Abstract
Background
Magnetic Resonance Imaging (MRI) plays an important role in the evaluation and management of adenomyosis. In this study, we first diagnosed the adenomyosis on MRI and then we analyzed the MRI changes in the uterus in pre and post intrauterine progesterone implants cases.
Method
All the patients with clinical diagnosis of menorrhagia or dysmenorrhea were screened by Ultrasonography (USG) of the pelvis. Patients with heterogeneous echo texture of the uterus were then evaluated by the MRI of the pelvis. All patients with MRI findings suggestive of adenomyosis formed the study group.
Result
On MRI study 60 patients were diagnosed as adenomyosis, 68.33% had diffuse adenomyosis and 31.66% had focal adenomyosis. 83% of diagnosed adenomyosis cases had high intensity signal foci which were seen in 75% cases of diffuse adenomyosis and 100% cases of focal adenomyosis. 50 diagnosed adenomyosis cases were then reviewed after 03 months, 06 months and 12 months to see for any change in the MRI findings in the post intrauterine implant cases. On follow up MRI after post progesterone intrauterine implant, 50% of the cases showed reduction in the high intensity signals, 10% of the cases showed mild reduction in the junctional zone thickness with no significant change in the uterine size.
Conclusions
It is inferred that MR imaging is not only helpful in diagnosing but also helpful in monitoring the effects of hormonal therapy in adenomyosis.
Keywords: Adenomyosis uterus, Intrauterine progesterone implant, MRI
Introduction
Adenomyosis is a common gynecologic condition that affects menstruating women. Diagnosis based on clinical findings is usually difficult because of the nonspecific nature of the symptoms and the frequent coexistence of other pelvic diseases.1 With the advent of high-resolution imaging techniques, like ultrasonography (USG) and Magnetic Resonance Imaging (MRI), accurate diagnosis of adenomyosis is now possible.2,3 Trans-abdominal sonography (TAS)/transvaginal sonography (TVS) is commonly used as the initial imaging modality for diagnosing adenomyosis. Improved spatial resolution TVS makes it is more accurate in diagnosing adenomyosis.2,4
MRI is an accurate, noninvasive modality for diagnosing adenomyosis and is thought to be more precise than TVS in distinguishing adenomyosis from various pathologies like leiomyoma that may simulate adenomyosis. This is perhaps clinically very relevant for planning appropriate treatment protocol.1,3 MRI allows excellent direct visualization and evaluation of zonal architecture of the uterus.5–8
The role of imaging in evaluating patients with suspected adenomyosis is to establish a correct diagnosis, to determine the extent and depth of myometrial penetration and to monitor the evolution of the disease in patients receiving conservative therapy.2
In earlier days, treatment of adenomyosis mainly consisted of hysterectomy. However, off late, conservative mode of treatment like intrauterine progesterone implants have become more popular. Amongst various intrauterine progesterone implants, the only levonorgestrel-releasing intrauterine system (LNG-IUS) that has been approved for general public use is Mirena1 (Schering AG). It is a T-shaped plastic intrauterine device (IUD) that releases levonorgestrel (20 mcg per day) directly into the uterine cavity for 5 years.9
Although MRI has been extensively used for the diagnosis of adenomyosis, to the best of our knowledge, literature is scant describing follow up changes in patients of adenomyosis treated with intrauterine progesterone implant. The present study was undertaken to evaluate post progesterone implant MRI changes in cases of adenomyosis.
In this study, we evaluated the MRI findings of suspected cases of adenomyosis and this was then followed by a comparative analysis of changes in the MRI findings in the pre & post intrauterine progesterone implants.
Materials and methods
This is a prospective diagnostic analytical study carried out in the Radiology and Gynecology Departments of a tertiary care teaching hospital. Approval of institutional ethical committee was taken prior to this study. The study period was from Aug 2010 to Aug 2012.
We have calculated the sample size from the previous study by Bragheto et al10 to assess the difference of 5 mm with alfa of 1% and power of 90% with pretext mean of 17.7 mm (SD = 0.90) and post treatment mean of 13.1 (SD = 0.8). The minimum required sample size was 4, however we studied 60 patients.
All the patients with clinical diagnosis of menorrhagia or dysmenorrhea referred to the radiology department by the gynecologists were screened by per abdominal & transvaginal USG of the pelvis. Patients with normal sized uterus or bulky uterus with heterogeneous echo texture of the myometrium were then further evaluated by the MRI of the pelvis. All patients with MRI findings suggestive of diffuse or focal adenomyosis formed the study group.
Exclusion criteria were-
-
1)
Patients with normal sized uterus on USG with normal echo texture of the myometrium.
-
2)
A maximum junctional zone thickness (JZT) of 10 mm or less on MRI.
-
3)
Patients who were claustrophobic
-
4)
Patients lost to follow up/operated/expulsion of IUD during the study and follow up period.
Ultrasonography of the pelvis was done on Logic-P5 (Wipro-GE) machine. TAS of the pelvis was done by 2–5 Mega-Hz Convex probe and the TVS was done frequencies of 7–11 Mega Hz.
MRI of the pelvis was done with Magnetom Harmony 1 T (Siemens) machine with CP body array flex coils. Following sequences were taken for all patients: T1 and T2 Weighted axial, coronal and sagittal and T1W FS sagittal. The Imaging parameters were as follows: field of view – 230 to 260; number of acquisitions – 2; matrix size – 256 × 128 or 256 × 192; section thickness – 3–5 mm; and intersection gap of 10–20 mm.
Criteria for MRI diagnosis11,12 of adenomyosis included:
-
1)
Diffuse or focal widening of the JZ (14 mm or greater) on T2WI.
-
2)
Poor definition of junctional zone (JZ) borders or indistinct margins with the myometrium.
-
3)
High-signal-intensity (HSI) foci (usually a few millimeters in diameter) within the JZ on T2-and/or T1-weighted images.
-
4)
JZ thickness 11–13 mm with poor definition of borders along with HSI foci on T1W and/or T2W
Adenomyosis was diagnosed on MRI when two of first three criteria were met or on the basis of criteria no 4.
All cases diagnosed to have adenomyosis on the basis of MRI findings were referred back to gynecologists for implantation of the LNG-IUS (Mirena1). The final decision for insertion of the IUD in appropriate cases was left to the treating gynecologist.
All patients who had IUD inserted were then followed clinically as well as with MRI after 03, 06 & 12 months respectively. The MRI of the pelvis was done using the same protocol as described above to assess for any change in the size of uterus, JZ thickness, T1 &/or T2 HSI foci in the JZ. The results were then analyzed.
MR images were reviewed by two experienced radiologists and decisions were reached by consensus. The following parameters were assessed on MRI.
-
a)
Uterus size
-
b)
JZ thickness on T2W Sagittal scan
-
c)
T1W &/or T2W HSI foci
-
d)
Associated uterine lesions if present
Results (Tables 1–5)
Table 1.
Types of adenomyosis as detected on initial MRI.
| Type of adenomyosis | No. of cases (%) N = 60 (100%) |
|---|---|
| Diffuse adenomyosis | 41 (68.3%) |
| Focal adenomyosis | 19 (31.7%) |
Table 2.
Subtypes of diffuse adenomyosis on initial MRI.
| S.No | Diffuse adenomyosis | No. of cases (%) N = 41 (100%) |
|---|---|---|
| 1. | Diffuse & even | 16 (39%) |
| 2. | Diffuse & uneven | 25 (61%) |
Table 3.
Junctional zone thickness in adenomyosis on initial MRI.
| S.No | JZ thickness | Diffuse uneven adenomyosis N = 25 (100%) |
Diffuse even adenomyosis N = 16 (100%) |
Focal adenomyosis No = 19 (100%) |
|---|---|---|---|---|
| 1. | <14 mm | 01 (4%) | 01 (6.25%) | 2 (10.52%) |
| 2. | 14–20 mm | 15 (60%) | 15 (93.75%) | 13 (68.42%) |
| 3. | 21–30 mm | 08 (32%) | 00 (00%) | 3 (15.80%) |
| 4. | >30 mm | 01 (4%) | 00 (00%) | 1 (5.26%) |
Table 4.
T1W &/or T2W HIS foci within the junctional zone.
| S.No | HSI foci | No. of cases N = 50 (100%) |
|---|---|---|
| 1. | Diffuse adenomyosis | 31 (62%) |
| 2. | Focal adenomyosis | 19 (38%) |
Table 5.
Paired samples test.
| Paired differences |
t | df | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. deviation | Std. error mean | 95% Confidence interval of the difference |
|||||
| Lower | Upper | |||||||
| Pair 1 JZT – JZT reduction |
0.235 | 0.737 | 0.103 | 0.028 | 0.443 | 2.279 | 49 | 0.027 |
Seventy patients were provisionally diagnosed as adenomyosis on the basis of TAS and/or TVS. However, on subsequent MRI study, 60 patients were diagnosed as having adenomyosis. 10 patients out of 70 had normal sized uterus with normal JZ thickness & zonal anatomy on MRI and hence were excluded from the study. Out of sixty patients, ten patients were lost to follow up, 04 patients had spontaneous expulsion of the IU implant, 03 patients were posted out, 03 got it removed due to the side effects such as interval spotting and failure of relief from menorrhagia. Patient's age ranged from 23 yrs to 52yrs with a mean age of40.92 yrs with standard deviation of 5.902.
Out of the 60 patients who had positive findings of adenomyosis on MR imaging, ten patients (16.7%) had normal size uterus (<9 cm in length), 28 patients (46.7%) had bulky uterus (>9–10 cm) and 22 patients (36.6%) had enlarged uterus (>10–14 cm).
Out of the sixty patients of adenomyosis on MR imaging, 14 patients had associated findings of fibroids. Out of 14 patients with fibroids, 11 patients had solitary fibroid while 03 had multiple fibroids. Out of 14 patients of adenomyosis with fibroids, 03 patients had focal adenomyosis while 11 had diffuse adenomyosis.
Out of the 60 patients who had positive findings of adenomyosis on MR imaging, 41 (68.33%) had diffuse adenomyosis and 19 (31.66%) had focal adenomyosis.
Diffuse adenomyosis
The JZT was either diffuse and even (n = 16, 39%) or diffuse and uneven (n = 25, 61%). One patient had endometrial polyp as an additional finding. In 16 of the 41 cases of diffuse adenomyosis, the JZ was evenly thickened and in 25 cases the JZ was unevenly thickened. Out of 16 cases of the diffuse & even adenomyosis, the JZ thickness measured 14–20 mm (mean = 17 mm) in 15 cases (93.75%) and it measured 12 mm in one case (6.25%)with associated T2 HI. Out of 25 cases of the diffuse and uneven adenomyosis, the JZ thickness measured 14–20 mm (mean = 17 mm) in 15 cases (60%) and measured 21–30 mm in 08 cases (32%). In one case (4%) it measured 37 mm while in one case (4%) it measured 12 mm with T2 HI.
Focal adenomyosis
Out of 19 cases of the focal adenomyosis, the JZT measured 14–20 mm (mean = 17 mm) in 13 cases (68.42%) and measured 21–30 mm in 03 cases (15.80%). It measured 37 mm in one case (5.26%) and in two cases (10.52%) it measured 12 & 13 mm respectively with T2 HI.
Signal Intensity, Size, Shape, and Location – Out of 19 patients of focal adenomyosis, one patient had bicornuateunicollis uterus with focal adenomyosis noted in the right cornu. The left cornu had 08 mm JZT with distinct margins. Out of 19 patients with focal adenomyosis, 08 patients (42.11%) had focal bulge as contour abnormality while 11 patients (57.89%) had smooth contour of the uterus. All 19 cases of focal adenomyosis manifested as a localized, low-signal-intensity mass within the myometrium on T2-weighted MR images. On T1-weighted images, all of the masses were isointense relative to the surrounding myometrium. All focal lesions were round or oval.
Out of 19 patients of focal adenomyosis, 12 patients (63.15%) had focal mass in the anterior wall, 05 (26.3%) in the posterior wall, one (5.3%) each in the right lateral wall and the fundus.
T1W &/or T2W HSI foci within the JZ
Out of 60 patients of adenomyosis, 50 cases had HSI foci (usually a few millimeters in diameter) within the low-signal-intensity lesions. HSI foci were noted in 31 patients (62%) of diffuse adenomyosis and 19 patients (38%) of focal adenomyosis.
In all the adenomyosis cases HSI foci on T2-weighted images were seen in 33 cases (66%), on both T1-and T2-weighted images in 15 cases (30%) and only on T1-weighted image in 02 cases (4%).
All the cases of focal adenomyosis had indistinct margins that blended imperceptibly with the surrounding myometrium. HSI foci within the focal masses were seen in all 19 cases, 12 on T2-weighted images only and 07 on both T1- and T2-weighted images.
Out of 60 diagnosed adenomyosis cases 10 patients were lost to follow up due to following reasons-
-
a.
Spontaneous expulsion – Four patients (6%)
-
b.
Posted out – Three patients (5%)
-
c.
Removal of LNG-IUS – Three patients (5%)
(Due to side effects such as interval spotting and failure of relief from menorrhagia)
50 patients of Adenomyosis were then reviewed after 03 months, 06 months and 12 months by MRI to see for any change in the MRI findings after the implantation of the Mirena1. In the follow up the same sequences and parameters were used for the MRI of the pelvis to see for any change.
Clinically there was significant improvement in the symptoms of the patients, in the form of marked reduction in the duration and amount of the bleeding per vagina and significant improvement in the lower abdominal pain. The pain relief was observed in 79.3% in 03 months, 94% in 06 months and in 96% in 12 months. Reduction in the duration and amount of the bleeding per vagina was observed in 67.2% in 03 months, 83.1% in 06 months and in 94% in 12 months. There was progressive improvement due to reduction in menstrual blood loss in the follow up period. The commonest side effects of LNG-IUS were irregular spotting and amenorrhea. Irregular spotting was seen in 35.8% in 06 months and in 42% in 12 months. Amenorrhea was seen in 3.4%in 03 months, 7.5% in 06 months and in 10% in 12 months. The acceptability improved with the use despite the increase in incidence of amenorrhea or irregular spotting. This was attributed by the patients to relief from pain and heavy menses which these individuals found acceptable.
We analyzed the morphology of the uterus to see the change in the size of the uterus, Junctional zone thickness and change in the size and number of the T1 & T2 HSI at 3, 6 and 12 months post progesterone intrauterine implants cases. In 03 months, no change in all the three parameters was seen. In 06 months, in 15 patients showed reductions in the size and number of T1 & T2 HSI with no change in other two parameters. In 12 months, total of 25 patients showed reductions in the size and number of T1 & T2 HSI and 05 patients showed reduction in JZT with no change in the uterine size.
On follow up MRI after post progesterone intrauterine implant, there was no significant change in the size of the uterus noted. In five patients out of 50 there was mild reduction in the JZT (ranging from 02 to 03 mm). In 25 patients there were reduction in the number of the T1 & T2 HSI lesions seen (Figs. 1 and 2). In one case there was fresh appearance of the T1 & T2 HSI lesions noted in the post implant uterus with no change in the JZ thickness.
Fig. 1.
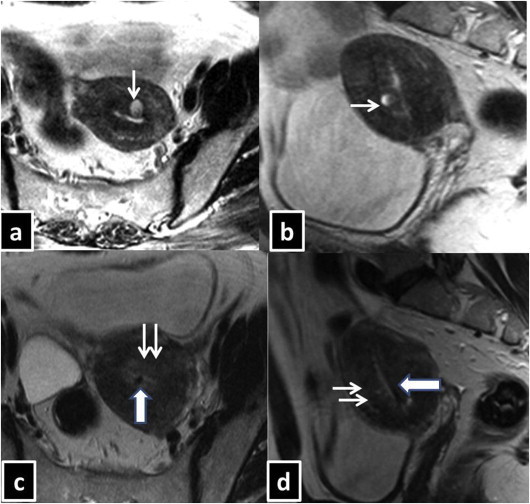
38 year old female presented with dysmenorrhea and menorrhagia. USG showed normal sized uterus with heterogeneous echo texture of the myometrium. Pre progesterone implant MRI-Uterus [T2WI, Transverse(a), Sagittal (b)] reveals diffuse uneven widening of the JZ thickness with focal T2W hyper-intensity in the anterior wall (single white arrow). Post progesterone implant MRI-Uterus [T2WI, axial (c), Sagittal (d)] reveals no significant change in the JZ thickness. However, focal T2W hyper-intensity as noted in the pre implant imaging is no longer visualized (double white arrow). Mirena is seen as black dot within the uterine cavity (Broad white arrow with blue margin) in the axial plane (c) as well as in sagittal plane(d).
Fig. 2.
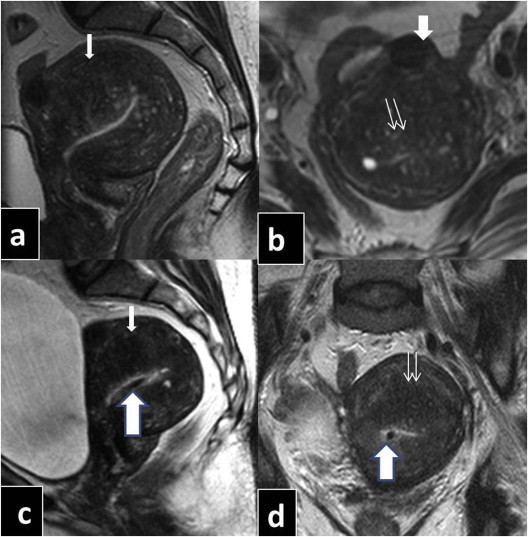
45 year old female presented with dysmenorrhea and menorrhagia of 6 months duration. USG revealed retroverted & retroflexed, enlarged uterus with heterogeneous echo texture of the myometrium. MRI preprogesterone implant (T2WI, Sagittal: a, Transverse: b) reveals enlarged uterus with diffuse & uneven JZ thickness (single white arrow) with indistinct margins associated with multiple T2W hyper intense foci within (double white arrow). Note solitary subserosal fibroid anteriorly (single white broad arrow). MRI post progesterone implant: (T2WI, Sagittal: c, Transverse: d) reveals mild reduction in the JZ thickness with reduction in the size and number of the T2W hyper intensities. Mirena is seen as black dot within the uterine cavity (Broad white arrow with blue margin) in the sagittal plane (C) and in axial plane (d).
Relationship of the MRI changes with other baseline covariates
Age was equally distributed across both the group (group with MRI changes and without MRI changes). In the group with MRI changes, 44% patients had diffuse uneven type of JZ thickness and 41% had focal type of JZ thickness which was in contrast with the group which showed no MRI changes (this group had 57% patients with diffuse even type of JZ thickness) (p = 0.007). 96% of patients in MRI changes group had MRI HI which was in contrast to the other group which had 35% MRI HI (p < 0.0001). The distribution of type of adenomyosis was similar to the JZ thickness. More number of patients had comorbidities in group with no MRI changes (48%) than with MRI changes (19%) (p = 0.027).
Discussion
MRI plays an important role in the evaluation and management of suspected cases of adenomyosis. MRI has been found particularly useful in the detailed evaluation and further characterization of various subtypes of adenomyosis. Zonal anatomy of the uterus particularly the JZ thickness is best evaluated by MRI even when compared with TVS. Small hemorrhagic and non-hemorrhagic cysts are also accurately delineated by MRI.11 Intrauterine progesterone implants have been used for more than a decade in the treatment of adenomyosis.
MRI and TVS are noninvasive modalities to diagnose adenomyosis. However, due to its superior spatial and contrast resolution not only the MRI is more sensitive and specific than TVS in diagnosing adenomyosis but also gives complete assessment of the pelvic organs. MRI has been reported to be the only noninvasive modality accurate for monitoring the response to the conservative therapy for adenomyosis.13 Charalampos et al reported high positive predictive value of MRI in the diagnosis of adenomyosis and uterine myomas.12
On T2W and/ or T1W sequences there are few or multiple punctate HSI foci noted within the JZ in adenomyosis cases, whereas they are rarely seen in patients without adenomyosis. These foci symbolize hemorrhagic or non-hemorrhagic foci and are very specific for the diagnosis of adenomyosis.7
In a study by Byun et al, 66.7% patients had diffuse adenomyosis and 33.3% had focal adenomyosis. HSI foci were seen 40% cases of diffuse adenomyosis and 100% cases of focal adenomyosis. While in our study 68%patients had diffuse adenomyosis and 31.66% had focal adenomyosis. 83% of diagnosed adenomyosis cases had HSI foci. These foci were seen in 75% cases of diffuse adenomyosis and 100% cases of focal adenomyosis. Thus, our study is in agreement with study of Byun et al13 except that in our series, more number of cases (75% vs 40%) of diffuse adenomyosis demonstrated HSI foci.
It is interesting to note that one of our patients had bicornuate uterus with focal adenomyosis in the right cornu while the left cornu showed normal JZ thickness with distinct margins.
More specific MRI criteria used by the Bazot et al were JZT >12 mm, HSI foci, maximum JZ thickness: overlying myometrial thickness ratio >40%. The ratio of maximum JZ thickness: overlying myometrial thickness ratio >40% was 65% sensitive and 92.5% specific in diagnosing adenomyosis.4 Dueholm et al; used the criteria of JZ differential calculated by measuring the difference in maximal and minimal thickness in both the anterior and posterior portions of the uterus. A JZ differential of 5–7 mm was found to improve the diagnostic accuracy of the MRI.14 However, out of these criteria, we have used absolute JZ thickness and HSI for diagnosing the adenomyosis on MRI, being the most tested criteria.
Fedele et al used LNG-IUS for the treatment of adenomyosis associated menorrhagia or dysmenorrhea.15 Sheng et al; used the LNG-IUS for the treatment of the dysmenorrhea associated with adenomyosis and followed up with TVS. There was significant reduction in the uterine volume at 06 and 12 months (p < .001) measured by TVS. It was found to be an effective method of medical treatment in alleviating adenomyosis associated dysmenorrhea.16 However, in our study there is no significant reduction in the size of the uterus even after 12 months of post progesterone intrauterine implant therapy. In this context, our study results differ from the previous study.
Imaoka et al reported that gonadotropin-releasing hormone analogs, when used for the treatment of diffuse adenomyosis, resulted in reduction of JZ thickness as noted on MRI over a follow up period of 06 months. The authors commented that asymmetric/focal adenomyosis associated with HSI foci were most sensitive to hormone therapy while adenomyosis with diffuse JZ thickening are more resistant to treatment.17
Bragheto et al monitored the use of LNG-IUS in the treatment of 29 cases of adenomyosis by MRI. He observed significant reduction of JZ thickness after 06 months with no significant change in the uterine volume.10 In our study 50% of the cases showed reduction in the HSI while 10% of the cases showed mild reduction in the JZ thickness. As far as the uterine size and volume is concerned, no significant change was noted in our study group.
Barrington et al have reported a significant relief in dysmenorrhea in 03 & 06 months follow up in their study.18 They reported 90% relief from menorrhagia in 03 months and 100% relief in 09 months. Sheng J et al have also reported a 90% relief from menorrhagia in 12 months. In our study the relief from menorrhagia was 39 (67.2%) in 03 months and 44 (83.1%) in 06 months and 47 (94%) in 09 months. Clinically there was significant improvement in the pain i.e. 79% at 03 months follow and 96% in 09 months.
Sheng J et al have reported an incidence of amenorrhea as 11% in 12 months and 13% in 36 months and incidence of irregular spotting as 10% in 12 months which declined to 05% in 36 months. Barrington et al have reported incidence of amenorrhea as 9% in 09 months. The incidence of amenorrhea was 03% in 03 months and 10% in 09 months and irregular spotting was 21% in 09 months in our study. The acceptability rates of MIRENA were 72% in 03 months and 76% in 09 months in our study. Sheng J et al have reported acceptability rates as 56% in 12 months and 72% in 36 months. The acceptability rate increased concurrently with symptomatic relief as the duration of use increased. This was attributable to the action of progesterone on the endometrial tissue causing thinning of the tissue and reduction in menstrual flow.
Limitations
Our study is limited by the fact that we followed up our patients only for one year whereas the therapeutic life of LNG-IUS is of five years. Hence long term effects of LNG – IUS on MRI findings cannot be commented upon. Future studies with longer observation period and follow up would enhance the overall accuracy and validity of the findings noted in our study.
Conclusions
Precise knowledge of the spectrum of MRI features in adenomyosis not only helps in making an accurate diagnosis but also helps in choosing an appropriate management protocol for such patients. Our study reveals that MRI not only helps in making a diagnosis of adenomyosis but also helps in successfully monitoring the effects of hormonal therapy. LNG-IUS showed significant clinical improvement in the symptoms of the patients, in the form of marked reduction in the duration and amount of the bleeding per vagina and significant improvement in the lower abdominal pain.
Conflicts of interest
All authors have none to declare.
References
- 1.Tamai K., Togashi K. MR imaging findings of adenomyosis: correlation with histopathology features and diagnostic pitfalls. Radiographics. 2005 Jan–Feb;25:21–40. doi: 10.1148/rg.251045060. [DOI] [PubMed] [Google Scholar]
- 2.Reinhold C., Tafazoli F., Mehio A. Uterine adenomyosis: endovaginal US and MR imaging features with histopathology correlation. Radiographics. 1999;19:S147–S160. doi: 10.1148/radiographics.19.suppl_1.g99oc13s147. [DOI] [PubMed] [Google Scholar]
- 3.Reinhold C., Tafazoli F., Wang L. Imaging features of adenomyosis. Hum Reprod Update. 1998;Jul–Aug;4:337–349. doi: 10.1093/humupd/4.4.337. [DOI] [PubMed] [Google Scholar]
- 4.Bazot M., Cortez A., Darai E. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. August 8, 2001;16:2427–2433. doi: 10.1093/humrep/16.11.2427. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.K.T., Gersell D.J., Balfe D.M., Worthington J.L., Picus D., Gapp G. The uterus: in vitro MR-anatomic correlation of normal and abnormal specimens. Radiology. 1985;Oct;157:175–179. doi: 10.1148/radiology.157.1.4034962. [DOI] [PubMed] [Google Scholar]
- 6.Hricak H. MRI of the female pelvis: a review. AJR. Jun 1986;16 doi: 10.2214/ajr.146.6.1115. [DOI] [PubMed] [Google Scholar]
- 7.Togashi K., Nishimura K., Itoh K. Adenomyosis: diagnosis with MR imaging. Radiology. 1988; Jan;166(1 Pt 1):111–114. doi: 10.1148/radiology.166.1.3336669. [DOI] [PubMed] [Google Scholar]
- 8.Togashi K., Ozasa H., Konishi I. Enlarged uterus: differentiation between adenomyosis and leiomyoma with MR imaging. Radiology. May 1989;171:531–534. doi: 10.1148/radiology.171.2.2704819. [DOI] [PubMed] [Google Scholar]
- 9.Varma R., Sinha D., Gupta J.K. Non-contraceptive uses of levonorgestrel-releasing hormone system (LNG-IUS), a systematic enquiry and overview. Eur J Obstet Gynecol Reprod Biol. October 2005;125:9–28. doi: 10.1016/j.ejogrb.2005.10.029. 04/2006. [DOI] [PubMed] [Google Scholar]
- 10.Bragheto A.M., Caserta N., Bahamondes L., Petta C.A. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. Sep 2007;76:195–199. doi: 10.1016/j.contraception.2007.05.091. [DOI] [PubMed] [Google Scholar]
- 11.Tamai K., Koyama T., Umeoka S., Saga T., Fujii S., Togashi K. Spectrum of MR features in adenomyosis. Best Pract Res Clin Obstet Gynaecol. Jan 2006;20:583–602. doi: 10.1016/j.bpobgyn.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Charalampos P.S., Themistoklis M., Grigoris F.G. Value of MRI in diagnosis of adenomyosis and myomas of the uterus. J Minim Invasive Gynecol. Jun 2012;19:620–626. doi: 10.1016/j.jmig.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Byun J.E., Kim S.E., Choi B.G., Ko G.Y., Jung S.E., Choi K.H. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. October 1999;19:S161–S170. doi: 10.1148/radiographics.19.suppl_1.g99oc03s161. Spec No. [DOI] [PubMed] [Google Scholar]
- 14.Dueholm M., Lundorf E., Hansen E.S., Sorensen J.S., Ledertoug S., Olesen F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril. Sep 2001;76:588–594. doi: 10.1016/s0015-0282(01)01962-8. [DOI] [PubMed] [Google Scholar]
- 15.Fedele L., Bianchi S., Raffaelli R. Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intrauterine device. Fertil Steril. Sep 1997;68:426–429. doi: 10.1016/s0015-0282(97)00245-8. [DOI] [PubMed] [Google Scholar]
- 16.Sheng J., Zhang W.Y., Zhang J.P., Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009 Mar;79:189–193. doi: 10.1016/j.contraception.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Imaoka I., Ascher S.M., Sugimura K. MR imaging of diffuse adenomyosis changes after GnRH analog therapy. J Magn Reson Imaging. Mar 2002;15:285–290. doi: 10.1002/jmri.10060. [DOI] [PubMed] [Google Scholar]
- 18.Barrington J.W., Bowen-Simpkins P. The levonorgestrel intrauterine system in the management of menorrhagia. Br J Obstet Gynaecol. 1997;104:614–616. doi: 10.1111/j.1471-0528.1997.tb11542.x. [DOI] [PubMed] [Google Scholar]


