosocomial Infection Surveillance 1984. MMWR Morb Mortal Wkly Rep, 1986; 35(SS 1):17SS–29SS. [PubMed]
- 11.Laboratory methods for detection of antibacterial resistance [chapter 18]. In: Forbes BA, Sahm DF, Weissfield AS eds. Bailey and Scott's Diagnostic Microbiology. 10th ed. London: Mosby; 1998:250–272.
- 12.Sood S., Malhotra M., Das B.K., Kapil A. Enterococcal infections and antimicrobial resistance. Indian J Med Res. 2008;128(2):111–121. [PubMed] [Google Scholar]
- 13.Mathur P., Kapil A., Chandra R., Sharma P., Das B. Antimicrobial resistance in Enterococcus faecalis at a tertiary care centre in Northern India. Indian J Med Res. 2003;118:25–28. [PubMed] [Google Scholar]
- 14.Karmarkar M.G., Gershom E.S., Mehta P.R. Enterococcal infections with special reference to phenotypic characterization & drug resistance. Indian J Med Res. 2004;119(suppl l):22–25. [PubMed] [Google Scholar]
- 15.Udo E.E., Al-Sweih N., Philips O.A., Chugh T.D. Species prevalence and antibacterial resistance of enterococci isolated in Kuwait hospitals. J Med Microbiol. 2003;52(2):163–168. doi: 10.1099/jmm.0.04949-0. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor L., Randhawa V.S., Deb M. Antimicrobial resistance of enterococcal blood isolates at a pediatric care hospital in India. Jpn J Infect Dis. 2005;58(2):101–103. [PubMed] [Google Scholar]
- 17.Padmasini E., Padmaraj R., Ramesh S.S. High level aminoglycoside resistance and distribution of aminoglycoside resistant genes among clinical isolates of Enterococcus species in Chennai, India. Sci World J. 2014 doi: 10.1155/2014/329157. http://dx.doi.org/10.1155/2014/329157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telkar A., Baragundi M.C., Raghavendra V.P., Vishwanath G., Chandrappa N.R. Change in prevalence and antibiotic resistance of enterococcal species isolated from blood cultures. J Clin Diagn Res. 2012;6:405–408. [Google Scholar]
- 19.Baragundi M.C., Sonth S.B., Solabannawar S., Patil C.S., Yemul V.L. The species prevalence and antimicrobial resistance pattern of enterococcal isolates in a tertiary health-care centre. J Clin Diagn Res. 2010;4:3405–3409. [Google Scholar]
- 20.Modi G.B., Soni S.T., Patel K.J., Goswami H.M., Vegad M.M. Prevalence of vancomycin resistant enterococci in a tertiary care hospital, Western India. Int J Microbiol Res. 2012;4(2):182–185. [Google Scholar]
- 21.Fernandes S.C., Dhanashree B. Drug resistance and virulence determinants in clinical isolates of Enterococcus species. Ind J Med Res. 2013;137(5):981–985. [PMC free article] [PubMed] [Google Scholar]
- 22.Shafiyabi S., Mariraj J., Sumathi S., Shanmugam, Krishna S. Emergence of vancomycin resistant enterococci in a tertiary care hospital in South India. Int J Pharm Biomed Res. 2013;4(2):111–113. [Google Scholar]
- 23.Adhikari L. High level aminoglycoside resistance and reduced susceptibility to vancomycin in nosocomial enterococci. J Glob Infect Dis. 2010;2(3):231–235. doi: 10.4103/0974-777X.68534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magiorakos A.P., Srinivasan A., Carey R.B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]


