Abstract
Asialoglycoprotein receptor (ASGPR) autoantibodies have been considered specific markers of autoimmune hepatitis (AIH). The exact mechanisms responsible for the development of these autoantibodies and leading to autoimmunity to this peculiar liver receptor remain elusive. Furthermore, loss of T cell tolerance to ASGPR has been demonstrated in patients with AIH, but it is poorly understood whether such liver-specific T cell responses bear a pathogenic potential and/or participate in the precipitation of AIH. Newly developed enzyme-linked immunosorbent assays have led to the investigation of the sensitivity and specificity of anti-ASGPR antibodies for AIH. The present review provides an overview of the diagnostic and clinical relevance of anti-ASGPR antibodies. A thorough investigation of the autoreactivity against ASGPR may assist efforts to understand liver autoimmunity in susceptible individuals.
Keywords: Autoantibody, Autoimmune hepatitis, Autoimmunity, Liver disease, Primary biliary cirrhosis
ASGPR: an introduction to the molecule
Asialoglycoprotein receptor (ASGPR) [1] is a C-type lectin, primary expressed on the sinusoidal surface of the hepatocyte [2–4]. ASGPR was discovered as early as 1965 by Gilbert Ashwell and Anatol Morell in USA [3–9]. These investigators isolated ASGPR from rabbit liver by affinity chromatography employing asialo-orosomucoid sepharose [10].
ASGPR is formed by a major 48 kDa (ASGPR-1) and a minor 40 kDa subunits (ASGPR-2) [11–13]. The major role of ASGPR is the binding, internalization, and subsequent clearance from the circulation of glycoproteins that contain terminal galactose or N-acetylgalactosamine residues (asialoglycoproteins) [11, 12, 14]. The binding of ligands to ASGPR depends on Ca2+ [15], the position of terminal galactose residues [16–18], and a pH optimum above 6.5 [19]. Mice lacking ASGPR are characterized by an impaired clearance of injected asialoglycoproteins, but do not accumulate glycoproteins in the serum suggesting that ASGPR is not the only regulator of glycoprotein levels in the blood.
ASGPR has been shown also to be involved in the clearance of IgA from circulation [10, 20–25], in the removal of apoptotic cells, in the clearance of low density lipoprotein (LDL) and chylomicron remnants [26, 27] and in the disposal of cellular fibronectin [28]. Recent data support the assumption that ASGPR is utilized by hepatotropic viruses for hepatocyte entry [29–35]. Furthermore, there is evidence that ASGPR participates in the elimination of activated lymphocytes [36–39].
From a pathophysiological point of view, ASGPR appears to be involved in the clearance of coagulation factors from circulation and to be involved in the establishment of lethal thrombocytopenia seen in sepsis caused by S. pneumonia infection [40]. In this disease setting, ASGPR-dependent clearance of platelets, which have been desialylated by the NanA sialidase of S. pneumonia, seems to play a pivotal role in sepsis precipitation. This may explain why ASGPR-knockout mice cannot survive inoculation with small doses of S. pneumonia [40–43].
ASGPR and liver inflammation
Liver inflammatory diseases irrespectively of the cause alter the expression levels of ASGPR, its synthesis, and binding activity. In normal hepatocytes, ASGPR is expressed in a polar manner on the sinusoidal and basolateral surface of the plasma hepatocyte membrane [11, 12, 44]. However, during liver inflammation, ASGPR’s expression shifts towards the canalicular membrane [45]. In end-stage liver disease (cirrhosis), ASGPR is over-expressed and serum levels of asialoglycoproteins are increased [46]. Cytokines appear to have a profound effect on the expression, synthesis, and functionality of the receptor [47, 48].
In immune-mediated liver diseases, ASGPR becomes the target of autoimmune responses, both at the B- and T cell level [49]. Thus, this review will mainly discuss the role of ASGPR as a liver autoantigen.
ASGPR as an autoantigen
In the 1970s, liver-specific membrane lipoproteins (LSP) have been found by Roger Williams’ group to bear antigenic epitopes for circulating autoantibodies in patients with acute and chronic active hepatitis [50]. Later on, ASGPR was identified as the major antigenic component of LSP by the same group in London and appears to be the main organ-specific autoantigenic target in autoimmune liver diseases reported so far [51]. Subsequent studies, both in human and animal biomaterial, have highlighted the autoimmunogenicity of ASGPR and its relevance to specific disease phenotypes [49, 52–68]. Soon after the experimental data provided evidence that ASGPR-specific monoclonal antibodies can induce liver damage, attempts to study the immunological potential of ASGPR have been accelerating [69]. ASGPR-specific T cell clones have been obtained [67] and ASGPR (or LSP) has been used in immunization experiments to induce liver damage in an animal setting [69–73]. The relevance of anti-ASGPR-specific T cell responses to the pathogenesis of AIH is still elusive [74, 75]. Other liver-specific B and T cell responses against AIH-relevant antigens appear to play an important role in the development of AIH in susceptible individuals, who are characterized by impaired immunoregulatory mechanisms [74–85]. Intriguingly, gastrointestinal receptors, such as the ASGPR with the potential to interact with pathogens appear to play an important role in gastrointestinal autoimmunity as recently demonstrated for the microfold cell-specific glycoprotein 2, the autoantigenic target in Crohn’s disease which has even an immunomodulating capacity [86–88].
One of the first efforts to elucidate the humoral loss of tolerance to ASGPR was to develop assays that could reliably detect anti-ASGPR antibody levels in patients with AIH. Due to the peculiar biochemical characteristics of ASGPR, this task proved to be rather difficult, but was nevertheless essential to study the diagnostic and clinical relevance of the tolerance loss to ASGPR.
Anti-ASGPR antibody testing
A variety of assays has been developed in order to detect anti-ASGPR antibody reactivity and such assays included solid-phase enzyme-linked immunosorbent assays (ELISA), liquid-phase radioimmunoassays, immunoblotting and dot blot assays. As an antigenic source, ASGPR purified from rabbit, rat or human liver preparations or recombinant ASGPR subunits have been utilized [52, 58, 60, 62, 89]. One of the major challenges in developing a molecular assay remained the access to highly purified ASGPR [90]. ASGPR obtained through affinity chromatography on galactose–sepharose has been considered a credible source of the antigen [68]. Recombinant antigen has been produced, but its immunogenicity appears poor [58]. In summary, data obtained by several studies produced inconsistent results, raising concerns as to whether a reliable immunoassay could ever be developed to assess the epitope structure of the antigenic preparation used for ASGPR-antibody testing [89]. As it was expected, the lack of a reliable, standardized assay for the detection of anti-ASGPR antibodies has led to a series of reports with significant variation in the prevalence of these autoantibodies in AIH, and other autoimmune and non-autoimmune liver diseases.
Nevertheless, anti-ASGPR antibodies have been included in the revised criteria of the International Autoimmune Hepatitis Group in 1999, but are missing in recently published diagnostic scores for AIH while other autoantibodies have been incorporated in the routine testing [91–93]. The assumption that ASGPR autoantibodies should not be part of the autoantibody specificities considered to be important for the serological diagnosis in patients with AIH deserves further discussion.
Diagnostic relevance of anti-ASGPR antibodies
Most studies recently published have tested anti-ASGPR antibodies in serum samples from patients with liver diseases, including autoimmune and non-autoimmune disorders of the liver. Anti-ASGPR autoantibodies have been predominantly found in patients with chronic active hepatitis, the term used in the past for what is currently known as AIH. Another key finding was that the level of these autoantibodies sharply decreases during immunosuppressive treatment [68]. This was an intriguing observation with profound implications regarding the broad range of anti-ASGPR seropositivity in various reports published over the years, as most studies included mixed sera from patients at diagnosis and during treatment. Thus, testing of cohorts that include more samples at diagnosis rather than under immunosuppression will most likely provide prevalence rates much higher than those of cohorts mainly containing samples tested after treatment [60].
The prevalence rates in children [94] and adult patients [59] with AIH-1 appear to be comparable, with anti-ASGPR antibodies being detectable in 75–82 % AIH-1 patients. However, in a recent study by Bulgakova et al. reported as an abstract at the 8th International Congress of Autoimmunity in Granada, a significantly higher prevalence of anti-ASGPR antibodies was found in children investigating 18 children and 28 adults with AIH (Fig. 1). However, only 2 out of the 18 children with AIH suffered from AIH-2 which demonstrate in general less prevalent anti-ASGPR antibodies (around 24–40 %) [94, 95].
Fig. 1.
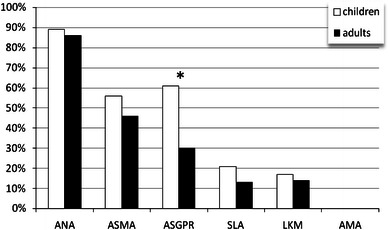
Prevalence of autoantibodies in children (n = 18, median of age 10 years) and adults (n = 28, median of age 46 years) with autoimmune hepatitis (AIH). Seropositivity for antinuclear antibodies (ANA), anti-smooth muscle antibodies (ASMA), liver–kidney microsomal type 1 (LKM-1) and anti-mitochondrial (AMA) antibodies were detected by indirect immunofluorescence on respective substrates. Antibodies to asialoglycoprotein receptor (ASGPR) and soluble liver antigen (SLA) were determined by commercial ELISA. 2 of 18 children with AIH suffered from type 2 AIH, whereas 2 of 28 adult patients with AIH demonstrated type 2 disease. All remaining patients had type 1 AIH. *P < 0.05
The diagnostic value of anti-ASGPR antibodies is supported by studies suggesting that up to 80 % of AIH patients, who are seronegative for conventionally tested autoantibodies such as antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA), anti-liver kidney microsomal 1 (LKM1) antibody, and even antibodies to soluble liver antigen (SLA), may have anti-ASGPR antibodies [61, 96]. In practical terms, these findings suggest that the recommended serological profile of patients with this disease may fail, if anti-ASGPR antibodies are not incorporated [97]. This has led some expert reports to underline the importance of anti-ASGPR antibody testing [92, 98]. However, the guidelines of the diagnostic criteria for AIH do not include anti-ASGPR antibodies in the scoring for the probable or definite diagnosis of AIH [93, 99, 100].
Considering the lack of commercially available assays, testing for anti-ASGPR antibodies has been limited to few university laboratories using laborious in house assays for research purposes. The reports so far published have also indicated that the specificity of anti-ASGPR for AIH is not as good as described for other autoantibodies, like anti-LKM1, anti-SLA, and anti-SMA filamentous actin. Anti-ASGPR antibodies have been reported in more than 10 % of patients with chronic hepatitis B or C, patients with PBC, and patients with alcoholic hepatitis [54, 56, 60, 62, 101, 102]. The inconsistent results may be due to the lack of a standardized assay. In recent years, a new commercially available ELISA based on purified rabbit ASGPR has been developed and a study has been published reporting on the sensitivity and the specificity of this assay. Remarkably, 70 % of naïve (untreated) AIH patients were tested positive for anti-ASGPR antibody, whereas only 30 % patients under immunosuppressive treatment demonstrated elevated anti-ASGPR antibody levels [68]. Detectable anti-ASGPR antibodies were found in approximately 10 % patients with chronic hepatitis B or C [68]. Anti-ASGPR antibodies were practically absent in other pathological controls (including patients with PBC and alcoholic hepatitis) [68]. The interesting finding of this study was the striking specificity of anti-ASGPR for AIH (up to 100 %) as compared to PBC excluding PBC AIH overlap syndromes and such findings have not been reported previously.
Clinical utility of anti-ASGPR antibodies
An early study assessing anti-ASGPR antibodies in AIH-1 patients from USA noted more frequent relapses on treatment cessation in anti-ASGPR antibody positive AIH patients compared to anti-ASGPR antibody negative [59]. Serum immunoglobulin levels appeared higher in those with anti-ASGPR antibodies [59]. The association of disease activity indices or response to treatment and the presence of anti-ASGPR antibodies has been reported by others [53, 59, 94, 100, 103].
The recently published study employing the new anti-ASGPR antibody ELISA has found a correlation between anti-ASGPR and the level of liver transaminases in patients followed for long period of time [68]. A further intriguing result was that anti-ASGPR antibodies may precede the onset of elevated transaminases. That finding and the fact that anti-ASGPR antibodies are decreased over the course of immunosuppression indicate that this autoantibody may be a prognostic marker of disease activity [54, 56, 60]. A recent report has also found that anti-ASGPR antibodies decrease during immunosuppression [91, 104].
Conclusion
In the years to come, the relevance of anti-ASGPR antibodies in the diagnosis of AIH will be understood better with the help of robust and user-friendly anti-ASGPR antibody assays used in routine laboratory analysis. At present, several laboratories over the world are testing the applicability of newly available anti-ASGPR antibody ELISA. The results of these tests will shed a light on the long discussed usefulness of this autoantibody in the serology of AIH. We need to remind ourselves that ASGPR is one of the very few autoantigens that is liver specific in AIH (if it is not the only liver-specific one). It is possible that the understanding of the pathogenesis of AIH lies within the understanding of the immunopathophysiology of this hepatic lectin [91]. We anticipate that we will learn more about the role of ASGPR in the near future.
Conflict of interest
Dirk Roggenbuck has a management role and is a shareholder of GA Generic Assays GmbH and Medipan GmbH. Both companies are diagnostic manufacturers. All other authors declare that they have no competing financial interests.
Abbreviations
- ANA
Anti-nuclear antibody
- ASGPR
Asialoglycoprotein receptor
- ASMA
Anti-smooth muscle antibody
- ELISA
Enzyme-linked immunosorbent assay
- LSP
Liver-specific membrane lipoproteins
- LKM
Liver kidney microsomal
- SLA
Soluble liver antigen
Contributor Information
Dirk Roggenbuck, Phone: +49-33708441716, FAX: +49-33708441725, Email: dirk.roggenbuck@hs-lausitz.de.
Maria G. Mytilinaiou, Email: maria.mytilinaiou@kcl.ac.uk
Sergey V. Lapin, Email: svlapin@mail.ru
Dirk Reinhold, Email: dirk.reinhold@med.ovgu.de.
Karsten Conrad, Email: k_conrad@mail.zih.tu-dresden.de.
References
- 1.Ashwell G, Morell AG. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41:99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- 2.Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 3.Morell AG, Gregoriadis G, Scheinberg IH, et al. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971;246:1461–1467. [PubMed] [Google Scholar]
- 4.Morell AG, Irvine RA, Sternlieb I, et al. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem. 1968;243:155–159. [PubMed] [Google Scholar]
- 5.Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988;263:9557–9560. [PubMed] [Google Scholar]
- 6.Drickamer K. Ca(2+)-dependent sugar recognition by animal lectins. Biochem Soc Trans. 1996;24:146–150. doi: 10.1042/bst0240146. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous Gilbert Ashwell: sweet on science. Nat Med. 2008;14:608. doi: 10.1038/nm0608-608. [DOI] [PubMed] [Google Scholar]
- 8.Morell AG, Van den Hamer CJ, Scheinberg IH, et al. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal d-galactose residues. J Biol Chem. 1966;241:3745–3749. [PubMed] [Google Scholar]
- 9.Van Den Hamer CJ, Morell AG, Scheinberg IH, et al. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970;245:4397–4402. [PubMed] [Google Scholar]
- 10.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 11.Spiess M. The asialoglycoprotein receptor: a model for endocytic transport receptors. Biochemistry. 1990;29:10009–10018. doi: 10.1021/bi00495a001. [DOI] [PubMed] [Google Scholar]
- 12.Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- 13.Diao J, Michalak TI. Composition, antigenic properties and hepatocyte surface expression of the woodchuck asialoglycoprotein receptor. J Recept Signal Transduct Res. 1996;16:243–271. doi: 10.3109/10799899609039951. [DOI] [PubMed] [Google Scholar]
- 14.Pricer WE, Jr, Hudgin RL, Ashwell G, et al. A membrane receptor protein for asialoglycoproteins. Methods Enzymol. 1974;34:688–691. doi: 10.1016/s0076-6879(74)34090-6. [DOI] [PubMed] [Google Scholar]
- 15.Weigel PH. Characterization of the asialoglycoprotein receptor on isolated rat hepatocytes. J Biol Chem. 1980;255:6111–6120. [PubMed] [Google Scholar]
- 16.Lee YC, Townsend RR, Hardy MR, et al. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J Biol Chem. 1983;258:199–202. [PubMed] [Google Scholar]
- 17.Hardy MR, Townsend RR, Parkhurst SM, et al. Different modes of ligand binding to the hepatic galactose/N-acetylgalactosamine lectin on the surface of rabbit hepatocytes. Biochemistry. 1985;24:22–28. doi: 10.1021/bi00322a004. [DOI] [PubMed] [Google Scholar]
- 18.Chiu MH, Tamura T, Wadhwa MS, et al. In vivo targeting function of N-linked oligosaccharides with terminating galactose and N-acetylgalactosamine residues. J Biol Chem. 1994;269:16195–16202. [PubMed] [Google Scholar]
- 19.Breitfeld PP, Rup D, Schwartz AL. Influence of the N-linked oligosaccharides on the biosynthesis, intracellular routing, and function of the human asialoglycoprotein receptor. J Biol Chem. 1984;259:10414–10421. [PubMed] [Google Scholar]
- 20.Stockert RJ, Kressner MS, Collins JC, et al. IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci USA. 1982;79:6229–6231. doi: 10.1073/pnas.79.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels CK, Schmucker DL, Jones AL. Hepatic asialoglycoprotein receptor-mediated binding of human polymeric immunoglobulin A. Hepatology. 1989;9:229–234. doi: 10.1002/hep.1840090211. [DOI] [PubMed] [Google Scholar]
- 22.Tomana M, Kulhavy R, Mestecky J. Receptor-mediated binding and uptake of immunoglobulin A by human liver. Gastroenterology. 1988;94:762–770. doi: 10.1016/0016-5085(88)90252-1. [DOI] [PubMed] [Google Scholar]
- 23.Rifai A, Fadden K, Morrison SL, et al. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med. 2000;191:2171–2182. doi: 10.1084/jem.191.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974;249:7260–7269. [PubMed] [Google Scholar]
- 25.Inamoto T, Brown WR. IgG is associated with the asialoglycoprotein receptor in the human liver. Hepatology. 1991;14:1070–1075. [PubMed] [Google Scholar]
- 26.Windler E, Greeve J, Levkau B, et al. The human asialoglycoprotein receptor is a possible binding site for low-density lipoproteins and chylomicron remnants. Biochem J. 1991;276(Pt 1):79–87. doi: 10.1042/bj2760079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi S, Perrey S, Chen Z, et al. Role of the low density lipoprotein (LDL) receptor pathway in the metabolism of chylomicron remnants. A quantitative study in knockout mice lacking the LDL receptor, apolipoprotein E, or both. J Biol Chem. 1996;271:22422–22427. doi: 10.1074/jbc.271.37.22422. [DOI] [PubMed] [Google Scholar]
- 28.Rotundo RF, Vincent PA, McKeown-Longo PJ, et al. Hepatic fibronectin matrix turnover in rats: involvement of the asialoglycoprotein receptor. Am J Physiol. 1999;277:G1189–G1199. doi: 10.1152/ajpgi.1999.277.6.G1189. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Bo XC, Ding XR, et al. Antisense oligonucleotides targeted against asialoglycoprotein receptor 1 block human hepatitis B virus replication. J Viral Hepat. 2006;13:158–165. doi: 10.1111/j.1365-2893.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Wang F, Tian L, et al. Fibronectin and asialoglyprotein receptor mediate hepatitis B surface antigen binding to the cell surface. Arch Virol. 2010;155:881–888. doi: 10.1007/s00705-010-0657-5. [DOI] [PubMed] [Google Scholar]
- 31.Treichel U, Meyer zum Buschenfelde KH, Dienes HP. Receptor-mediated entry of hepatitis B virus particles into liver cells. Arch Virol. 1997;142:493–498. doi: 10.1007/s007050050095. [DOI] [PubMed] [Google Scholar]
- 32.Treichel U, Meyer zum Buschenfelde KH, Stockert RJ, et al. The asialoglycoprotein receptor mediates hepatic binding and uptake of natural hepatitis B virus particles derived from viraemic carriers. J Gen Virol. 1994;75(Pt 11):3021–3029. doi: 10.1099/0022-1317-75-11-3021. [DOI] [PubMed] [Google Scholar]
- 33.Dotzauer A, Gebhardt U, Bieback K, et al. Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor. J Virol. 2000;74:10950–10957. doi: 10.1128/jvi.74.23.10950-10957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu GY, Wu CH. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988;263:14621–14624. [PubMed] [Google Scholar]
- 35.Wu GY, Zhan P, Sze LL, et al. Incorporation of adenovirus into a ligand-based DNA carrier system results in retention of original receptor specificity and enhances targeted gene expression. J Biol Chem. 1994;269:11542–11546. [PubMed] [Google Scholar]
- 36.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guy CS, Rankin SL, Michalak TI. Hepatocyte cytotoxicity is facilitated by asialoglycoprotein receptor. Hepatology. 2011;54:1043–1050. doi: 10.1002/hep.24477. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Soldevila G, Leeker M, et al. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–749. doi: 10.1016/s1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Sye K, Crispe IN. Proliferation and apoptosis of B220+CD4-CD8-TCR alpha beta intermediate T cells in the liver of normal adult mice: implication for lpr pathogenesis. Int Immunol. 1994;6:533–540. doi: 10.1093/intimm/6.4.533. [DOI] [PubMed] [Google Scholar]
- 40.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14:648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–1280. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grewal PK. The Ashwell–Morell receptor. Methods Enzymol. 2010;479:223–241. doi: 10.1016/S0076-6879(10)79013-3. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen AL, Rumjantseva V, Nayeb-Hashemi S, et al. Role of sialic acid for platelet life span: exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114:1645–1654. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker S, Spiess M, Klenk HD. The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J Gen Virol. 1995;76(Pt 2):393–399. doi: 10.1099/0022-1317-76-2-393. [DOI] [PubMed] [Google Scholar]
- 45.Burgess JB, Baenziger JU, Brown WR. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology. 1992;15:702–706. doi: 10.1002/hep.1840150425. [DOI] [PubMed] [Google Scholar]
- 46.Nakaya R, Kohgo Y, Mogi Y, et al. Regulation of asialoglycoprotein receptor synthesis by inflammation-related cytokines in HepG2 cells. J Gastroenterol. 1994;29:24–30. doi: 10.1007/BF01229069. [DOI] [PubMed] [Google Scholar]
- 47.Treichel U, Paietta E, Poralla T, et al. Effects of cytokines on synthesis and function of the hepatic asialoglycoprotein receptor. J Cell Physiol. 1994;158:527–534. doi: 10.1002/jcp.1041580319. [DOI] [PubMed] [Google Scholar]
- 48.Marshall JS, Green AM, Pensky J, et al. Measurement of circulating desialylated glycoproteins and correlation with hepatocellular damage. J Clin Invest. 1974;54:555–562. doi: 10.1172/JCI107792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poralla T, Treichel U, Lohr H, et al. The asialoglycoprotein receptor as target structure in autoimmune liver diseases. Semin Liver Dis. 1991;11:215–222. doi: 10.1055/s-2008-1040439. [DOI] [PubMed] [Google Scholar]
- 50.Jensen DM, McFarlane IG, Portmann BS, et al. Detection of antibodies directed against a liver-specific membrane lipoprotein in patients with acute and chronic active hepatitis. N Engl J Med. 1978;299:1–7. doi: 10.1056/NEJM197807062990101. [DOI] [PubMed] [Google Scholar]
- 51.McFarlane IG, McFarlane BM, Major GN, et al. Identification of the hepatic asialo-glycoprotein receptor (hepatic lectin) as a component of liver specific membrane lipoprotein (LSP) Clin Exp Immunol. 1984;55:347–354. [PMC free article] [PubMed] [Google Scholar]
- 52.McFarlane BM, McSorley CG, McFarlane IG, et al. A radioimmunoassay for detection of circulating antibodies reacting with the hepatic asialoglycoprotein receptor protein. J Immunol Methods. 1985;77:219–228. doi: 10.1016/0022-1759(85)90034-1. [DOI] [PubMed] [Google Scholar]
- 53.McFarlane IG, Hegarty JE, McSorley CG, et al. Antibodies to liver-specific protein predict outcome of treatment withdrawal in autoimmune chronic active hepatitis. Lancet. 1984;2:954–956. doi: 10.1016/s0140-6736(84)91167-x. [DOI] [PubMed] [Google Scholar]
- 54.McFarlane BM, McSorley CG, Vergani D, et al. Serum autoantibodies reacting with the hepatic asialoglycoprotein receptor protein (hepatic lectin) in acute and chronic liver disorders. J Hepatol. 1986;3:196–205. doi: 10.1016/s0168-8278(86)80026-5. [DOI] [PubMed] [Google Scholar]
- 55.McFarlane BM, Sipos J, Gove CD, et al. Antibodies against the hepatic asialoglycoprotein receptor perfused in situ preferentially attach to periportal liver cells in the rat. Hepatology. 1990;11:408–415. doi: 10.1002/hep.1840110312. [DOI] [PubMed] [Google Scholar]
- 56.Treichel U, Gerken G, Rossol S, et al. Autoantibodies against the human asialoglycoprotein receptor: effects of therapy in autoimmune and virus-induced chronic active hepatitis. J Hepatol. 1993;19:55–63. doi: 10.1016/s0168-8278(05)80176-x. [DOI] [PubMed] [Google Scholar]
- 57.Treichel U, McFarlane BM, Seki T, et al. Demographics of anti-asialoglycoprotein receptor autoantibodies in autoimmune hepatitis. Gastroenterology. 1994;107:799–804. doi: 10.1016/0016-5085(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 58.Treichel U, Poralla T, Hess G, et al. Autoantibodies to human asialoglycoprotein receptor in autoimmune-type chronic hepatitis. Hepatology. 1990;11:606–612. doi: 10.1002/hep.1840110413. [DOI] [PubMed] [Google Scholar]
- 59.Czaja AJ, Pfeifer KD, Decker RH, et al. Frequency and significance of antibodies to asialoglycoprotein receptor in type 1 autoimmune hepatitis. Dig Dis Sci. 1996;41:1733–1740. doi: 10.1007/BF02088738. [DOI] [PubMed] [Google Scholar]
- 60.Dejica D, Treichel U, Par A, et al. Anti asialoglycoprotein receptor antibodies and soluble interleukin-2 receptor levels as marker for inflammation in autoimmune hepatitis. Z Gastroenterol. 1997;35:15–21. [PubMed] [Google Scholar]
- 61.Lohse AW, Gerken G, Mohr H, et al. Relation between autoimmune liver diseases and viral hepatitis: clinical and serological characteristics in 859 patients. Z Gastroenterol. 1995;33:527–533. [PubMed] [Google Scholar]
- 62.Yoshioka M, Mizuno M, Morisue Y, et al. Anti-asialoglycoprotein receptor autoantibodies, detected by a capture-immunoassay, are associated with autoimmune liver diseases. Acta Med Okayama. 2002;56:99–105. doi: 10.18926/AMO/31695. [DOI] [PubMed] [Google Scholar]
- 63.Bedlow AJ, Donaldson PT, McFarlane BM, et al. Autoreactivity to hepatocellular antigens in primary biliary cirrhosis and primary sclerosing cholangitis. J Clin Lab Immunol. 1989;30:103–109. [PubMed] [Google Scholar]
- 64.Vento S, McFarlane BM, Vento TG, et al. Serial study of liver-directed autoantibodies and autoreactive T-lymphocytes in acute viral hepatitis B. J Autoimmun. 1988;1:299–307. doi: 10.1016/0896-8411(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 65.McFarlane BM, Bridger CB, Smith HM, et al. Autoimmune mechanisms in chronic hepatitis B and delta virus infections. Eur J Gastroenterol Hepatol. 1995;7:615–621. [PubMed] [Google Scholar]
- 66.Lohr H, Treichel U, Poralla T, et al. Liver-infiltrating T helper cells in autoimmune chronic active hepatitis stimulate the production of autoantibodies against the human asialoglycoprotein receptor in vitro. Clin Exp Immunol. 1992;88:45–49. doi: 10.1111/j.1365-2249.1992.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lohr H, Treichel U, Poralla T, et al. The human hepatic asialoglycoprotein receptor is a target antigen for liver-infiltrating T cells in autoimmune chronic active hepatitis and primary biliary cirrhosis. Hepatology. 1990;12:1314–1320. doi: 10.1002/hep.1840120611. [DOI] [PubMed] [Google Scholar]
- 68.Hausdorf G, Roggenbuck D, Feist E, et al. Autoantibodies to asialoglycoprotein receptor (ASGPR) measured by a novel ELISA—revival of a disease-activity marker in autoimmune hepatitis. Clin Chim Acta. 2009;408:19–24. doi: 10.1016/j.cca.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 69.Poralla T, Ramadori G, Dienes HP, et al. Liver cell damage caused by monoclonal antibody against an organ-specific membrane antigen in vivo and in vitro. J Hepatol. 1987;4:373–380. doi: 10.1016/s0168-8278(87)80548-2. [DOI] [PubMed] [Google Scholar]
- 70.Gonzales C, Cochrane AM, Eddleston AL, et al. Mechanisms responsible for antibody-dependent, cell-mediated cytotoxicity to isolated hepatocytes in chronic active hepatitis. Gut. 1979;20:385–388. doi: 10.1136/gut.20.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cochrane AM, Moussouros A, Thomsom AD, et al. Antibody-dependent cell-mediated (K cell) cytotoxicity against isolated hepatocytes in chronic active hepatitis. Lancet. 1976;1:441–444. doi: 10.1016/s0140-6736(76)91472-0. [DOI] [PubMed] [Google Scholar]
- 72.Hopf U, Meyer zum Buschenfelde KH, Arnold W. Detection of a liver-membrane autoantibody in HBsAg-negative chronic active hepatitis. N Engl J Med. 1976;294:578–582. doi: 10.1056/NEJM197603112941103. [DOI] [PubMed] [Google Scholar]
- 73.Meyer zum Buschenfelde KH, Treichel U, Lohr H, et al. Human asialoglycoprotein receptor as an autoantigen in chronic hepatitis. Immunol Res. 1991;10:497–502. doi: 10.1007/BF02919748. [DOI] [PubMed] [Google Scholar]
- 74.Vergani D, Longhi MS, Bogdanos DP, et al. Autoimmune hepatitis. Semin Immunopathol. 2009;31:421–435. doi: 10.1007/s00281-009-0170-7. [DOI] [PubMed] [Google Scholar]
- 75.Wen L, Ma Y, Bogdanos DP, et al. Pediatric autoimmune liver diseases: the molecular basis of humoral and cellular immunity. Curr Mol Med. 2001;1:379–389. doi: 10.2174/1566524013363672. [DOI] [PubMed] [Google Scholar]
- 76.Longhi MS, Hussain MJ, Bogdanos DP, et al. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 77.Longhi MS, Ma Y, Bogdanos DP, et al. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Longhi MS, Ma Y, Mitry RR, et al. Effect of CD4+CD25+regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun. 2005;25:63–71. doi: 10.1016/j.jaut.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Ma Y, Bogdanos DP, Hussain MJ, et al. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130:868–882. doi: 10.1053/j.gastro.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 80.Ma Y, Okamoto M, Thomas MG, et al. Antibodies to conformational epitopes of soluble liver antigen define a severe form of autoimmune liver disease. Hepatology. 2002;35:658–664. doi: 10.1053/jhep.2002.32092. [DOI] [PubMed] [Google Scholar]
- 81.Ma Y, Thomas MG, Okamoto M, et al. Key residues of a major cytochrome P4502D6 epitope are located on the surface of the molecule. J Immunol. 2002;169:277–285. doi: 10.4049/jimmunol.169.1.277. [DOI] [PubMed] [Google Scholar]
- 82.Mullighan CG, Bogdanos DP, Vergani D, et al. Cytochrome P450 1A2 is a target antigen in hepatitic graft-versus-host disease. Bone Marrow Transplant. 2006;38:703–705. doi: 10.1038/sj.bmt.1705510. [DOI] [PubMed] [Google Scholar]
- 83.Vergani D, Choudhuri K, Bogdanos DP, et al. Pathogenesis of autoimmune hepatitis. Clin Liver Dis. 2002;6:727–737. doi: 10.1016/s1089-3261(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 84.Hintermann E, Holdener M, Bayer M, et al. Epitope spreading of the anti-CYP2D6 antibody response in patients with autoimmune hepatitis and in the CYP2D6 mouse model. J Autoimmun. 2011;37:242–253. doi: 10.1016/j.jaut.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Christen U, Hintermann E, Jaeckel E. New animal models for autoimmune hepatitis. Semin Liver Dis. 2009;29:262–272. doi: 10.1055/s-0029-1233536. [DOI] [PubMed] [Google Scholar]
- 86.Werner L, Paclik D, Fritz C, et al. Identification of pancreatic glycoprotein 2 as an endogenous immunomodulator of innate and adaptive immune responses. J Immunol. 2012;189:2774–2783. doi: 10.4049/jimmunol.1103190. [DOI] [PubMed] [Google Scholar]
- 87.Roggenbuck D, Hausdorf G, Martinez-Gamboa L, et al. Identification of GP2, the major zymogen granule membrane glycoprotein, as the autoantigen of pancreatic antibodies in Crohn’s disease. Gut. 2009;58:1620–1628. doi: 10.1136/gut.2008.162495. [DOI] [PubMed] [Google Scholar]
- 88.Bogdanos DP, Rigopoulou EI, Smyk DS, et al. Diagnostic value, clinical utility and pathogenic significance of reactivity to the molecular targets of Crohn’s disease specific-pancreatic autoantibodies. Autoimmun Rev. 2011;11:143–148. doi: 10.1016/j.autrev.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 89.Hajoui O, Martin S, Alvarez F. Study of antigenic sites on the asialoglycoprotein receptor recognized by autoantibodies. Clin Exp Immunol. 1998;113:339–345. doi: 10.1046/j.1365-2249.1998.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Treichel U, Schreiter T, Meyer zum Buschenfelde KH, et al. High-yield purification and characterization of human asialoglycoprotein receptor. Protein Expr Purif. 1995;6:251–255. doi: 10.1006/prep.1995.1032. [DOI] [PubMed] [Google Scholar]
- 91.Rigopoulou EI, Roggenbuck D, Smyk DS et al (2012) Asialoglycoprotein receptor (ASGPR) as target autoantigen in liver autoimmunity: lost and found. Autoimmun Rev [DOI] [PubMed]
- 92.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 93.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 94.Hajoui O, Debray D, Martin S, et al. Auto-antibodies to the asialoglycoprotein receptor in sera of children with auto-immune hepatitis. Eur J Pediatr. 2000;159:310–313. doi: 10.1007/s004310051278. [DOI] [PubMed] [Google Scholar]
- 95.Gregorio GV, Portmann B, Reid F, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997;25:541–547. doi: 10.1002/hep.510250308. [DOI] [PubMed] [Google Scholar]
- 96.Johnson PJ, McFarlane IG, McFarlane BM, et al. Auto-immune features in patients with idiopathic chronic active hepatitis who are seronegative for conventional auto-antibodies. J Gastroenterol Hepatol. 1990;5:244–251. doi: 10.1111/j.1440-1746.1990.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 97.Bogdanos DP, Mieli-Vergani G, Vergani D. Autoantibodies and their antigens in autoimmune hepatitis. Semin Liver Dis. 2009;29:241–253. doi: 10.1055/s-0029-1233533. [DOI] [PubMed] [Google Scholar]
- 98.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 99.Vergani D, Alvarez F, Bianchi FB, et al. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677–683. doi: 10.1016/j.jhep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Chantran Y, Ballot E, Johanet C (2012) Autoantibodies in autoimmune hepatitis: anti-asialoglycoprotein receptor (anti-ASGPR) antibodies. Clin Res Hepatol Gastroenterol [DOI] [PubMed]
- 101.Hilgard P, Schreiter T, Stockert RJ, et al. Asialoglycoprotein receptor facilitates hemolysis in patients with alcoholic liver cirrhosis. Hepatology. 2004;39:1398–1407. doi: 10.1002/hep.20172. [DOI] [PubMed] [Google Scholar]
- 102.Husa P, Chalupa P, Stroblova H, et al. Autoantibodies to asialoglycoprotein receptor in chronic hepatitis C patients. Acta Virol. 2001;45:7–11. [PubMed] [Google Scholar]
- 103.Sasaki M, Yamauchi K, Tokushige K, et al. Clinical significance of autoantibody to hepatocyte membrane antigen in type 1 autoimmune hepatitis. Am J Gastroenterol. 2001;96:846–851. doi: 10.1111/j.1572-0241.2001.03630.x. [DOI] [PubMed] [Google Scholar]
- 104.Mytilinaiou MG, Roggenbuck D, Grammatikopoulos A, et al. Anti-asialoglycoprotein receptor antibody measured by ELISA is a specific marker of liver autoimmunity and mirrors disease activity in patients with autoimmune hepatitis. Hepatology. 2011;54:919A. [Google Scholar]


