Abstract
Purpose
Before expanding our indications for laparoscopic gastrectomy to advanced gastric cancer and adopting reduced port laparoscopic gastrectomy, we analyzed and audited the outcomes of laparoscopy-assisted distal gastrectomy (LADG) for adenocarcinoma; this was done during the adoptive period at our institution through the comparative analysis of short-term surgical outcomes and learning curves (LCs) of two surgeons with different careers.
Materials and Methods
A detailed comparative analysis of the LCs and surgical outcomes was done for the respective first 95 and 111 LADGs performed by two surgeons between July, 2006 and June, 2011. The LCs were fitted by using the non-linear ordinary least squares estimation method.
Results
The postoperative morbidity and mortality rates were 14.6% and 0.0%, respectively, and there was no significant difference in the morbidity rates (12.6% vs. 16.2%, P=0.467). More than 25 lymph nodes were retrieved by each surgeon during LADG procedures. The LCs of both surgeons were distinct. In this study, a stable plateau of the LC was not achieved by both surgeons even after performing 90 LADGs.
Conclusions
Regardless of the experience with gastrectomy or laparoscopic surgery for other organs, or the age of surgeon, the outcome was quite acceptable; the learning process differ according to the surgeon's experience and individual characteristics.
Keywords: Laparoscopy, Gastrectomy, Learning curve, Treatment outcome
Introduction
Minimally invasive procedures, such as endoscopic resection and laparoscopic gastrectomy for early gastric cancer (EGC) were introduced in the 1990s. These procedures, which are currently standard for EGC, have led to an era of tailored therapy for gastric cancer, especially in East Asia. Furthermore, experienced surgeons are attempting to extend laparoscopic procedures to advanced gastric cancer.1,2 In addition, robotic and single port laparoscopic gastrectomies have respectively been developed to overcome the technical drawbacks and to reduce the invasiveness of laparoscopic gastrectomy.3,4
The emergence of new operative procedures has a significant impact on the professional activities of surgeons, who must acquire them as soon as possible to provide the best care to their patients and to remain competitive.5 However, new surgical procedures in their introductory period are difficult to learn. Educational opportunities and structured training programs, as in residency or fellowship, that support the acquisition of skills for a new procedure cannot be anticipated during that period. In addition, securing an adequate annual volume of experience is another difficulty in low volume hospitals. It has been suggested previously that the high incidence of bile duct injuries in the early series of laparoscopic cholecystectomy could be related to the learning curve (LC).6 Self-assessment of one's LC based on statistical analysis may therefore be essential and helpful in ensuring quality patient care. Before expanding our indications for laparoscopic gastrectomy to advanced gastric cancer and adopting reduced-port laparoscopic gastrectomy, we analyzed and audited the outcomes of laparoscopy-assisted distal gastrectomy (LADG) for adenocarcinoma; this was done during the adoptive period at our institution through the comparative analysis of outcomes and LCs between two surgeons with different careers.
Materials and Methods
This comparative, retrospective study was based on the prospectively maintained database of the first 206 consecutive patients with gastric adenocarcinoma who underwent LADG for adenocarcinoma at the Department of Surgery, Korea University Ansan Hospital between July 2006 and June 2011.
Our indication for LADG was adenocarcinoma at the lower or middle third of the stomach, staged as T1N0M0 by a preoperative workup including esophagogastroduodenoscopy and abdominal computed tomography. The indication was further expanded to include cases of T1N1M0 and T2N0M0 adenocarcinoma in the later study period.
All LADG procedures were performed by one of two dedicated gastric surgeons (surgeon A or B). Surgeon A is senior with extensive experience in open gastrectomy for adenocarcinoma but limited laparoscopic training (cholecystectomy only) during residency. However, he has performed laparoscopic appendectomies and total extraperitoneal laparoscopic herniorrhaphies since March 2007 to promote acquisition of skills. Surgeon B is junior with limited experience in open gastrectomy (about 60 procedures), but more experience than the senior surgeon in laparoscopic procedures, such as cholecystectomy, colectomy, herniorrhaphy and appendectomy during residency. Neither had experience in laparoscopic surgery for gastric cancer before they joined this hospital. Thus, it is safe to say that LADG during this time period exhibits a true LC. The surgeons learned the technical details of LADG through active participation in numerous video conferences, workshops, and wet animal laboratories held by the Laparoendoscopic Gastrointestinal Study Group of the Korean Gastric Cancer Association. They also observed a few LADGs performed by pioneering surgeons in Korea. All operations included in the study were performed in the same hospital, and the two surgeons shared the same operative apparatus and surgical residents of equal ability as assistants.
The two surgeons in this study used basically the same technique of LADG, including partial omentectomy and extracorporeal anastomosis, as previously described.7 A decision on the extent of lymphadenectomy was made independently by each surgeon on the basis of preoperative T stage and his self-estimation of technical proficiency. Except for an initial few cases with modified gastrectomy A, which included dissections of lymph node groups 1, 3, 4sb, 4d, 5, 6, and 7 according to the Japanese Classification of Gastric Carcinoma,8 both surgeons performed modified gastrectomy B during the LADG procedure; this added dissections of lymph node groups 8 and 9 to modified gastrectomy A. Surgeon B further included lymph node groups 12a and/or 11p to modified gastrectomy B after the 80th procedure, while surgeon A continued with modified gastrectomy B. The patients made the choice between conventional open gastrectomy and laparoscopic gastrectomy after they were provided with detailed information and they gave written informed consent.
The outcome variables subjected to comparative analysis included operative time, estimated blood loss, mode of anastomosis, number of lymph nodes retrieved, LADG failure, reoperation and postoperative hospital days. Estimated blood loss was based on the anesthesiologist's notes. Conversion, inadvertent injury to the major vessels or other organs around the stomach, and transfusion during LADG were defined as intraoperative complications. Conversion was defined as extension of the mini-laparotomy for various reasons, such as difficult dissection and bleeding control. Only short-term postoperative complications of grade 2 or greater as defined by Dindo et al.,9 were included in this study. Occurrence of intraoperative or postoperative complications and inadequate number (fewer than 15) of lymph nodes retrieved from the specimen were de fined as failure of LADG. To determine differences, the Pearson's chi-squared test or Fisher's exact test was used to compare numerical variables, and the independent two-sample t-test or the Kruskal-Wallis H test was used to compare quantitative variables, as appropriate. Continuous variables are presented as means±standard deviations or as medians and interquartile ranges (IQR); categorical variables are expressed as counts (percentages).
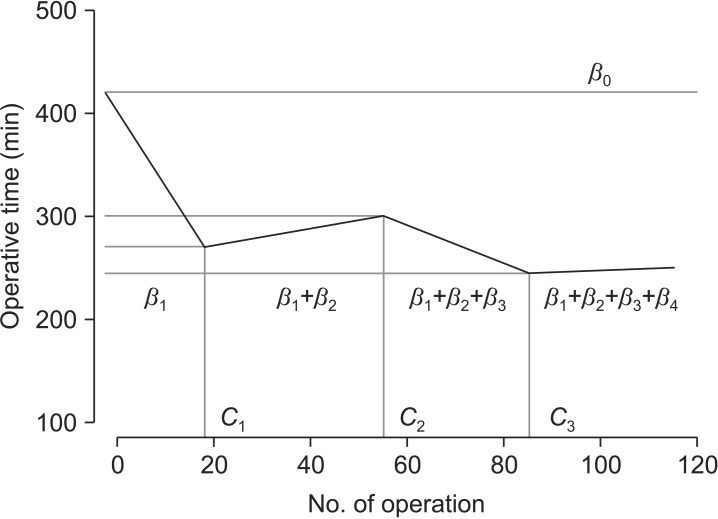
To determine the LC of the LADG, the operative time was analyzed with respect to the chronological order of each patient who had undergone LADG by their respective surgeon. Operative time was defined as the time from sub-umbilical incision for a trocar to the time of closure of all abdominal wounds. If a patient received a combined procedure during LADG, the time required for the combined procedure was subtracted from the operative time. The LCs of the surgeons were fitted using the regression analysis of the operative time. The authors here assumed that some linear changes would comprise the LCs and these would connected together, as illustrated in Fig. 1. The displayed underlying LCs inherent in the observed operative times for each surgeon were fitted by non-linear regression analysis based on the following non-linear regression model (phase-wise linear regression model):
|
For t = 1, 2, ..., T and k≥0, |
Equation (1) |
Fig. 1. Four-phase regression model of the learning curves shown in Equation (2) of Materials and Method section. C1, C2, and C3 are cut-off values dividing the learning curves into four phases; β0 denotes the intercept; β1, β2, β3, and β4 denote the partial changes of slope compared to the previous phases.
where Yt is the operation time of tth patient, I[t≥c] is an indicating function which is 1 if t≥c, and 0 elsewhere, C1, C2, ..., Ck are structural changing time points, and β0, β1, ..., βk+1 are regression parameter coefficients. This is one of the main advantages of the regression model in Equation (1) over conventional methods such as moving-average technique and cumulative sum analysis, none of which explicitly provide any statistical inferences, as, for instance, hypothesis testing for the parameters of our interest. For ease of understanding, we transformed Equation (1) into a simple form of the regression model with k=3 represented as follows:
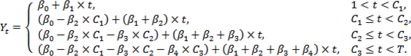 |
Equation (2) |
As shown in Equation (2), β1, ..., β4 stand for partial changes of slope compared to their previous phases and C1, C2, ..., C3 divide the LCs into 4 phases (Fig. 1). The Pearson's chi-squared test or Fisher's exact test was used to check any difference of categorical variables among the estimated phases for each surgeon. The Kruskal-Wallis H test was used to investigate any phase-wise difference of numerical variables. Finally, as some basic characteristics of each patient were quite heterogeneous, the phase-wise regression model was refitted, along with some confounding factors, such as sex, body mass index (BMI), previous abdominal surgery, extent of lymph node dissection, and mode of anastomosis, each of which was selected by means of univariate analysis. The authors believe that this phase-wise linear regression model is very helpful to estimate not only the LC effects but also the structural changing time points, which are assigned by a researcher's experience. This type of model results from the concept that a surgeon's performance will have some phases in which the operative time is assumed to change linearly.
All the statistical outcomes based on two-sided tests were obtained using SAS software version 9.2 (SAS Institute, Cary, NC, USA). P-values <0.05 were regarded as statistically significant.
Results
1. Patient demographics and tumor characteristics between the patient groups by surgeon
Surgeons A and B performed 95 and 111 LADG procedures, respectively. The details of patient demographics and tumor characteristics are summarized in Table 1. The variables of both groups were statistically comparable to each other.
Table 1. Comparative analysis of patient demographics and tumor characteristics between patient groups by surgeons.
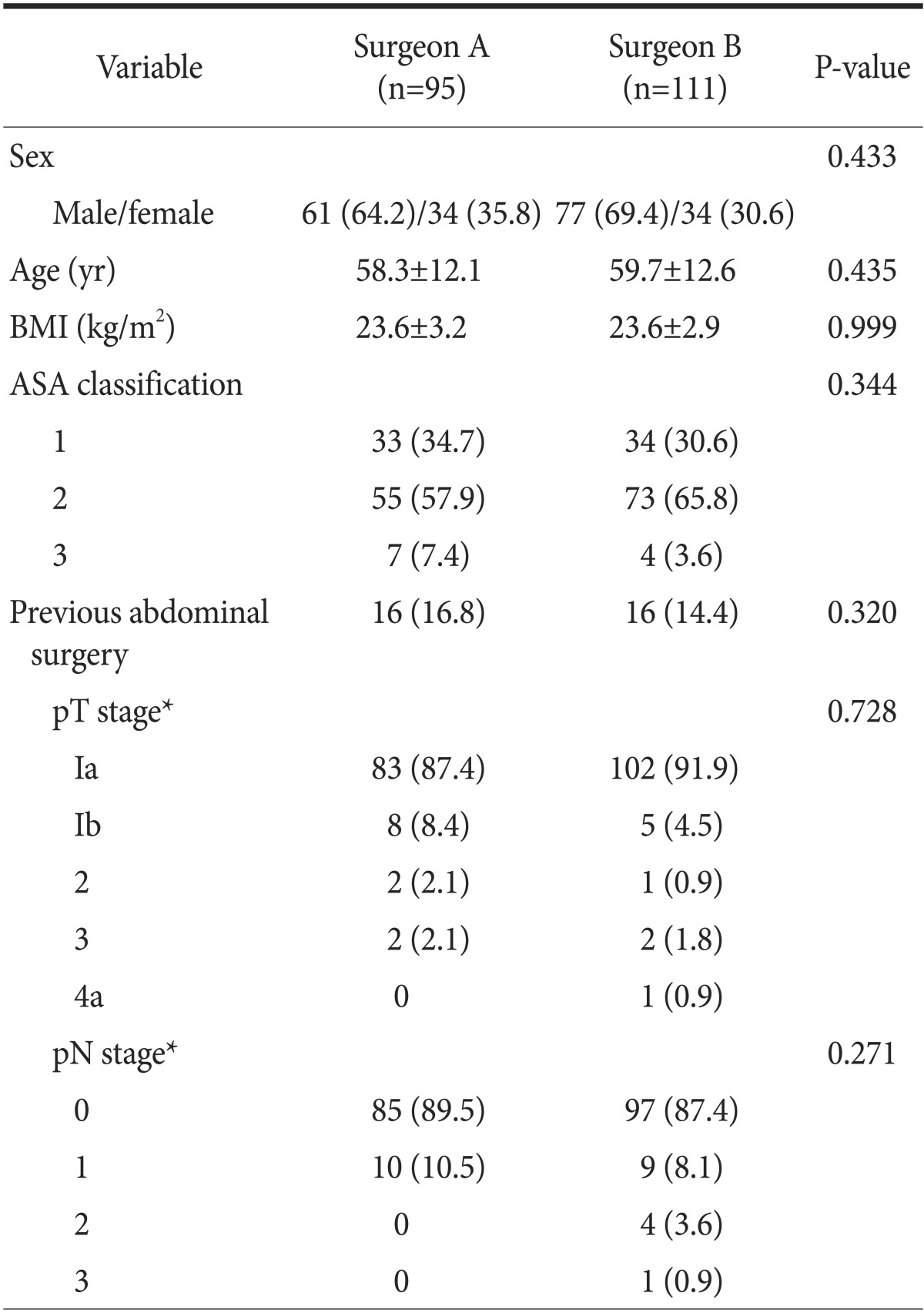
Values are presented as number (%) or mean±standard deviation. BMI = body mass index; ASA = American Society of Anesthesiologists. *The stage was based on the 7th edition of the American Joint Committee on Cancer staging system.
2. Comparative analysis of operative and outcome data by surgeon
Table 2 shows the comparative operative and surgical outcome data. The median operative time and the estimated blood loss of the surgeon A group were significantly less than those of the surgeon B group (250 minutes vs. 295 minutes, P<0.001; and 200 ml vs. 250 ml, P=0.002). Surgeon A performed stapled gastroduodenostomy (48 patients, 50.5%) more frequently than handsewn gastrojejunostomy (47 patients, 49.5%); surgeon B performed hand-sewn gastrojejunostomy with Braun anastomosis (23 patients, 20.7%) more frequently than the stapled gastroduodenostomy (88 patients, 79.3%, P<0.001). The median postoperative hospital stay of the surgeon A group was 1 day shorter than that of the surgeon B group (8 days vs. 9 days, P=0.009). The other variables including the number of lymph modes retrieved, rates of LADG failure, and reoperation were comparable between both groups.
Table 2. Comparative analysis of operative and surgical outcome data by surgeons.
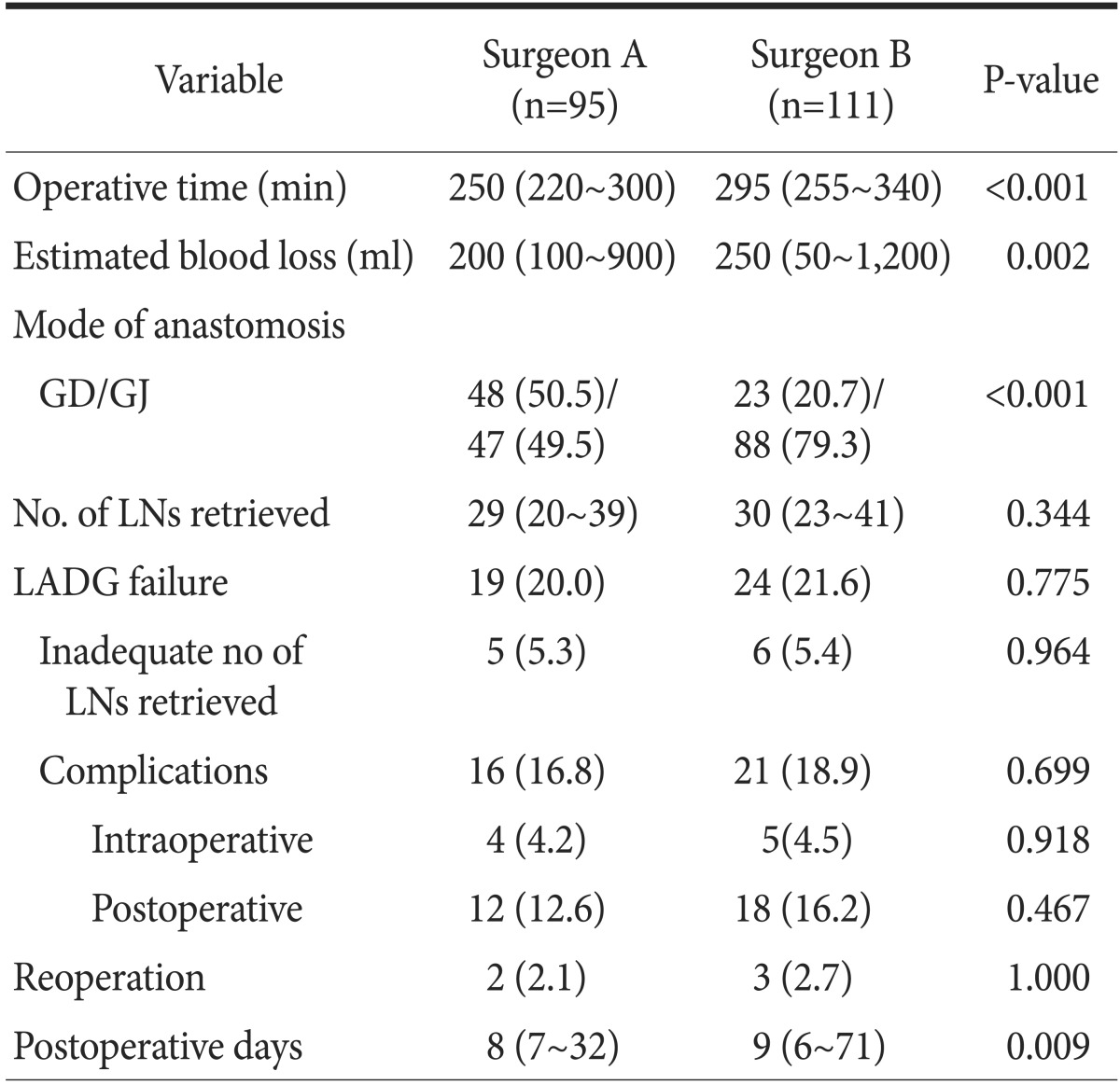
Values are presented as median (interquatile range) or number (%). GD/GJ = gastroduodenostomy/gastrojejunostomy; LN = lymph node; LADG = laparoscopy-assisted distal gastrectomy.
Of a total of 206 patients, intraoperative and postoperative complications occurred in 9 patients (4.4%) and 30 patients (14.6%), respectively. There was no postoperative death during the study period. Conversion to open gastrectomy occurred during three LADG procedures at the beginning of surgeon A's experience. The reasons for these conversions were severe adhesions due to a previous abdominal surgery, bleeding from the injured spleen, and difficulty in dissection around the duodenum in a patient with a history of duodenal ulcer. Significant bleeding requiring transfusion occurred during the procedure in 5 patients, i.e., in 2 and 3 in surgeon A and B groups, respectively. Two cases of transection of the common hepatic artery occurred during lymphadenectomy along the artery by surgeon B. These cases required no additional procedure to establish blood flow to the liver, since hepatic blood flow was from the superior mesenteric artery through the gastroduodenal artery. The common postoperative complications were intra-abdominal bleeding (5 patients, 2.4%), intraluminal bleeding (4 patients, 1.9%), duodenal stump leakage (4 patients, 1.9%), delayed gastric emptying (4 patients, 1.9%), wound infection (4 patients, 1.9%), and anastomotic stricture (3 patients, 1.5%). A complete list of complications is provided in Table 3. Five patients (2.6%) underwent reoperation due to postoperative complications. There was no difference in the postoperative complication rate (12.6% vs. 16.2%, P=0.467).
Table 3. List of complications of laparoscopy-assisted distal gastrectomy.
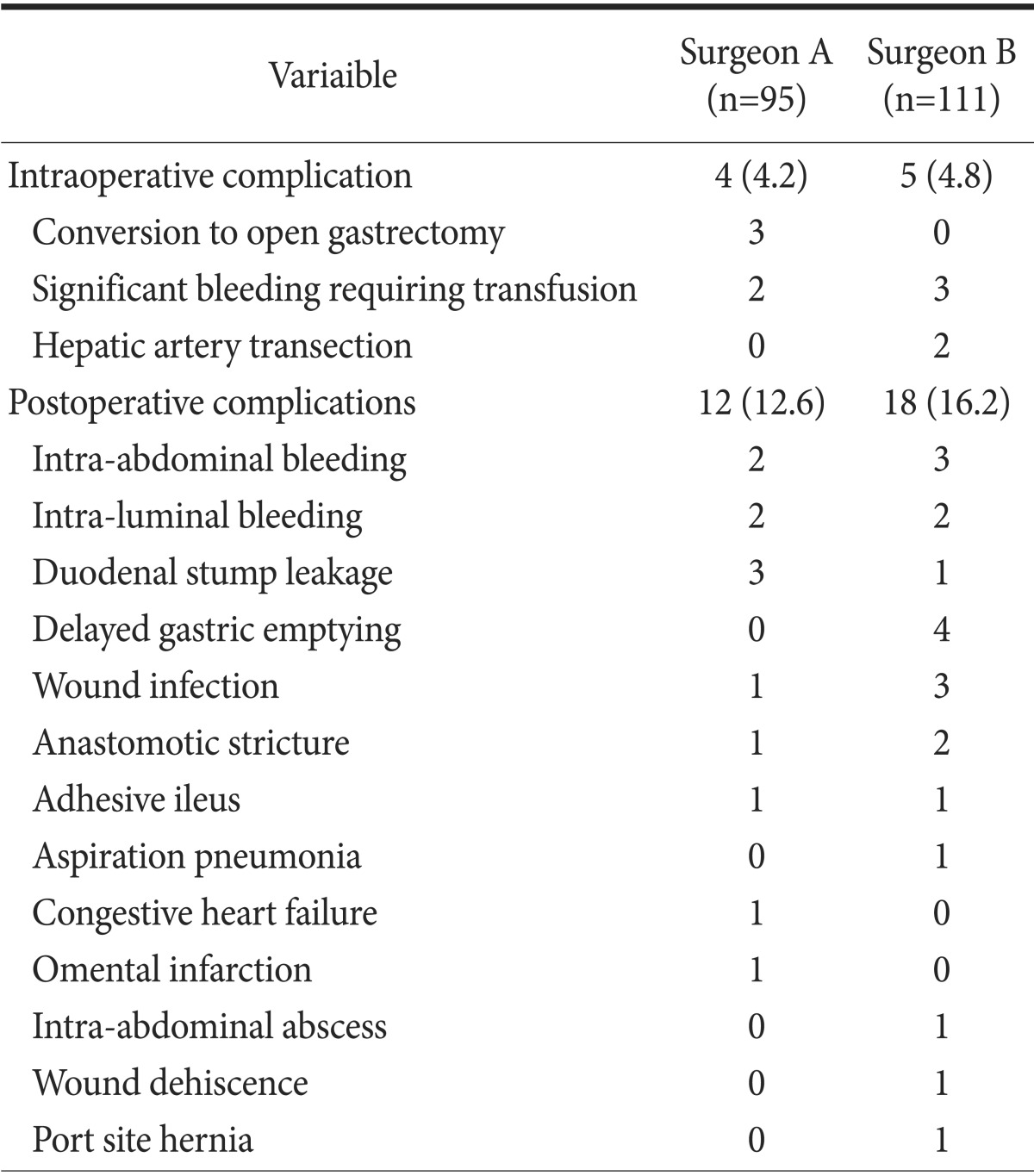
Values are presented as number (%) or number only. Total numbers of intraoperative and postoperative complications exceeded sums of the individual complications because some patients had more than one complications.
3. Comparative analyses of learning curves between surgeons
Statistical results for sequential operative times fitted by the non-linear least squares estimation method without adjusting for confounding factors are shown in Table 4. As some important demographic and operative characteristics of each group were heterogeneous, linear combinations of the confounding factors were added to adjust for these factors. Among those listed earlier, no one confounding factor had any influence on surgeon B's operative time; however, the operative time for surgeon A increased when BMI increased, and when gastrojejunostomy was done for reconstruction (Table 5).
Table 4. Results of statistical analyses of sequential operative times fitted by the non-linear least squares estimation method, based on a nonlinear regression model without adjustment for confounding factors.
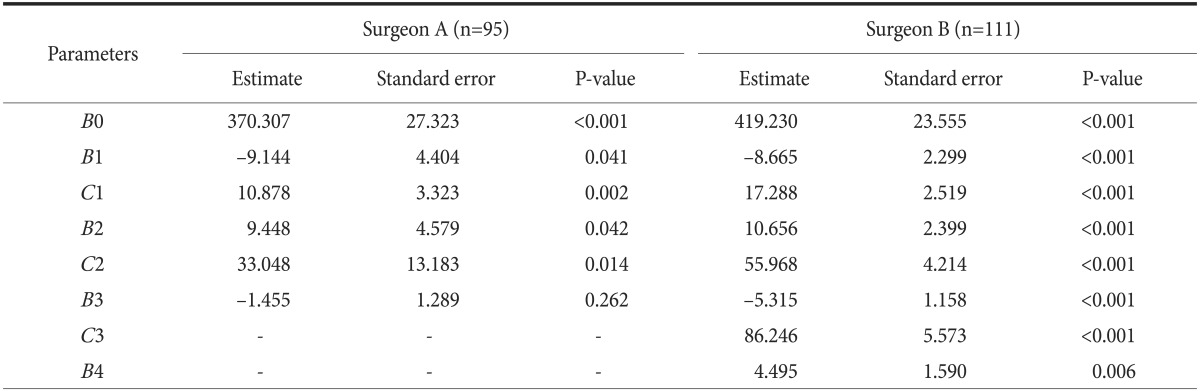
All the statistics were derived from the non-linear regression (phase-wise linear regression) analysis based on Equation (1) shown in the Materials and Methods section. B0, B1, B2, B3, and B4 stand for the regression parameter estimates; C1, C2, and C3 stand for the structural changing time points. R2: the coefficient of determination (Surgeon A's R2=0.395, Surgeon B's R2=0.402).
Table 5. Results of statistical analyses of sequential operative times fitted by the non-linear least squares estimation method based on a nonlinear regression model after adjustment for confounding factors.
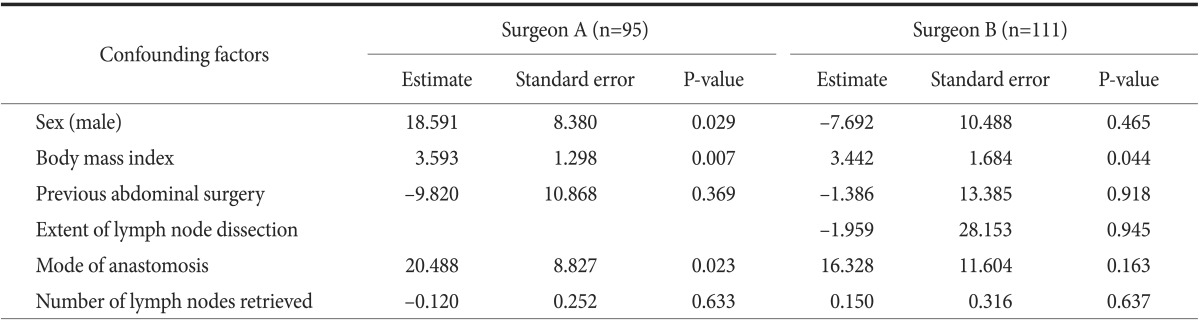
All the statistics were derived from the non-linear regression (phase-wise linear regression) analysis based on Equation (1) shown in the Materials and Methods section. B0, B1, B2, B3, and B4 stand for the regression parameter estimates; C1, C2, and C3 stand for the structural changing time points. R2: the coefficient of determination (Surgeon A's R2=0.460, Surgeon B's R2=0.506).
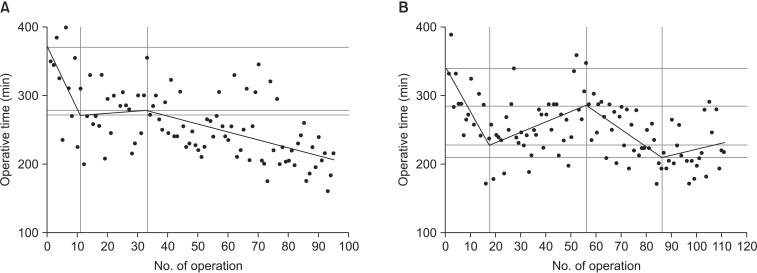
LCs constructed using the results in Table 4 are shown in Fig. 2. The LCs of both surgeons were distinct from each other. The LC of surgeon A had 3 phases while that of surgeon B had 4 phases. A decrease in the slope for LADG operative time by surgeon A was noted after the 10th procedure; after the slope of the curve plateaued with subsequent procedures, the operative time progressively decreased from the 34th procedure (Fig. 2A). For surgeon B, however, after the initial steep slope, the LC showed a seesaw pattern. Furthermore, there even appeared to be an increase in operative time after the 86th procedure (Fig. 2B). A stable LC plateau was still not achieved by either surgeon by the end of this study.
Fig. 2. The phase-wise learning-curve models fitted by non-linear least squares method. The learning curve of the surgeon A had 3 phases (A), while that of the surgeon B had 4 phases (B).
4. Comparative analysis of operative and surgical outcome data among the learning curve phases by surgeon
Table 6 summarizes comparative operative and surgical outcome data according to the LC phases for each surgeon. For surgeon A, the median operative time decreased from the first phase (median [IQR]: 270 [225~335] minutes) to the second phase (245 [200~280] minutes) and the third phase (210 [160~240] minutes) (P<0.001); the rate of LADG failure significantly decreased from the first phase (5/10, 50%) to the second phase (5/23, 21.7%) and the third phase (9/62, 14.5%) (P=0.038). For surgeon B, neither the median operative time nor the rate of LADG failure decreased with respect to phase. A progressive decrease in the estimated blood loss during LADG was found for both surgeons with respect to phase order. Of note, in both surgeons, the number of lymph nodes retrieved was not significantly different with respect to phase order.
Table 6. Comparative analysis of operative and surgical outcome data among the learning curve phases by surgeons.
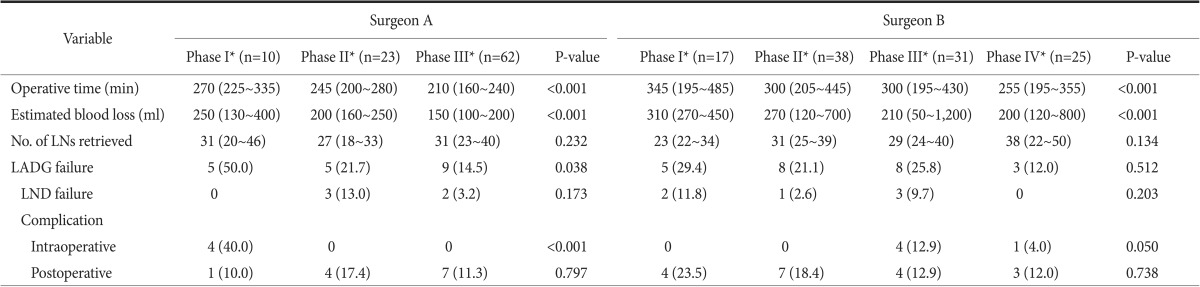
Values are presented as median (interquatile range) or number (%). LN = lymph node; LADG = laparoscopy-assisted distal gastrectomy; LND = lymph node dissection. *Each phase was identified by individual learning curve analysis.
Discussion
Many surgeons have accepted that laparoscopic gastrectomy is associated with a better postoperative course than is seen with open gastrectomy in the patients with EGC.10,11,12,13,14 A recent meta-analysis evaluated the safety and efficacy of LADG in patients with EGC compared to open distal gastrectomy; LADG is a technically feasible, acceptable alternative to EGC when performed by experienced surgeons.15 Long-term oncologic outcomes of laparoscopic gastrectomy for patients with gastric cancer were comparable to those of open gastrectomy in a recently reported multicenter, retrospective study.16
However, laparoscopic gastrectomy is technically more difficult than open gastrectomy, and surgeons are required to overcome a substantial LC. The adoption of complicated laparoscopic surgery in the treatment of cancer has been hampered by multiple factors, such as lack of training and surgical skills and long operative time.17 Surgeons operate on a patient by using inconvenient instruments in a two-dimensional view. Considering these limitations, it is important to emphasize surgical teaching programs beyond residency and fellowship.5 Early adopters, however, have no opportunity for formal training in LADG. They can only learn technical details of the procedures through video conferences, workshops, experience in wet animal laboratories, and observation of procedures performed by pioneering surgeons.
The number of procedures required to be performed by a surgeon to master LADG has been debated. The number to overcome the LC for any new surgical procedure may further vary with choice of end-point, such as short-term surgical outcome or operative time.18,19 These variations may also arise from differences in the annual volume of the surgeon, patient characteristics, the surgeon's prior experience and skill with laparoscopic procedures, and innate ability. Although many studies of the LC for laparoscopic gastrectomy have been reported, none has reported their subsequent influence on the LC. This study provided a detailed comparative analysis of LCs for LADG by two surgeons, each with different experiences in open gastrectomy and skill with laparoscopy. Although the two surgeons shared the same operative apparatus and surgical residents of equal ability as assistants, the LCs differed: one surgeon had three phases and the other had four. Previous studies have estimated the LC for laparoscopic gastrectomy to be approximately 40 to 90 procedures.16,20,21,22,23 In a report from a large volume medical center in Korea, experience with 30 LADGs (7 months) was the point at which the LC plateau was reached.24 However, this should be interpreted with caution, because it was achieved by a few experienced surgeons in a high-volume center in Korea, where a large number of gastric cancer patients are treated by a small number of experts. A stable LC plateau was still not achieved by either surgeon after 90 procedures in this study. This is probably owing to low annual LADG volume for both surgeons. With an average of less than two LADGs per month by each surgeon, it was difficult to master the LC in a short period. However, the postoperative morbidity and mortality rates in this study are comparable to those of two large-scale Korean multicenter studies (morbidity 12.5% and 14.0%, and mortality 0.5% and 0.6%, respectively).16,25
Operative time-based LC analysis may not be completely accurate and sufficient to determine proficiency in a surgical procedure.26,27 Thus, analyses of intra- and postoperative data according to LC phases were added in this study. Intraoperative and postoperative complications, estimated blood loss, number of lymph nodes retrieved and duration of postoperative hospital stay are other qualitative and technical indicators for the measurement of the LC.15,28 In this study, occurrence of intraoperative or postoperative complications and inadequate number of lymph nodes retrieved from the specimen were defined as failure of LADG. The rate of LADG failure was similar. However, for surgeon A, the LADG failure rate significantly decreased with progression from the first phase to the second and third phases; for surgeon B, the LADG failure rate did not decrease with respect to the phase order.
Seemingly, surgeon A's LC had better characteristics, and the surgeon's experience in open gastrectomy might have favorably affected the LC for LADG. However, the comparative analyses of individual LCs in this study were biased because of the differences in the procedures. First, although both surgeons used basically the same LADG technique, including partial omentectomy and extracorporeal anastomosis, all procedures were not completed with the same extent of lymphadenectomy. For surgeon B, the extent of lymphadenectomy progressed as he gained comfort with the procedure. In the later study period, while surgeon A continued with modified gastrectomy B, surgeon B included the lymph nodes along the proper hepatic artery and/or proximal splenic artery, which seemed to explain the upward LC slope in the later period. This might also cause differences in the median operative time and in the number of LC phases. Adequate lymphadenectomy for oncological safety was one of our major concerns throughout the adoptive period of LADG. There should be no compromise in the principles of oncological surgery for completing a gastrectomy laparoscopically. Martinez-Ramos et al.29 showed that pN0 misclassification was very low when 26 negative lymph nodes were examined. In this study, the mean and median numbers of lymph nodes retrieved at LADG by the surgeons were about 30, and the failure rate of lymphadenectomy was about 5%, comparable to other studies.15,20,23,30 These results imply that adequate lymphadenectomy was performed during the adoptive period. In addition to the different strategies for extension of lymphadenectomy, the analysis of individual LCs is further biased by differences in the mode of anastomosis: surgeon B performed the more time-consuming handsewn gastrojejunostomy with Braun anastomosis more frequently than surgeon A. The above biases accounted for different operative time-based LCs and surgical outcome data between surgeons.
The surgeons had different criteria for conversion. The conversion rate in our study was about 1.5%. Notably, conversion only occurred in the first phase of surgeon A. Similar to our findings, Kye et al.26 demonstrated that the conversion rate of a senior surgeon was somewhat higher than that of a junior surgeon in their study on the LC of laparoscopic right hemicolectomy conversion was regarded as an intraoperative complication in the present study. On the contrary, Belizon et al.31 suggested that it should be viewed as a wise decision when the technical limitations of a laparoscopic procedure have been exceeded; their study showed that conversions performed in the earlier part of the operation have better clinical outcomes than conversions carried out later in the procedure. Furthermore, inadvertent vessel injury during lymphadenectomy was found only in surgeon B's group; this suggests that experience in open gastrectomy would facilitate a timely decision regarding conversion, when a laparoscopic procedure has been deemed technically limited.
A limitation in this study was that the comparative analyses of individual LCs was biased because of procedural differences. In addition to the surgeons' experience with open gastrectomy and skill with laparoscopy, the mode of preferred anastomosis, strategy for extending lymphadenectomy and policy of conversion differed. These might also cause differences in the median operative time and in the LC shape and number of phases.
In conclusion, our study showed that LADG, including an adequate number of lymph node harvest, could be performed safely and with acceptable morbidity and mortality rates during the adoptive period. More procedures than those previously suggested by a few large-volume centers in Korea were required to overcome LC; however, the authors believe that the surgical outcomes of this study would be comparable. Although two surgeons at our hospital shared the same operative apparatus and assistants of equal ability, each surgeon had a different LADG LC. This might be owing to differences in the experience with open gastrectomy, skill with laparoscopy, mode of preferred anastomosis, strategy for extending lymphadenectomy and criteria for conversion. Regardless of the experience in gastrectomy or laparoscopic surgery for other organs, or the age of surgeon, the outcome is quite acceptable; the learning process varies according to each surgeon's experience and individual characteristics.
References
- 1.Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years' experience. J Surg Oncol. 2008;98:515–519. doi: 10.1002/jso.21155. [DOI] [PubMed] [Google Scholar]
- 2.Nam BH, Kim YW, Reim D, Eom BW, Yu WS, Park YK, et al. Laparoscopy assisted versus open distal gastrectomy with D2 lymph node dissection for advanced gastric cancer: design and rationale of a phase II randomized controlled multicenter trial (COACT 1001) J Gastric Cancer. 2013;13:164–171. doi: 10.5230/jgc.2013.13.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc. 2009;23:1204–1211. doi: 10.1007/s00464-009-0351-4. [DOI] [PubMed] [Google Scholar]
- 4.Park do J, Lee JH, Ahn SH, Eng AK, Kim HH. Single-port laparoscopic distal gastrectomy with D1+β lymph node dissection for gastric cancers: report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2012;22:e214–e216. doi: 10.1097/SLE.0b013e318253df9b. [DOI] [PubMed] [Google Scholar]
- 5.Sachdeva AK. Acquiring skills in new procedures and technology: the challenge and the opportunity. Arch Surg. 2005;140:387–389. doi: 10.1001/archsurg.140.4.387. [DOI] [PubMed] [Google Scholar]
- 6.MacFadyen BV, Jr, Vecchio R, Ricardo AE, Mathis CR. Bile duct injury after laparoscopic cholecystectomy. The United States experience. Surg Endosc. 1998;12:315–321. doi: 10.1007/s004649900661. [DOI] [PubMed] [Google Scholar]
- 7.Park HA, Park SH, Cho SI, Jang YJ, Kim JH, Park SS, et al. Impact of age and comorbidity on the short-term surgical outcome after laparoscopy-assisted distal gastrectomy for adenocarcinoma. Am Surg. 2013;79:40–48. [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 12.Kim MC, Kim KH, Kim HH, Jung GJ. Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol. 2005;91:90–94. doi: 10.1002/jso.20271. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi N, Yasuda K, Kitano S. Laparoscopic gastrectomy with lymph node dissection for gastric cancer. Gastric Cancer. 2006;9:167–176. doi: 10.1007/s10120-006-0380-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 15.Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39–52. doi: 10.1097/SLA.0b013e3182583e2e. [DOI] [PubMed] [Google Scholar]
- 16.Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627–633. doi: 10.1200/JCO.2013.48.8551. [DOI] [PubMed] [Google Scholar]
- 17.Mabrouk M, Frumovitz M, Greer M, Sharma S, Schmeler KM, Soliman PT, et al. Trends in laparoscopic and robotic surgery among gynecologic oncologists: a survey update. Gynecol Oncol. 2009;112:501–505. doi: 10.1016/j.ygyno.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinçler S, Koller MT, Steurer J, Bachmann LM, Christen D, Buchmann P. Multidimensional analysis of learning curves in laparoscopic sigmoid resection: eight-year results. Dis Colon Rectum. 2003;46:1371–1378. doi: 10.1007/s10350-004-6752-5. [DOI] [PubMed] [Google Scholar]
- 19.Forbes TL. A cumulative analysis of an individual surgeon's early experience with elective open abdominal aortic aneurysm repair. Am J Surg. 2005;189:469–473. doi: 10.1016/j.amjsurg.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007;21:28–33. doi: 10.1007/s00464-005-0634-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc. 2009;23:1259–1264. doi: 10.1007/s00464-008-0142-3. [DOI] [PubMed] [Google Scholar]
- 22.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunisaki C, Makino H, Yamamoto N, Sato T, Oshima T, Nagano Y, et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2008;18:236–241. doi: 10.1097/SLE.0b013e31816aa13f. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Jung YS, Kim BS, Jeong O, Lim JT, Yook JH, et al. Learning curve of a laparoscopy assisted distal gastrectomy for a surgeon expert in performing a conventional open gastrectomy. J Korean Gastric Cancer Assoc. 2006;6:167–172. [Google Scholar]
- 25.Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale Korean multicenter study. Ann Surg Oncol. 2008;15:2692–2700. doi: 10.1245/s10434-008-0075-z. [DOI] [PubMed] [Google Scholar]
- 26.Kye BH, Kim JG, Cho HM, Kim HJ, Suh YJ, Chun CS. Learning curves in laparoscopic right-sided colon cancer surgery: a comparison of first-generation colorectal surgeon to advance laparoscopically trained surgeon. J Laparoendosc Adv Surg Tech A. 2011;21:789–796. doi: 10.1089/lap.2011.0086. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Sailhamer E, Berger DL, Rattner DW. Operative time is a poor surrogate for the learning curve in laparoscopic colorectal surgery. Surg Endosc. 2007;21:238–243. doi: 10.1007/s00464-006-0120-6. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 29.Martinez-Ramos D, Calero A, Escrig-Sos J, Mingol F, Daroca-Jose JM, Sauri M, et al. Prognosis for gastric carcinomas with an insufficient number of examined negative lymph nodes. Eur J Surg Oncol. 2014;40:358–365. doi: 10.1016/j.ejso.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, et al. Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol. 2008;15:1625–1631. doi: 10.1245/s10434-008-9845-x. [DOI] [PubMed] [Google Scholar]
- 31.Belizon A, Sardinha CT, Sher ME. Converted laparoscopic colectomy: what are the consequences? Surg Endosc. 2006;20:947–951. doi: 10.1007/s00464-005-0553-3. [DOI] [PubMed] [Google Scholar]