Abstract
The aim of this review is to focus attention on high quality diagnostics of systemic inflammatory rheumatic diseases. Though many steps in the diagnostic process from the first visit in a doctor’s office till a final diagnosis have been established a lot of things still must be done to improve quality assurance and secure fast and safe transmission of data from one step to the next. Some procedures inherent in early high quality diagnostics need to be worked out. A number of elements can be improved, some stumble stones can be removed, and a tighter collaboration between actors at different levels in the line of action in clinical and laboratory medicine can be organized. Several proposals have been made by international working groups such as the IUIS International Autoantibody Standardization Committee, and the EASI steering group in collaboration with their national EASI teams. Practical exercises carried out for more than three decades by the European Consensus Finding Study Group have proven to very useful. The review points at several principles worked out by these international expert groups can be useful in actual daily practice also in rheumatology. The hope is that the presentation will give rise to a continued discussion on how to link different parts of the diagnostic process together and strengthen collaboration between all teams involved in the diagnostic chain. The ultimate measure of success will be better clinical outcomes for patients and increased satisfaction in their families.
Keywords: Autoimmunity, Antinuclear antibodies, Laboratory diagnostics, Harmonization, Standardization, Quality assurance
Introduction
It is well recognized that changes slowly taking place over a long time are not perceived as easily as changes happening within a short period of time. During the last 6 decades laboratory diagnostics has changed dramatically, different habits in carrying out clinical diagnostics and different strategies for follow-up of patients with systemic inflammatory rheumatic diseases (SRD) have developed over time. The ever changing methodologies and platforms used for autoantibody detection are often not realized in the clinical realm. From a positive antinuclear antibody (ANA) test recorded by the indirect immunofluorescence (IIF) microscopic reading of nuclear staining of rat kidney or liver cryostat sections, an ANA test has evolved into a sophisticated recognition of a broad range of autoantibodies detected by IIF using human epithelial carcinoma cells (HEp-2 cells) as cell substrate [1, 2].
In this presentation, the popular term “ANA” will be used for all antibodies giving a positive IIF signal on HEp-2 cells even if the binding is not confined to the nuclei, and thus the broad usage of the term can even include autoantibodies to cytoplasmic organelles or mitotic spindle structures. Determination of antibody specificity has changed from the original methods, e.g. double immunodiffusion, counter-immunoelectrophoresis, Farr co-precipitation and immunoblotting techniques to a broad range of fast result/high throughput technologies not always driven by a clear knowledge about the clinical utility of results derived from these new assays [3].
The diagnostics of SRD is based on previous events and present clinical manifestations as well as serological findings some of which are now used as classification criteria. The diagnostic criteria of autoimmune SRD used today have been presented very recently in a book publication [4]. Some ANA are being used as disease-specific criteria [3–6], but nevertheless highly characteristic autoantibodies used in the diagnosis of an SRD have not yet been included as diagnostic criteria even though they are rarely found in other SRDs. The mere presence of an ANA expressed at a pathological level is likely to reflect particular disease manifestations and tissue lesions. The autoantibody content of serum can thus be regarded as a biological “fingerprint” or “bio-signature” of an inflammatory disease process indicating on-going disease. Such bio-signatures can be regarded as skewed B-cell responses to cell and tissue breakdown products and modified self-antigens and thus a sign of inflammatory tissue damage. Thus, ANA reflects lesional pathology and do not respect conventional diagnostic terms.
In routine diagnostics other bio-signatures, e.g. markers of T-cell and B-cell activation, cytokine and chemokine production markers are not used in routine diagnostics though characteristic profiles of such bio-markers can be present both in serum and in other biological fluids.
In the 1950–1960s serological laboratories were started up in the clinic and surveyed by clinicians themselves, techniques were few and interchange of information between clinical and laboratory scientists took place frequently. A major problem today is the fact that the functional distance between the clinical setting and the laboratory has increased, and thus it is more difficult to keep up the information on important developments in laboratory medicine. Clinical signs and symptoms have been described in extensive studies on patients with a particular prototype disease as compared to patients with other rheumatic disorders that commonly mimic the prototype disease. Such data have been retrieved in the ARAMIS (American Rheumatism Association Medical Information System) data bank [7]. However, in the serological field thorough differential-diagnostic studies have had less precedence, and studies of large enough patient cohorts are few. To complicate the picture presence of a particular autoantibody may overlap between several clinical diagnoses.
This review will focus on what has been done and what can be done in the future to improve diagnostics of SRD. As mentioned above major medical challenges relate to the fact that different SRD have overlapping clinical and serological features, but also that both healthy and sick people produce autoantibodies. There is a fast proliferation of autoantibodies being described in each SRD. High throughput techniques for specific ANA detection are now flooding the market often without proper post-marketing studies having been done [3, 4]. To some extent experts speak non-sense language to non-experts instead of explaining how results can be interpreted and used. The cost of laboratory testing has been considered more important than a thorough selection of the clinically most valuable assays for differential diagnostics. In addition, medical education in patient related long term health cost estimation has been lacking.
Rather than looking at details in the processes essential for diagnosis of a SRD, the focus of this review has been to take a bird’s eye view on the coherence and flow of actions that will affect quality, efficiency as well as the transit time from the first visit in a doctor’s office or a clinic till a firm diagnosis has been established. It will also deal with some of the shortcomings of diagnostic tests due to use of different commercial test kits, variation in handling the kits by different technical personnel, and different interpretation of positive versus negative results. To arrive at an accurate phenotypic diagnosis, experienced scientists in the laboratory and in the clinic must collaborate, and persons with advisory function and expertise in translational medicine must be available [5, 6, 8–10].
A major focus will be on the inter-dependence of the work done in the clinic and the work performed in the clinical laboratory and to stress that both medical and laboratory personnel have an ethical responsibility toward the patient under study [11].
Clinical evaluation of early diagnosis
The main goal in the early phase of clinical diagnosis of an SRD is to recognize characteristic features that appear in the incipient development of disease. Usually, the first medical personnel that sees the patient will be the family doctor. It is thus important to equip the family practitioner with simple tools to alleviate the setting of a tentative diagnosis by recognizing early signs and features of a SRD. To aid in this endeavor, it can be practical to use an algorithm showing the most common and characteristic clinical signs of an SRD and link it with a simple schedule for laboratory testing that may be relevant to support or turn down the clinical suspicion. The goal is an early referral of the patient to a specialist in the most relevant field of medicine.
An algorithm can thus contain some clinical eye-opening signs of each of the major SRDs e.g. systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD), scleroderma (SSc), Sjögren’s syndrome (SjS), poly-and dermato-myositis (PM/DM), antiphospholipid syndrome (APS), rheumatoid arthritis (RA), juvenile chronic arthritis (JCA) and systemic small vessel vasculitis (SVV). Preliminary laboratory support suggesting the presence of an inflammatory disease can be sought through simple tests, e.g. acute phase reactants, erythrocyte sedimentation rate (ESR), leukocyte and platelet counts. If clinical suspicion of an SRD is still upheld a simple screening for autoantibodies such as IIF ANA, rheumatoid factors IgM and IgA, anti-citrullinated peptide antibodies (ACPA), anti-neutrophil cytoplasmic antibodies (ANCA), and anti-cardiolipin can be done at this level of medical exploration, but if clinical suspicion is maintained the patient should be referred to a specialist even before serology is done.
Algorithms produced to alleviate rational test ordering for specialists have been published previously [3, 5, 6, 8]. As above, the principle of such an algorithm is to start out with medical history, symptoms and manifestations that are present in the patient on presentation with the aim to set a tentative diagnosis based on the early clinical picture. After having addressed the most likely diagnosis (tentative diagnosis), the doctor can choose screening test(s) that are likely to reveal whether further tests are needed and subsequently order meaningful specific autoantibody tests. Detection of a specific autoantibody in serum may then lead to diagnosis, subtype of diagnosis (phenotype), estimated prognosis and planning of follow-up strategy to predict outcome and start possible treatment. The optimal laboratory tests are those that reflect disease variables so well that rational decisions in the clinic can be based on credible serological findings, since in the end the main result of importance for the patient, the family and the health system is a clinical outcome [4, 11].
Both of the algorithms proposed for use in private practice and for use by specialists have been discussed and agreed upon in the IUIS/WHO/AF/CDC Committee for the Standardization of Autoantibodies in Rheumatic and Related Disorders (for shortness here called the IUIS Standardization Committee) [5, 12]. The principles have also been used in the European Autoimmunity Standardization Initiative (EASI) [9, 13], and in the European Autoantibody Consensus Finding Study Group (ECFSG) that started its activities in 1988 [14].
Screening for antinuclear antibodies
The ideal screening assay for ANA does not exist. Until further, the indirect immunofluorescence (IIF) test for ANA using HEp-2 cells as cellular substrate is the test recommended for screening as suggested both by the American College of Rheumatology and the European League against Rheumatism [15]. The majority of antibodies to nuclear membrane, nucleoli, nucleoplasm, mitotic spindle structures and cytoplasm components are recorded as positive signals on most HEp-2 cell substrates commercially available. However, there may be disagreement between the laboratory and the clinic about what to report as a positive ANA result (are antibodies to a mitotic spindle or a cytoplasm structure to be reported as a positive ANA). In addition, the IIF ANA test has some limitations since several antigens become non-reactive after the use of various fixatives for keeping the cells on the slide and for permeabilizing its membranes. Thus, among several shortcomings of commercial HEp-2 cell, some kits show no or weak reactivity with anti-Jo-1, anti-ribosomal P proteins, and anti-Ro-60 antibodies [5]. Thus, if the diagnosis of either PM/DM or SjS is suspected specific tests for anti-Jo-1 and anti-Ro 60 should be ordered already at the screening stage.
In most countries of the world, IIF ANA screening is performed in the laboratory where also the tests for specific ANA are being done. Such an approach allows for a comprehensive and coherent evaluation of the agreement between the IIF staining pattern and the subsequent specific ANA result before reporting it [5]. In certain parts of the world, e.g. the USA the screening for ANA is often done in pathology laboratories remote from the clinical laboratory, and therefore, the latter is not aware of the specific ANA findings. An overall laboratory conclusion is thus not made in one laboratory and an estimate of the accuracy of the laboratory result is left to the clinician and not the laboratory scientist who knows the strengths and weaknesses of the tests used [16]. This often leaves the interpretation and credibility of ANA results to clinicians with very varied knowledge about autoimmune serology.
In a local setting of small hospitals and clinics, laboratory results are often reported with notes by a laboratory scientist on duty who may or may not have clinical information about the patient prior to testing and in addition may have little clinical experience. When different scientists add personal comments a great variability in the quality and content of comments are to be expected. Such communication between laboratories and clinicians can cause confusion, and efforts to attain official laboratory accreditation can be impossible.
In a larger setting such as a big hospital with several clinics, information about the patient is usually lacking on referral of serum for laboratory testing. Tests are done on a large scale following a fixed scheme for testing, and close surveillance of the laboratory reports leaving the laboratory is difficult. In this situation, it is important to add fixed agreed comments on how to use the result in clinical diagnostics e.g. “this antibody is typically found in …” followed by one or a few diagnoses.
It is important to realize that the IIF ANA test is much more valuable for excluding a diagnosis of SRD than for supporting such diagnosis [17, 18]. Now that composite solid phase-based commercial methods have been introduced as a substitute for IIF ANA screening, a large number of false negative ANA results can be anticipated since some autoantigens coated on the plastic or glass surface are simply not present. However, some antigens are present in a form that is not recognized by the autoantibody because the reactive epitope is hidden or the native molecular structure has been denatured during the purification procedures used [3, 5, 6, 8, 11].
What is a positive and what is a negative IIF ANA result?
As stated above, there needs to be an agreement between clinical and laboratory scientists on what results are considered important for the clinical work-up and what should be called a negative or positive ANA result. Some rheumatologists would like to have information about antibodies reacting with nuclear and cytoplasm structures of HEp-2 cells, because they can use that information in the clinical work-up of diagnosis, while other clinicians only want to know whether antibodies reacting strictly with nuclei are found.
Generally, a “false negative” ANA result using HEp-2 cell IIF is quite infrequent in a patient with an active SRD. A positive IIF ANA result should not be regarded as “false positive” just because the antibody is found out of a presumed clinical context [5]. A positive ANA in an apparently healthy individual may represent the first immunological signature of an incipient disease even before clinical signs have been manifested, but the specific ANA spectrum already at this stage reflects the disease that will appear later [19]. In contrast, negative results derived from an ANA screening ELISA or another solid phase-based composite solid phase assay are quite common and thus to be considered as real “false negative” (see below).
In a few cases, a positive IIF ANA may be found in what appears to be a healthy person who does not develop an SRD on follow-up [20]. Since such ANA are rather easy to recognize by people with experience in IIF ANA reading, they should be reported with a notice that the patient may not suffer from an SRD. This is one of the reasons why a strictly defined terminology for IIF ANA staining patterns needed to be agreed on.
Nomenclature of HEp-2 cell staining patterns
A unified and strictly defined classification of HEp-2 cell staining patterns has yet not been developed. In a European Union supported study involving three different expert centers during the period 1998–2000, a preliminary nomenclature with agreed definitions was set up with the main aim to evaluate how the participants having different levels of prior knowledge about HEp-2 cell reading (experts, experienced, un-experienced) could recognize and correctly classify digitized IIF ANA images of 27 different positive and two negative HEp-2 cell staining patterns [21, 22]. These images had been selected and agreed upon at repeated sessions involving an expert panel. The IIF staining patterns were chosen as representative prototype patterns from existing literature [21]. The study included the use of a software developed by Percepton, Copenhagen, to quantitatively measure IIF ANA image recognition skills by a method called perceptometry as described earlier [21, 22]. The harmonized nomenclature with definitions and corresponding IIF images were incorporated in the computer-assisted procedures aimed at calibrating and improving recognition skills of participants having very different experience with IIF ANA reading.
The taxonomy reached is shown in Table 1. The terms were first of all linked to main structural areas of the cell such as nuclear membrane (nuclear envelope), nucleoli, nucleoplasm, mitotic spindle apparatus and cytoplasm. Then, the different sub-types of staining in these areas (for example homogeneous, fine grainy, fine speckled, coarse speckled etc.) were added to characterize the staining details using a harmonized glossary [21]. An important goal for agreeing on selected unique terms for positive reactions was to avoid any overlap with of earlier terms that have led to confusion and imprecision. Where necessary the term includes the precise location of the staining (nucleoplasmic, nucleolar, cytoplasmic etc.). The taxonomy with the terms, definitions, positive and negative characteristics, annotations and corresponding IIF images can be seen on the Percepton website http://www.percepton.com/wisecase/download/documents/atlasHEp-2 patterns.
Table 1.
Nomenclature of HEp-2 cell staining patterns
| Membranous nuclear patterns |
| Smooth membranous nuclear |
| Punctate membranous nuclear |
| Nucleoplasmic patterns |
| Homogeneous nucloplasmic |
| Large speckled nucleoplasmic |
| Coarse speckled nucleoplasmic |
| Fine speckled nucleoplasmic |
| Fine grainy Scl-70 like nucleoplasmic |
| Pleomorphic speckled (anti-PCNA) |
| Centromere |
| Multiple nuclear dots |
| Coiled bodies (few nuclear dots) |
| Nucleolar patterns |
| Homogeneous nucleolar |
| Punctate nucleolar |
| Clumpy nucleolar |
| Spindle apparatus patterns |
| Centriole (centrosome) |
| Spindle pole (NuMa/MSA-1) |
| Spindle fiber |
| Midbody (MSA-2) |
| CENP-F (MSA-3) |
| Cytoplasmic patterns |
| Diffuse cytoplasmic |
| Fine speckled cytoplasmic |
| Mitochondrial-like |
| Lysosomal-like |
| Golgi-like |
| Contact proteins |
| Vimentin-like |
| Negative |
| Undeterminable |
Practical impact of using the agreed nomenclature
A very practical impact of this study has been the use of the harmonized terms and definitions in the European Autoantibody Consensus Finding studies where interpretation of IIF ANA patterns has been included as part of the serological exercises. More than 40 laboratory centers have participated in the work on harmonization and consensus finding in autoimmune serology. The results attained each year can be used for audits by accreditation bodies and quality assurance surveys. Discussions on the influence of using different IIF ANA screen kits and methods for detecting specific ANA have taken place after each year’s exercise.
The terms, definitions and their disease associations were published in a textbook in 2008 [8] and the ANA taxonomy, terminology, details of staining characteristics and corresponding prototype IIF images followed in 2010 based on the above-mentioned European multi-center study [2]. This nomenclature can of course only be regarded as an initial step toward further harmonization and standardization of a useful IIF ANA terminology [21]. An excellent overview of different staining patterns, their cognate autoantigens, and their potential disease associations was published in a book in 2007 authored by European and Canadian experts [23]. As mentioned above, existing clinical and laboratory criteria for the different SRDs and suggested modes of therapy have been published recently [4].
Some ANA occur in less than 1–2 % of ANA-positive sera, directed to structures that until further can only be distinguished by their unique IIF ANA pattern, since no routine assays are available to determine the antigenic targets. Many of these autoantibodies are infrequently found in the well-known SRD syndromes mentioned earlier (SLE, SjS, SSc, PM/DM, RA, SSV and APS), and these ANA have been collected under the collective term “esoteric autoantibodies”. Such ANA can be of great importance for clinical work-up, as they are associated with particular disease expression patterns that characterize partly new groups/subgroups of autoimmune diseases [24, 25]. Antibodies to CENP-F, PCNA, spindle fibers, NuMa, Golgi, GW bodies, PML bodies, early endosomes, and coiled bodies are thus infrequent. However, to give an example Golgi, GW bodies, early endosome antibodies among others have been found to be associated with autoimmune neurological diseases and especially ataxia.
Automated reading of HEp-2 cell slides
Recent work has been done to elaborate an automated IIF ANA reading system where the screening of images can replace labor-intensive and potentially uncertain reading by eye. This has lead to publications which indicate that there are still great challenges in developing this technology before credible results can be trusted for routine laboratory work [25, 26]. A major challenge is the ability to distinguish homogenous staining from different forms of grainy, fine and coarse speckled patterns in cell nuclei and/or the neighboring cytoplasm. The discrimination between nuclear and cytoplasmic staining is still not distinct enough. Another difficulty is the not uncommon presence of more than one ANA in one patient serum, each of them giving rise to different staining patterns in the same slide [17]. ANA directed to a multiplicity of structures are not infrequent in conditions like SLE, SSc, different overlap syndromes, and chronic hepatic disorders, and up to 4–5 different staining patterns can be found in one patient serum. When an experienced person reads an ANA result, the overall picture of negative and positive features is taken into account, including staining characteristics of cells in different stages of cell division. Thus, the cellular structures, and the cell compartments targeted gives rise to an integrated interpretation of the result. Ideally, this also ought to be achieved by automatic slide reading.
There is little doubt that this technology will be useful for future IIF ANA screening. As of now, the main potential of automated reading is the differentiation between positive and negative samples, the advantage being that only positive samples need to be read by eye.
Setting an IIF ANA cut-off value between healthy individuals and SRD patients
The presence of a positive ANA in serum is not in itself an abnormality, since many healthy people have low titers of IIF ANA in serum. At an age of 50–60 years, the production of low levels of ANA is quite common. The cut-off between abnormal and pathological levels of ANA encountered in immunoinflammatory patients is usually set such that 95 % of healthy donor sera are negative. A study from the IUIS Standardization Committee in 1997 established that a cut-off of 1:160 would be advisable in routine serology for ANA, since up to 31.7 % were positive at 1:40 and 13.3 % at 1:80 while less than 5 % were positive at a dilution 1:160 [27]. Very importantly, the cut-off should be determined in each clinical laboratory using a population of healthy control persons from the same area since values may differ from one area in the world or in a country to another. In addition, fluorescence detection is heavily dependent on the light source of the microscope, the numerical aperture of the objectives used and the degree of labeling of the conjugate used for detection.
Guidelines for ANA testing
Guidelines have been produced by a panel of experts from the College of American Pathologists, the American College of Rheumatology, the Clinical Immunology Society and Clinical Center of the National Institutes of Health to help clinicians set diagnosis with the aid of ANA [28]. These guidelines involve technical issues and quality assurance of ANA testing, regulatory requirements for IIF ANA and enzyme immunoassays, and demands for the fluorescent anti-IgG conjugate for use in the test. A flowchart for antinuclear antibody testing, a list of SRD conditions associated with presence of a positive ANA test and the expected frequency of ANA in these different conditions were also presented. Finally, there was a short summary about the use of different specific autoantibodies for diagnostics. Similar advice based on international experience about assays, testing and standardization in patients with SRD was published in 2006 [29]. These American guidelines for ANA testing are pretty much similar to those suggested for use in Europe and other parts of the world [3, 9, 18].
The European autoimmunity standardization initiative
If tight collaboration between family practitioners, specialized clinicians and laboratory experts can be organized and practiced in a fluent sequence of diagnostic steps following the recommendations mentioned here, the time span between the first appearance of the patients in a doctor’s office and decision on a final diagnosis can be shortened. Facilitation of this process has been the aim of the work in the European Autoimmunity Standardization Initiative (EASI) [9, 13]. The EASI working group has initiated work on an overall European level and has subsequently motivated work on national levels to create agreed models for diagnosing SRD. In general, the EASI steering group has presented workable models for diagnosing SRD for discussion in each national EASI group, and then the national group has had the responsibility to present national guidelines agreed among themselves in a national forum. To finish the work each hospital would finally have to construct, approve and test out the optimized model for the local diagnostic work-up. In this way European, national and local guidelines can be formulated and put into use.
Such work is not new. In Italy, work has been ongoing for many years to put together guidelines for autoantibody testing. This work has been done in the FIRMA (Forum Interdisciplinare per la Ricerca sulle Malattie Autoimmune) group that has published among others Italian multi-center studies and guidelines on optimal determination of anti-dsDNA antibodies [30]. Participants involved in this work were representatives for clinical immunology, autoimmune serology and clinical immunopathology organizations.
Evidently, local models for diagnostics need to fit into local and national recommendations and conform with approved habits of testing. Some of the work from national EASI working groups has been published [31, 32]. A practical guide on autoantibody demonstration and clinical use has been published in booklet form from Spain [33].
How do we secure appropriateness of ANA testing?
Appropriateness in medicine can be defined by the ability to provide necessary care and to avoid unnecessary care. The chance to secure appropriate testing has to do with the ability of a clinician to envision a likely fit of symptoms into a clinically anticipated context. This chance of obtaining early useful laboratory findings is strongly augmented if more than one clinical symptom or objective finding is the basis for ordering screen tests such as an ANA test [18].
Appropriateness in medical diagnostics may be a true challenge [34]. However in the field of SRD diagnostics, the challenge may be especially great since the diagnosis of a disease or a syndrome in the first place has to be made up of a certain number of clinical features and signs that may be used as disease criteria. Manifestations often occur over some time and what is believed to represent characteristic diagnostic features may overlap between those seen in several different syndromes. Just a few of the clinical criteria used for diagnosis are truly pathognomonic signs for one given condition, and thus multiple criteria must be fulfilled to confirm a diagnosis. The more clinical and para-clinical criteria have been found in one patient the greater the chance of reaching at a definite diagnosis [4, 18, 35, 36]. Classification and diagnostic criteria for SRDs have been published in Ref. [4].
From the laboratory point of view, appropriateness means promoting awareness of correct procedures and guidelines related to autoantibody testing; developing effective diagnostic algorithms avoiding the execution of unnecessary or redundant tests; predisposing adequate forms for test request; suggesting diagnostic rules based on reflex tests; providing interpretative comments on reports for improving interpretation and utilization of laboratory results and the capacity to counsel the clinician on diagnostics and monitoring of therapy for individual patients [10].
Indeed, there is an increasing need from physicians ordering laboratory tests for a patient-specific narrative interpretation from the clinical laboratory that includes information about test results and other relevant clinical details [37]. Other major drivers for advice on laboratory test selection and result interpretation are the need to reduce medical errors and cost containment.
An aspect that is little known is that most of the tests performed in laboratories have a negative result. If the majority of tests is normal, does it perhaps mean that these tests are useless? The answer is no. There are some good reasons why a normal laboratory result has instead its significant clinical value: (1) the predictive value of a negative test that can rule-out the disease, (2) the low pretest probability of a test performed to diagnose a disease with very low prevalence; in this situation, the test result is far more likely to be negative rather than positive; and (3) the different clinical context of each patient.
This latter point, which answers to the axiom “think globally but act locally”, underlines that appropriateness is strongly dependent on the clinical context. One example for all: as it may be considered appropriate any action that produces a useful effect in the diagnosis or treatment of the patient, in the case of a very anxious patient with pressing demands on test execution, a negative test result may be more useful than a lot of reassurance from the doctor.
Diagnostic stages
At the initial stage of diagnosing, an SRD or related autoimmune syndrome the first step is to set a tentative diagnosis based on existing information and actual findings [5]. If this is not possible a single or two manifestations or laboratory screen results may be of help for leading further steps. When an ANA has been detected by HEp-2 cell IIF, the next step is to go on with a rational screening for autoantibodies (sometimes called reflex testing) that would cover the specific ANA known to be concordant with the IIF staining pattern. The specific antibodies most commonly found in the suspected condition can be visualized in an algorithm for ordering cascade testing according to the best laboratory expertise [5] (Fig. 1).
Fig. 1.
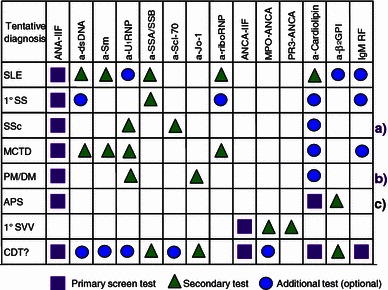
Algorithm proposed for ordering screen tests and specific autoantibodies in some classical inflammatory systemic rheumatic diseases. A proposal of the EASI steering group. a Ideally should include SSc panel testing anti-RNA polymerase I and III, anti-U3RNP, and anti-Th/To antibodies. b Ideally should include PM/DM panel testing for anti-aminoacyl synthetase tRNA, anti-SRP, anti-Mi-2, anti-PM/Scl, and anti-Ku antibodies. c Must also include lupus anti-coagulant. APS anti-phospholipid syndrome, CTD connective tissue disease, MCTD mixed connective tissue disease, PM/DM polymyositis-dermatomyositis, SjS Sjögren’s syndrome, SVV systemic small vessel vasculitis, SLE systemic lupus erythematosus, SSc systemic sclerosis
There is another logic way to go about: in case a particular clinical diagnosis is suspected the laboratory order form can be outlined that enables the clinician to tick the suspected diagnosis (1st level), yet still leaving the opportunity to order one or two relevant autoantibodies using the same order form (2nd level) (Fig. 2). In cases where a so-called “esoteric autoantibody” has been found by HEp-2 IIF, cascade testing is usually not done since chances are few that a specific antibody will be detectable by ordinary laboratory routine techniques.
Fig. 2.
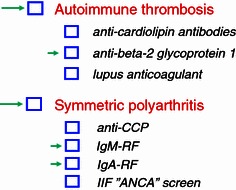
Proposed order form for screen tests for a suspected autoimmune rheumatic disease and/or for specific autoantibody tests. Long arrow shows suspected diagnosis and small arrow shows a specific autoantibody that can be ordered independent of diagnosis
It must be remembered that a negative IIF HEp-2 cell test does not at all exclude SLE or another SRD and the negative result may represent a false negative result due to the presence of antibodies directed to native conformationally labile autoantigens e.g. Ro60, Ro52, Jo-1 or PM/Scl.
Who should guide the autoantibody testing?
It is preferable that the clinician surveying the clinical course of the patient during follow-up drives the ordering process according to the accumulated clinical occurrence of signs and symptoms [5, 6]. This will also minimize the chance of getting false negative laboratory results [8]. Repeated requests for a screen test such as an IIF ANA are usually not rational since few changes occur in the overall IIF ANA profile in a patient over time. Only if the disorder changes from quiescent to active disease or if new features appear it may be rational to repeat the screen test.
In contrast, if an antibody screen test or a specific test has come out with a questionable result repeated testing or testing by a different assay should be done [5, 6]. If it is indicated to follow the level of a particular antibody e.g. anti-dsDNA the test—selected to be most credible and apt for quantification—should be the test used to follow the level of the antibody. As reported from Italy the choice of more than one assay for anti-dsDNA antibody detection is decisive for determining whether a result is positive or negative [6, 30]. Still, an overall correlation between the results of different methods used was reasonably good. Even quantification of the anti-dsDNA level may require more than one test method.
Testing for antibodies to specific autoantigen targets
The literature on tests for relevant specific autoantibodies after obtaining a positive or negative ANA report is vast [mentioned in 4, 8, 13, 18, 29], and details cannot be mentioned in a review like this. The steps going from a positive IIF ANA screen test to the level of specific antibody detection are very diverse and must be chosen at a local level. Comprehensive practical advice has been proposed recently, representing one way of solving the problem [36]. Among other important information should be stressed that awareness of the technique used to detect an antibody to so-called “ENA” (an inappropriate old name for “extractable nuclear antigens”) is imperative for interpreting a positive or negative result.
Generally, no single technology can reveal all autoantibodies of clinical value and there are clearly preferred methods for detection and quantification of specific antibodies. Broad information on the subject is available in Refs. [24] and [38].
The classical techniques used for specific antibody detection (double immunodiffusion in agarose gel, counter-immunoelectrophoresis, co-precipitation assay, e.g. the Farr assay, Western blotting, haemagglutination) have been exchanged with new methods aimed at fast and efficient antibody detection, e.g. enzyme-immunoassay, line immunoassay, dot blot assays, addressable laser bead immunoassay and protein array immunoassays using purified native or recombinantly expressed proteins as targets. Protein arrays can be prepared on planar surfaces of plastic, glass or nitrocellulose, or on polymer micro-beads or bar-coded micro-particles coated by individual antigens in a solution can be used. The methods range from single strip blotting to fully automated robot multiplex analyzers.
Radio-immunoprecipitation
The most accurate and sensitive detection method for specific autoantibody is generally considered to be radioactive immunoprecipitation. There are several variants of this method, but they all seem to surpass other methods in revealing molecular details about proteins targeted by the autoantibody. To give an illustration of its impressive potential for detecting unique details about protein targets one recent publication from 2011 relating to autoantibodies to the survival of motor neuron (SMN) complex will be given [39]. These autoantibodies were found in some patients with inflammatory myositis. A uniting IIF ANA feature was the staining coiled bodies (Cajal bodies) just like antibodies to p80 coilin. The antibodies, however, precipitated proteins D, E, F, and G of small nuclear ribo-nucleoproteins plus four proteins that are contained in the SMN complex. No reactivity with the A, B/B’ and C nucleoproteins targeted in SLE and overlap syndromes was seen. The use of a (³²P)-labeled cell extract for detection of antibodies to ribo-nucleoprotein parts of these particles, e.g. anti-SSA/SSB can complement the results seen by immunoprecipitation [40].
Two major problems arise if such very sensitive assays were to be used in conventional laboratory work up:
most laboratories tend to avoid the use of radio-labeled components in a routine laboratory, although radio-active tracers can be avoided by exchange of the tracer by use of e. g. a fluorescent or colored label.
extensive clinical studies would have to be done to reveal the relationship between the antibodies detected by such sensitive techniques and the clinical value of positive results.
Association of specific ANA with diagnosis and prognosis
In a short review like this, only a few studies illustrating the association between ANA and diagnosis/prognosis can be mentioned. First, we will focus on some studies linking sub-types of two SRDs with characteristic serological profiles, starting with SSc.
In 1994, Kuwana et al. [41] published an important study of Japanese patients with SSc where serum samples from the early onset of disease were available for analysis. The specific ANA found at baseline of disease in the early phase as shown by of disease using radio-immunoprecipitation was found to relate directly to a particular sub-type of disease and the mortality over a time course of 20 years. A number of studies published later on have confirmed these findings. The serological autoantibody profile in sera of SSc patients is generally assumed to be quite stable and include just one SSc-associated ANA. Thus, it was quite unexpected that a thorough long-term serological follow-up of Japanese SSc patients showed that anti-topoisomerase 1 (anti-Scl 70) could change from positive to negative in some patients as they went into spontaneous remission [42], indicating that waxing and waning of SSc-related ANA production may reflect disease activity. Accordingly, it may be preferable to order ANA tests not only at baseline but also during follow-up.
A recent large German study dealt with the association of different ANA and the clinical phenotypes of SSc. It underlines the importance of determining ANA profiles in defining not only the SSc diagnosis but also the subset allocation and prognosis of the SSc patients [43]. Antibodies to centromere, topoisomerase I, PM-Scl, U1RNP and RNA polymerases covered more 95 % of the known SSc-associated ANAs in ANA-positive SSc patients. Simultaneous presence of more than one SSc-associated ANA expectedly was <2 %.
In contrast to these assumptions, a study 4 years earlier conducted by the EULAR EUSTAR group seemed to indicate that autoantibody status predicts SSc complications rather than the development of a characteristic subset of the disease [44]. Both of these assumptions may be true.
To illustrate diagnostics and prognostics at a very early stage of disease, an example will be given from a publication on Canadian patients with Raynaud’s syndrome [45]. ANA was studied in 586 patients with yet no clear diagnosis presenting with Raynaud’s syndrome and the patients were followed up for a long period of time (3,197 person-years). Vascular damage was evaluated by looking at nail-fold changes by capillaroscopy. 12.6 % of the patients developed some form SSc. Presence of anti-CENP-B and/or anti-Th/To antibodies predicted loss of capillaries, while anti-RNAP III antibodies predicted destruction of capillaries. At final follow-up, 79.5 % of the patients with capillary abnormalities and at least one specific ANA at presentation had developed SSc. Those with both predictors at baseline were 60 times more likely to develop SSc, while no patient showing absence of such antibodies developed the disease. This thorough study gave rise to a proposal of new diagnostic criteria for early SSc [45].
Switching to another SRD, a study of 100 Canadian patients with an established diagnosis of inflammatory myositis (PM, DM, overlap myositis and cancer-associated myositis) looked at the presence of ANA using HEp-2 IIF and several modern techniques for ANA detection, e.g. multiplex addressable laser bead immunoassay, line blot, immunoprecipitation of translated recombinant protein, protein A-assisted immunoprecipitation, and enzyme immunoassay to determine the ANA specificity [46]. An unexpected finding was the common existence of more than one PM- or SSc-related autoantibody in one-third of the patients. 80 % of the patients expressed at least one ANA, the most frequent being anti-Ro60 and anti-Ro52. In one particular patient, six different myositis-specific and myositis-associated antibodies were found. Distinct clinical syndromes and therapeutic responses were associated with anti-Jo-1, anti-fibrillarin, anti-U1RNP, anti-Ro60, anti-Ro52 and SSc-related antibodies. These data would indicate that ANA profiling can be useful for estimation of diagnostic phenotype and potentially help to guide the choice of therapy in Raynaud’s syndrome patients developing myositis.
Realizing the properties and limitations of using test kits to detect ANA
The most commonly used commercially available test kits for detection of specific ANA in a clinical routine laboratory is EIA (enzyme immunoassay). Several critical evaluation studies have been published by the IUIS Standardization Committee on the performance characteristics of different commercially available EIA. In the first investigation, nine manufacturers of EIA kits had agreed to receive and test coded sera from Centers of Disease Control and Prevention in Atlanta where the repository for autoantibody reference sera of the IUIS Standardization Committee is kept [47] (see below). Coded sera contained autoantibodies commonly used in the evaluation of diagnosis of different SRDs. Dilutions of antibody of one ANA specificity were mixed with sera containing ANA of other defined specificities coming from the IUIS Standardization Committee repository in Atlanta. These samples were to be analyzed for autoantibody content using the standard method of the individual company producing the kits. The mixed serum samples containing anti-dsDNA, anti-ssDNA, anti-histone, anti-Sm, anti-U1RNP, anti-La, anti-Ro, anti-topoisomerase I (anti-Scl-70), anti-centromere and anti-Jo-1 antibodies were thus analyzed by the companies themselves, and sensitivity and specificity of each antibody were calculated after results had been received by the IUIS Standardization Committee.
Generally, anti-Ro, anti-La, anti-Scl-70, anti-centromere and anti-Jo-1 kits performed well, however, anti-dsDNA and anti-Sm kits lacked sensitivity and specificity in most cases, and precision varied from poor to very good. Some kits achieved good sensitivity and specificity, but no single manufacturer had been able to provide kits that were superior with regard to all antibodies tested for. The data from the laboratories of each manufacturer were presented to the company individually to allow for correction of deficiencies in order to improve quality.
The second study looked at the potential of different EIAs for quantification of autoantibody content [48]. Nine out of twenty purveyors of EIA kits for detection of ANA of defined specificities decided to participate in the study of coded sera with mixtures of anti-dsDNA, anti-La, anti-Sm and anti-Scl-70 antibodies. The kits of certain manufacturers showed very good accuracy in three out of four antibody specificities and poor performance for the 4th kit. It was evident that no manufacturer produced kits with uniformly good performance for all specificities. Clinicians should be aware of the large variation in the performance of EIA kits used with the aim to assist in diagnosis of SRD. Reliable quantification of antibody levels in serial samples from SRD patients is only meaningful if kits can be shown to be improved in the future.
The third study was done to see whether laboratory investigators in commercial companies produced results comparable to the results coming from academic institutions that had done research in the area of ANA and ran laboratory routine serology [49]. Nine commercial kit providers and 12 academic laboratories took part in this study. Kits were used according to prescribed procedures and the analysts were blinded to the content and concentration of four autoantibodies (anti-dsDNA, anti-La, anti-Sm, and anti-Scl-70) in the mixture sent to them from the repository as mentioned above. Coefficients of variation were calculated for each participant.
We found the range of performance characteristics to vary a lot from laboratory to laboratory, a variation that could be disastrous in a clinical diagnostic setting. This indicates that clinicians as well as laboratories need to be aware of the often doubtful quality of the ANA results used for diagnostics of SRD patients, and both manufacturers of kits and clinical laboratories need to assure the quality and survey the performance of commercial kits in the hands of medical technologists doing routine testing.
Need for differential diagnostic borderline setting
To be able to determine a clinically meaningful a positive cut-off value between that found in one particular SRD compared to values found in differential diagnostic populations (other SRDs and/or chronic infections) real patient sera must be available for critical testing [4, 5, 8]. To illustrate the practicality of choosing a differential diagnostic cut-off, we will focus for a short while on another set of autoantibodies namely those directed to citrulline-modified peptides by looking at some recent publications [50–52]. Most clinicians are aware of the fact that the diagnosis of RA does not represent one clinically uniform population of arthritis patients but probably several sub-populations with different autoantibodies and different prognoses [51]. The largest population of RA patients developing erosive arthritis is the sub-type producing considerable amounts of ACPA [51, 52].
Discrepancies between tests for ACPA can be attributed to the occurrence of borderline results, inter-assay variability and inter-test variability, and the use of one particular ACPA substrate and one test system gives better agreement between results.
The technique most widely researched and now being used by most clinical laboratories is the anti-cyclic citrullinated antibody EIA (the CCP2 test) that uses an artificial cyclic peptide (a mimotope) as substrate [52, 53]. The content of anti-CCP is determined as optical density (OD) values found in sera from RA patients and non-RA arthritic patients. OD values are ranked into a receiver operating characteristics curve (ROC curve) using the ODs obtained in one particular assay.
First of all, it is important that clinicians and the laboratory agree on a pre-selected diagnostic specificity (f. ex. 98 %). Then a ROC curve with the data from sera derived from RA patients and differential diagnostic arthritis populations using an individual assay is created 98 % is marked on the horizontal axis of the curve. Repeating the analysis with the same sera in each assay specific ROC curves are drawn and the cut-off is set the same 98 % level of specificity. The sensitivity for the diagnosis of RA can now be seen on the vertical axis (ordinate) of the each ROC [53].
Such comparisons between the sensitivity of different assays for the same specific antibodies only become valid if the same mixed group of sera from differential diagnostic patients are used in all assays studied (stratified studies) [50, 52, 54]. The assay that most accurately reflects the sensitivity the clinician wants to diagnose an accurate anti-CCP positive RA patient is then chosen (usually a satisfactory high sensitivity).
Obviously, the same strategy of evaluating assays for ANA with defined specificity is applicable although the level of pre-selected specificity level often has to be set somewhat lower because of lower diagnostic specificity.
Quality assurance
The concept of quality can be summarized with two aphorisms: “do the things right” and “do the right thing”. In laboratory diagnostics, and therefore, also that relating to autoimmunity tests, the analytical quality control meets the first condition, and quality assurance (broader concept that includes the clinical appropriateness of testing) meets the second requirement. In other words, quality control is the ability to ensure performance adhering to the state of the art, while quality assurance represents the effectiveness of laboratory services in determining outcomes useful to patients.
In practice, obtaining a material control, analyzing and recording the results might be enough to say that you have a quality control. To ensure quality assurance you need something more. Indeed, the laboratory’s aim is not only to provide accurate results but also to do so within a reasonable turn-around time, with traceability of all laboratory procedures, a respect for ethics and to assure the safety of patients and staff alike.
Regarding the analytical quality, also in autoimmunology laboratories as in all other sectors of laboratory diagnostics, there are two essential elements in the quality system: the internal quality control and the external quality assessment (EQA). Rules addressing these issues are contained in ISO 15189 [55]. In the chapter dedicated to Quality of Examination Procedures, the document provides some indication on how a quality program should be implemented and run. First, the laboratory shall design internal quality control systems that verify the attainment of the intended quality of results. It is important to note that the control system provide staff members with clear and easily understood information on which to base technical and medical decisions. Special attention should be paid to the elimination of mistakes in the process of handling samples, requests, examinations, reports, etc.
Second, the laboratory shall participate in inter-laboratory comparisons such as those organized by EQA schemes. Laboratory management shall monitor the results of EQA and participate in the implementation of corrective actions when control criteria are not fulfilled. EQA programs should, as far as possible, provide clinically relevant challenges that mimic patient samples and have the effect of checking the entire examination process, including pre- and post-examination procedures.
Third, whenever a formal inter-laboratory comparison program is not available, the laboratory shall develop a mechanism for determining the acceptability of procedures not otherwise evaluated. Whenever possible, this mechanism shall utilize externally derived challenge materials such as exchange of samples with other laboratories.
However, while in other areas of laboratory diagnostics, as in those of clinical chemistry and immunochemistry, thanks also to automated instruments and tools with highly sophisticated on-board computers, it is easier to achieve a good quality monitoring, in other areas such as autoimmunology, this can be more difficult.
In fact in autoimmune diagnostics, we deal with methods with a relevant content of manual operation, using materials which are not well defined (antibodies) or based on procedures that are not well standardized, such as the indirect immunofluorescence (IIF) technique. With this method, which still plays an important role in the basic autoimmune diagnostics, not only the technological aspects of production and preparation of substrates, but also the training and preparation of the operator and the quality of the fluorescence microscope have a major impact on the results.
Critical aspects not only concern tests performed in IIF but also in general all assays for antibody detection. Multicentre studies conducted in clinical immunology laboratories have confirmed that the analytical intra- and inter-laboratory assay variability in autoantibody detection is very large and different for each type of autoantibody [56, 57].
In this regard, a guideline for better standardization of autoimmune tests (document I/LA2-A2 [58]) has been developed by the Clinical and Laboratory Standards Institute (CLSI), which contains several items for the proper performance of the ANA test by IIF or by immunoenzymatic assays (ELISA) (Table 2), and particularly for the internal quality control (Table 3).
Table 2.
Items in the clinical and laboratory standards institute’s document I/LA2-A2 on the quality in the autoimmunology laboratory
| Part I. Indirect immunofluorescence test for antinuclear antibodies (IF-ANA) |
| Principles of the IF-ANA test |
| Patient specimen and collection procedure |
| Substrate and fixative variations |
| Fixation of substrate tissues |
| SS-A/Ro antigen |
| Fluorochrome-labeled conjugates |
| Working dilution |
| Polyvalent and IgG-specific conjugates |
| Reference preparation of fluorochrome-labeled conjugates |
| Microscope optics |
| Part II. The enzyme immunoassay test (ELISA-ANA) |
| Assay requirements |
| Solid phase with adsorbed nuclear antigens |
| Enzyme-labeled (second stage) detection antibody (conjugate) |
| Standards, calibrators, and controls |
| Wash solutions and other reagents |
| Assay validation |
| Assay validation from the manufacturer’s perspective |
| Assay validation from the user’s perspective |
| ELISA enzyme-labeled conjugates |
| ELISA detection methods |
| Technical considerations |
| Alternative, emerging solid-phase technologies |
| Part III. Quantitation of antibodies, reference intervals and reporting of test results, and intralaboratory quality control |
| Quantitation of antibodies |
| Reference intervals and reporting of results |
| Intralaboratory quality control |
| Reference preparations for ANA tests |
| Definitions and nomenclature |
| WHO/IUIS reference preparations |
| AF/CDC reference sera for autoantibodies to nuclear and intracellular antigens |
| College of American pathologists reference serum for anti-SS-A/Ro antibodies |
| ANA reference preparations of association of medical laboratory immunologists (AMLI) |
Table 3.
Recommendations of the Clinical and Laboratory Standards Institute’s document I/LA2-A2 for Intralaboratory Quality Control on IIF-ANA and ELISA-ANA
| • Use in-house negative and positive controls with each assay |
| • Positive control should be chosen at a level that is important for clinical decision making |
| • Put in the analytical series a number of previously tested negative and positive sera for the purpose of making lot-to-lot comparisons of test reagents |
| • Definition of acceptable variability defined by the laboratory |
| • In terms of overall (qualitative) concordance/discordance with previous results or additional quantitative criteria, e.g., at least 85 % of results giving numerically equivalent results with both lots |
| • Monitor the frequencies of test results that fall in different reference range categories, e.g., negative, borderline (equivocal), or positive |
Among others, special attention is given to define rules for patient specimen collection and handling, type of substrate and fixation procedures, working dilution, characteristics of fluorochrome-labeled conjugates and microscope optics. The document also provides indication on quantitation of antibodies, reference preparations, reference intervals and reporting of test results.
Without going into detail, some critical points, however, deserve to be highlighted.
In the immunofluorescence methods, sera provided by kit manufacturers are often used as negative and positive controls. These controls by their nature look more like calibrators than control material, as they are not treated as patients’ sera, and do not undergo any dilution. It is therefore recommended the use of serum samples collected in the in-house routine work-up, to be included as positive and negative controls in the analytical series.
CLSI further recommends that antibody level of the positive control corresponds to values close to those for clinical decision making, but this occurs very rarely in the controls provided in the kits. Almost always the positive control is at high level.
A part of the CLSI document is dedicated to the ANA-ELISA systems. Various studies have shown that these methods cannot recognize the presence of autoantibodies against clinically relevant nuclear antigens [59–61]. In these cases, EQA programs could be very useful, by selecting rare but clinically important antibodies. However, distributors of EQA programs seldom distribute sera containing rare antibodies.
A final recommendation indicates that the EQA samples are inserted within the routine analytical series, among patients’ samples, without any special treatment. Performing duplicate testing in the EQA sample is permissible only if practiced routinely for patients.
In conclusion, these brief notes, which are necessarily incomplete (for a more thorough knowledge of the matter it is appropriate to refer to the documents cited), emphasize the complexity of the task and the importance that each autoimmunology laboratory adopts systems and procedures for quality assessment. As a study at the Mayo Clinic has found that up to 70 % of clinical diagnosis is based on the result of laboratory tests [62], it is evident that the laboratory has a very important role in medical diagnostics and that the guarantee that the data are of high quality is the prerequisite for a correct clinical and therapeutic decision.
Use of serum reference materials
Since the 1980s, the IUIS International Autoantibody Standardization Committee has worked to establish a repository of ANA reference sera that could be made available to research and diagnostic laboratories worldwide [11, 63]. In the beginning, when IIF microscopy and double immunodiffusion were the main techniques used to detect ANA, the sera were used as international standards, but as techniques for demonstrating and quantifying antibodies have multiplied and developed so much that the reagents are now to be considered as reference reagents to substantiate the specificity and the content of a defined ANA. It is very important to understand that the older serum standards for various ANA for IIF and double immunodiffusion contain several specific autoantibodies if analyzed by another method than the one it was develop for e.g. immunoblotting. [64].
Another use of reference sera is the potential of allowing quantification of an autoantibody level in international units (IU) instead of in different arbitrary units that cannot be compared between the methods and kits. Thus, a reference reagent can be used to align values of serum standards that are included in commercial kits and the internal standard serum used by the clinical laboratory. The international reference reagents are only to be used once or twice a year for quality assurance purposes and not for daily routine use, since the resources of the repository are limited. The long-term evaluation of a kit should involve monthly surveys of the highest and borderline levels of the internal serum standard from day to day. Clinical and laboratory guidelines for verification of assay performance are also available for use in the attempt to secure precision and trueness of serological findings [65].
A list of the 16 reference reagents available today can be seen on the present website of the IUIS Standardization Committee http://asc.dental.ufl.edu/home.html. Ten of these reference reagents are used in ANA diagnostics. Publications from the standardization committee are also visible on the website.
Collaboration with the diagnostic industry
The most satisfactory solution in daily practice is to focus on the use of a few well-studied commercial kits that have been proven by internal control measures to be of satisfactory diagnostic value, satisfactory stability and reproducible quality in long-term internal surveys. Another factor is the development of assays in close collaboration with the diagnostic industry producing kits for autoantibody detection and quantification. Commercially independent laboratory and clinical specialists need to offer advisory functions to kit providers to secure that all of the most important elements of quality, reliability, and comparability of results are met. The introduction of international units for measurement of antibody levels in quantitative commercial assays would be an enormous advantage.
Conclusions
Implementation of measures to facilitate fast track high quality diagnostics of inflammatory SRD is highly warranted. Several steps towards harmonization have been taken by international working groups such as the IUIS Autoantibody Standardization Committee, the EASI steering group and their collaborative national teams, and the European Consensus Finding Study Group. However, there are still road blocks on the path toward fast high quality diagnostics in this field, often starting at the earliest steps of diagnostics. A closer tie between primary care teams and secondary care specialists to deliver what is needed to reach at a final diagnosis before irreversible tissue damage has taken place is paramount. A rational plan for follow-up of patients and advice on best practice guidelines for timely drug therapy can also be worked out.
A correct early diagnosis and prognostic phenotype of disease can only be set if all links in the chain of diagnostic procedures work optimally. High quality serological evaluation is an important integrated part in the chain and needs to be coordinated, tested out and proven to be effective in actual life. The communication between all responsible persons participating in this diagnostic chain should be strengthened wherever possible e.g. by giving those diagnosticians involved in the process easy and fast access to all data relating the patient electronically. It is great that the enthusiasm for following some of the procedures mentioned here is spreading and that harmonized guidelines are being produced and discussed, however, the final proof of the efficiency of improved diagnostics is that a better outcome for the patient can be quantitatively documented.
It is all about the patient!
Acknowledgments
The collaboration of members of the IUIS International Autoantibody Standardization Committee, members of the European Autoimmunity Standardization Initiative steering group and national EASI teams, and the European Autoantibody Consensus Finding Study Group is gratefully acknowledged for their great efforts to help accomplish high quality diagnostics in rheumatology. Statens Serum Institut in Copenhagen is thanked for support and openness to allow the Department of Clinical Biochemistry, Clinical Immunology and Biomarkers to be involved in the broad collaboration that has led to this work.
Conflict of interest
None.
References
- 1.Humbel RL (1993) Detection of antinuclear antibodies by immunofluorescence, part 1. In: Maini RN, van Venrooij WJ (eds) Manual of biological markers of disease, section A2. Kluwer, Dordrecht, pp 1–16
- 2.Wiik A, Høier-Madsen M, Forslid J, Charles P, Meyrowitsch (2010) Antinuclear antibodies: a contemporary nomenclature using HEp-2 cells. J Autoimmun 35:276–90 [DOI] [PubMed]
- 3.Fritzler MJ, Wiik A, Fritzler ML, Barr SG. The use and abuse of commercial kits used to detect autoantibodies. Arthritis Res Ther. 2003;5:192–201. doi: 10.1186/ar782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoenfeld Y, Cervera R, Gershwin ME. Diagnostic criteria in autoimmune diseases. Totowa: Humana Press; 2008. [Google Scholar]
- 5.Wiik AS, Gordon TP, Kavanaugh AF, Lahita RG, Reeves W, van Venrooij WJ, Wilson MR, Fritzler M, The IUIS/WHO/AF/CDC Committee for the Standardization of Autoantibodies in Rheumatic and Related Diseases Cutting edge diagnostics in rheumatology: the role of patients, clinicians, and laboratory scientists in optimizing the use of autoimmune serology. Arthritis Rheum (Arthritis Care Res) 2004;51:291–298. doi: 10.1002/art.20229. [DOI] [PubMed] [Google Scholar]
- 6.Wiik AS. Anti-nuclear autoantibodies: clinical utility for diagnosis, prognosis, monitoring, and planning of treatment strategy in systemic immunoinflammatory diseases. Scand J Rheumatol. 2005;34:260–268. doi: 10.1080/03009740500202664. [DOI] [PubMed] [Google Scholar]
- 7.Fries JF. The chronic disease data bank: first principles to future directions. J Med Philos. 1984;9:161–180. doi: 10.1093/jmp/9.2.161. [DOI] [PubMed] [Google Scholar]
- 8.Wiik AS, Fritzler MJ. Laboratory tests in rheumatic disorders. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 4. Edinburg: Mosby Elsevier; 2008. pp. 219–232. [Google Scholar]
- 9.Wiik A, Cervera R, Haass M, Kallenberg C, Khamashta M, Meroni PL, et al. European attempts to set guidelines for improving diagnostics of autoimmune rheumatic disorders. Lupus. 2006;15:391–396. doi: 10.1191/0961203306lu2322oa. [DOI] [PubMed] [Google Scholar]
- 10.Tozzoli R, Bizzaro N (2012) The clinical autoimmunologist and the laboratory autoimmunologist: the two sides of the coin. Autoimmun Rev. Epub ahead of print [DOI] [PubMed]
- 11.Bossuyt X, Louche C, Wiik A. Standardisation in clinical laboratory medicine: an ethical reflection. Ann Rheum Dis. 2008;67:1061–1063. doi: 10.1136/ard.2007.084228. [DOI] [PubMed] [Google Scholar]
- 12.Chan EKL, Fritzler MJ, Wiik A, Andrade LEC, Reeves WH, Tincani A, Meroni PL, The IUIS/WHO/AF/CDC Committee for the Standardization of Autoantibodies in Rheumatic and Related Diseases AutoABSC.Org—Autoantibody Standardization Committee in 2006. Autoimmun Rev. 2006;6:577–580. doi: 10.1016/j.autrev.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Shoenfeld Y, Cervera R, Haass M, Kallenberg C, Khamashta M, Meroni PL, Piette J-C, Schmidt R, Wiik A. EASI—The European Autoimmunity Standardisation Initiative: a new initiative that can contribute to agreed diagnostic models of diagnosing autoimmune disorders throughout. Ann N Y Acad Sci. 2007;1109:138–144. doi: 10.1196/annals.1398.016. [DOI] [PubMed] [Google Scholar]
- 14.van Venrooij WJ, Charles P, Maini RN. The consensus workshops for the detection of autoantibodies to intracellular antigens in rheumatic diseases. J Immunol Methods. 1991;140:181–189. doi: 10.1016/0022-1759(91)90369-Q. [DOI] [PubMed] [Google Scholar]
- 15.Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69:1420–1422. doi: 10.1136/ard.2009.127100. [DOI] [PubMed] [Google Scholar]
- 16.Kavanaugh A. The utility of immunologic laboratory tests in patients with rheumatic diseases. Arthritis Rheum. 2001;44:2221–2223. doi: 10.1002/1529-0131(200110)44:10<2221::AID-ART383>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Fritzler MJ. The antinuclear antibody test: last or lasting gasp? Arthritis Rheum. 2010;63:19–22. doi: 10.1002/art.30078. [DOI] [PubMed] [Google Scholar]
- 18.Bizzaro N, Wiik A. Appropriateness in antinuclear antibody testing: from clinical request to strategic laboratory practice. Clin Exp Rheumatol. 2004;22:349–355. [PubMed] [Google Scholar]
- 19.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James J, et al. Development of auto-antibodies before the clinical onset of systemic lupus erythematosus. New Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 20.Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LEC. Pattern on the antinuclear antibody HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:191–200. doi: 10.1002/art.30084. [DOI] [PubMed] [Google Scholar]
- 21.Wiik A, Lam K (2000) On the usability of extended DOORS for education and training, quality assurance and consensus formation. The EU CANTOR project HC 4003 (HC). Deliverable D09.1, version 2.1. The European Commission; 2001
- 22.Wiik A, Charles P, Meyrowitsch J (2011) Multi-centre collaboration is needed to reach a unified and strictly defined classification of IIF HEp-2 cell staining patterns. In: Conrad K, Chan EKL, Meroni PL, Shoenfeld Y (eds) Autoantigens, autoantibodies, autoimmunity, vol 7. Pabst, Lengerich, pp 634–646
- 23.Conrad K, Schössler W, Hiepe F, Fritzler MJ (2007) Autoantibodies in systemic autoimmune diseases. A diagnostic reference, 2nd edn. Pabst, Lengerich
- 24.Stinton LM, Eystathioy T, Selak S, Chan EK, Fritzler MJ. Autoantibodies to protein transport and messenger RNA processing pathways: endosomes, lysosomes, Golgi complex, proteasomes, assemblyosomes, exosomes and GW bodies. Clin Immunol. 2004;110:30–44. doi: 10.1016/j.clim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Hiemann R, Büttner T, Krieger T, Roggenbuck D, Sack U, Conrad K. Challenges of automated screening and differentiation of non-organ specific autoantibodies on HEp-2 cells. Autoimmunity Rev. 2009;9:17–22. doi: 10.1016/j.autrev.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Egerer K, Roggenbuck D, Hiemann R, Weyer MG, Büttner T, Radau B. Automated evaluation of autoantibodies on human epithelial-2 cells as an approach to standardize cell-based immuno-fluorescence tests. Arthritis Res Ther. 2010;12:R40. doi: 10.1186/ar2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 28.Kavanaugh A, Russell T, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. Arch Pathol Lab Med. 2000;124:71–81. doi: 10.5858/2000-124-0071-GFCUOT. [DOI] [PubMed] [Google Scholar]
- 29.Fritzler MJ, Wiik A. Autoantibody assays, testing, and standardization. In: Rose NR, Mackay IR, editors. The autoimmune diseases. 4. Amsterdam: Elsevier; 2006. pp. 1011–1022. [Google Scholar]
- 30.Ghirardello A, Villalta D, Morozzi G, Galeazzi M, Gerli R, et al. Diagnostic accuracy of currently available anti-double-stranded DNA antibody assays. An Italian multicentre study. Clin Exp Rheumatol. 2011;29:50–56. [PubMed] [Google Scholar]
- 31.Sack U, Conrad K, Csernok E, Frank I, Hiepe GF, Krieger T, German EASI (European Autoimmunity Standardization Initiative) et al. Autoantibody detection using indirect immunofluorescence on HEp-2 cells. Dtsch Med Wochenschr. 2009;134:1278–1282. doi: 10.1055/s-0029-1225278. [DOI] [PubMed] [Google Scholar]
- 32.Damoiseax J, Tervaert JW, Derksen R, Hamann D, Hooijkaas H, Klasen I, et al. Autoantibody standardization in the Netherlands. Ann N Y Acad Sci. 2009;1173:10–14. doi: 10.1111/j.1749-6632.2009.04631.x. [DOI] [PubMed] [Google Scholar]
- 33.Cervera R, Plaza A. Guias de pratica clinica y laboratorio.Anticuerpos y enfermedades autoimmune. Barcelona: Sweden Diagnostics; 2009. [Google Scholar]
- 34.Brooks RH. Appropriateness: the next frontier. BMJ. 1994;308:218–219. doi: 10.1136/bmj.308.6923.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiik AS. Appropriateness in autoantibody testing in clinical medicine. Clin Chim Acta. 2003;333:177–180. doi: 10.1016/S0009-8981(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 36.Damoiseaux JGMC, Cohen Tervaert JW. From ANA to ENA: how to proceed? Autoimmun Rev. 2006;5:10–17. doi: 10.1016/j.autrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Laposata M. Patient-specific narrative interpretations of complex clinical laboratory evaluations: who is competent to provide them? Clin Chem. 2004;50:471–472. doi: 10.1373/clinchem.2003.028951. [DOI] [PubMed] [Google Scholar]
- 38.Shoenfeld Y, Gershwin ME, Meroni PL. Autoantibodies. 2. Amsterdam: Elsevier; 2007. [Google Scholar]
- 39.Satoh M, Chan JY, Ross SJ, Cerebelli A, Cavazzana I, Franceschini F, et al. Autoantibodies to survival of motor neuron complex in patients with polymyositis: immunoprecipitation of D, E, F and G proteins without other components of small nuclear ribonucleoproteins. Arthritis Rheum. 2011;63:1972–1978. doi: 10.1002/art.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zieve G, Fury M, Janssen EJR (1993) Analysis of autoimmune sera by immuno-precipitation of cellular RNPs. In: van Venrooij WJ, Maini RN (eds) Manual of biological markers of disease, section A7. Kluwer, Dordrecht
- 41.Kuwana M, Kaburaki J, Okano Y, Tojo T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum. 1994;37:75–83. doi: 10.1002/art.1780370111. [DOI] [PubMed] [Google Scholar]
- 42.Kuwana M, Kaburaki J, Mimori T, Kawakami Y, Tojo T. Longitudinal analysis of autoantibody response to topoisomerase I in systemic sclerosis. Arthritis Rheum. 2000;43:1074–1084. doi: 10.1002/1529-0131(200005)43:5<1074::AID-ANR18>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 43.Mierau R, Moinzadeh P, Riemekasten G, Melchers I, Reichenberger F, et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German network for systemic scleroderma: correlation with clinical features. Arthritis Res Ther. 2011;13:R172. doi: 10.1186/ar3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker UA, Tyndall A, Czirja′k L, Denton C, Farge-Bancel D, Kowal-Bielecka O, Müller-Ladner U, Bocelli-Tyndall C, Matucci-Cerinic M, EUSTAR et al (2007) Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR scleroderma trials and research group database. Ann Rheum Dis 66:754–763 [DOI] [PMC free article] [PubMed]
- 45.Koenig M, Joyal F, Fritzler MJ, Roussin A, Abrahamowicz M, Boire G, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 568 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58:3902–3912. doi: 10.1002/art.24038. [DOI] [PubMed] [Google Scholar]
- 46.Koenig M, Fritzler MJ, Targoff IN, Troyanov Y, Sénecal J-L. Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights in clinical features and outcomes. Arthritis Res Ther. 2007;9:R78. doi: 10.1186/ar2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan EM, Smolen JS, McDougal JS, Butcher BT, Conn D, Dawkins R, et al. A critical evaluation of enzyme immunoassays for detection of antinuclear antibodies of defined specificity I. Precision, sensitivity, and specificity. Arthritis Rheum. 1999;42:455–464. doi: 10.1002/1529-0131(199904)42:3<455::AID-ANR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Tan EM, Smolen J, McDougal JS, Fritzler MJ, Gordon T, Hardin JA, et al. A critical evaluation of enzyme immunoassays for detection of antinuclear antibodies of defined specificities. II. Potential for quantitation of antibody content. J Rheumatol. 2002;29:68–74. [PubMed] [Google Scholar]
- 49.Fritzler MJ, Wiik A, Tan EM, Smolen JS, McDougal JS, Chan EK, et al. A critical evaluation of enzyme immunoassay kits for detection of antinuclear antibodies of defined specificities. III. Comparative performance characteristics of academic and manufacturer’s laboratories. J Rheumatol. 2003;30:2374–2381. [PubMed] [Google Scholar]
- 50.Van der Cruyssen B, Hoffman IE, Zmierczak H, van den Berghe M, Kruithof E, de Rycke L, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis. 2005;64:1145–1149. doi: 10.1136/ard.2004.032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 52.van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol. 2011;7:391–398. doi: 10.1038/nrrheum.2011.76. [DOI] [PubMed] [Google Scholar]
- 53.Wiik AS, van Venrooij WJ, Pruijn GJM. All you wanted to know about anti-CCP but were afraid to ask. Autoimmun Rev. 2010;10:90–93. doi: 10.1016/j.autrev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Bizzaro N, Tonutti E, Tozzoli R, Villalta D. Analytical and diagnostic characteristics of 11 2nd- and 3rd-generation immunoenzymatic methods for the detection of antibodies to citrullinated proteins. Clin Chem. 2007;53:1527–1533. doi: 10.1373/clinchem.2007.087569. [DOI] [PubMed] [Google Scholar]
- 55.ISO 15189:2003. Medical laboratories: particular requirements for quality and competence. ISO, Geneva, 2003
- 56.Bizzaro N, Tozzoli R, Tonutti E, Piazza A, Manoni F, Ghirardello A, et al. Variability between methods to determine ANA, anti-dsDNA and anti-ENA autoantibodies: a collaborative study with the biomedical industry. J Immunol Methods. 1998;219:99–107. doi: 10.1016/S0022-1759(98)00140-9. [DOI] [PubMed] [Google Scholar]
- 57.Villalta D, Bizzaro N, Platzgummer S, Antico A, Tampoia M, Camogliano L, et al. Accuracy of semiquantitative immunoenzymatic methods in quantitation of anti-topoisomerase I (Scl-70) antibodies. Clin Rheumatol. 2005;24:453–459. doi: 10.1007/s10067-004-1054-9. [DOI] [PubMed] [Google Scholar]
- 58.Clinical and Laboratory Standards Institute (2006) Quality Assurance of Laboratory Tests for Autoantibodies to Nuclear Antigens: (1) Indirect Fluorescence Assay for Microscopy and (2) Microtiter Enzyme Immunoassay Methods; Approved Guideline, 2nd ed. CLSI document I/LA2-A2. Wayne, PA: CLSI
- 59.Bayer PM, Bauerfeind S, Bienvenu J, Fabien N, Frei PC, Gildburd B. Multicenter evaluation study on an new HEp2 ANA screening enzyme immune assay. J Autoimmun. 1999;13:89–93. doi: 10.1006/jaut.1999.0298. [DOI] [PubMed] [Google Scholar]
- 60.Rondell JMM, Van Gelder M, Van der Leeden H, Dinkelaar RB. Different strategies in the laboratory diagnosis of autoimmune disease: immunofluorescence, enzyme-linked-immunosorbent assay or both? Ann Clin Biochem. 1999;36:189–195. doi: 10.1177/000456329903600209. [DOI] [PubMed] [Google Scholar]
- 61.Tonutti E, Bassetti D, Piazza A, Visentini D, Poletto M, Bassetto F, et al. Diagnostic accuracy of ELISA methods as an alternative test to indirect immunofluorescence for the detection of antinuclear antibodies. Evaluation of five commercial kits. Autoimmunity. 2004;37:171–176. doi: 10.1080/08916930310001657010. [DOI] [PubMed] [Google Scholar]
- 62.Forsman RW. Why is the laboratory an afterthought for managed care organizations? Clin Chem. 1996;42:813–816. [PubMed] [Google Scholar]
- 63.Tan EM (1993) International cooperative activities in the standardization of antinuclear antibodies. Manual of biological markers of disease, vol A1. Kluwer Academic Publishers, Dordrecht, pp 1–5
- 64.Smolen JS, Butcher B, Fritzler MJ, Gordon T, Hardin J, Kalden JR, et al. Reference sera for antibuclear antibodies. II. Further definition of antibody specificities in international antinuclear antibody reference sera by immunofluorescence and Western blotting. Arthritis Rheum. 1997;40:413–418. doi: 10.1002/art.1780400304. [DOI] [PubMed] [Google Scholar]
- 65.Carey RN (2006) User verification of performances for precision and trueness; approved guideline. Clinical and Laboratory Standard Institute (CLSI) document EP15-A2, 2nd edn. CLSI:ISBN 1-56238-000-0, Wayne


