Abstract
Purpose
The aim of this study was to compare the short-term surgical and long-term functional outcomes of Billroth I, Billroth II, and Roux-en-Y reconstruction after laparoscopic distal gastrectomy.
Materials and Methods
We retrospectively collected data from 697 patients who underwent laparoscopic distal gastrectomy for operable gastric cancer between January 2009 and December 2012. The patients were classified into three groups according to the reconstruction methods: Billroth I, Billroth II, and Roux-en-Y. The parameters evaluated included patient and tumor characteristics, operative details, and postoperative complications classified according to the Clavien-Dindo classification. Endoscopic findings of the remnant stomach were evaluated according to the residue, gastritis, bile (RGB) classification and the Los Angeles classification 1 year postoperatively.
Results
Billroth I, Billroth II, and Roux-en-Y were performed in 165 (23.7%), 371 (53.2%), and 161 patients (23.1%), respectively. Operation time was significantly shorter (173.4±44.7 minute, P<0.001) as was time to first flatus (2.8±0.8 days, P=0.009), time to first soft diet was significantly faster (4.3±1.0 days, P<0.001), and postoperative hospital stay was significantly shorter (7.7±4.0 days, P=0.004) in Billroth I in comparison to the other methods. Postoperative complications higher than Clavien-Dindo grade III occurred in 61 patients (8.8%) with no statistically significant differences between groups (P=0.797). Endoscopic findings confirmed that gastric residue, gastritis, bile reflux, and reflux esophagitis were significantly lower in Roux-en-Y (P<0.001) patients.
Conclusions
Roux-en-Y reconstruction after laparoscopic distal gastrectomy for middle-third gastric cancer is beneficial in terms of long-term functional outcome, whereas Billroth I reconstruction for distal-third gastric cancer has a superior short-term surgical outcome and postoperative weight change.
Keywords: Gastrectomy, Surgical anastomosis, Laparoscopy, Stomach neoplasms
Introduction
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death.1 In South Korea, gastric cancer is the most prevalent cancer and radical gastrectomy is the treatment of choice.2,3
Since laparoscopic distal gastrectomy (LDG) was first devised by Kitano et al.4 in 1994, the number of laparoscopic surgeries has rapidly increased for the treatment of gastric cancer.5 Several studies have supported the oncologic safety and surgical efficacy profile of laparoscopic surgery for gastric cancer. In comparison to open gastrectomy, laparoscopic gastrectomy generally has several important advantages, such as less intraoperative blood loss and postoperative pain, smaller surgical openings, faster postoperative recovery, and shorter hospital stay.6,7,8,9
After LDG, three reconstruction methods are commonly used Billroth I (B-I), Billroth II (B-II), and Roux-en-Y (RY).10 In Japan, B-II reconstruction is rarely applied due to the relative likelihood of bile reflux which can potentially result in inflammation and increased risk of gastric cancer of the remnant stomach.11,12 On the contrary, in Korea, B-II reconstruction remains the dominant method for patients with obesity or those with more advanced tumors.13 Postoperative complications such as delayed gastric empting, enterogastric reflux, and reflux esophagitis are important factors to be considered in choosing the reconstruction procedure since these factors are strongly correlated with the quality of life of patients after surgery.14,15 However, consensus on the definitive reconstruction method after LDG has not been established at present.
The aim of this study was to compare and distinctively analyze the short-term surgical and long-term functional outcomes of B-I, B-II, and RY reconstruction after LDG.
Materials and Methods
We retrospectively collected data from two hospitals of the same ins titution, the Incheon St. Mary's Hospital and the Seoul St. Mary's Hospital, of the Catholic University of Korea. A total of 2,476 patients were diagnosed preoperatively with gastric cancer between January 2009 and December 2012. Among these patients, 825 underwent LDG in either laparoscopy-assisted distal gastrectomy (610 cases) or totally LDG (215 cases). Ninety-eight patients were excluded because of incomplete medical records, 25 patients because of open conversion, and 9 patients because of death within one year from other than gastric cancer or surgical causes. Overall, a total of 697 patients who underwent LDG for operable gastric cancer were enrolled in the present study. Four surgeons from the two hospitals performed the surgery and a R0 resection was achieved in all patients. The patients were classified into three groups according to the reconstruction methods used: B-I, B-II and RY.
This study was approved by the Catholic University Institute's review board (XC15RIMI0016O).
1. Perioperative data
The evaluated parameters included patient demographics, comorbidity, operative details, postoperative complications, time to first flatus, time to first soft diet, length of hospital stay, and endoscopic findings, nutritional parameters such as serum albumin level, serum hemoglobin level, and body weight. Tumor depth, nodal status, and stage were classified according to the 7th American Joint Committee on Cancer Staging System.16 Lymph node dissection was performed according to the Guidelines of the Japanese Gastric Cancer Association.17
2. Surgical procedures
A laparoscopy-assisted distal gastrectomy anda totally LDG were performed in the same manner as previously described.18,19 The choice of the reconstruction method was based on the tumor location or surgeon's preference.
3. Classification of postoperative complications
Postoperative complications were defined as any deviation from the normal postoperative course. These were graded according to the Clavien-Dindo classification20 and all complications higher than grade III were collected and analyzed.
4. Nutritional status
Body weight, albumin levels, and serum hemoglobin were collected retrospectively to investigate the changes in preoperative nutritional status and at 1 year postoperatively.
5. Classification of endoscopic findings
Postoperative follow-up endoscopic findings were classified according to the residue, gastritis, bile (RGB) classification21 and the Los Angeles classification.22
6. Short-term surgical and long-term functional outcome
The short-term surgical outcome was measured using perioperative surgical parameters such as operation time, blood loss, extent of lymph node dissection, number of harvested lymph nodes, time to first flatus, time to first soft diet, postoperative hospital stay, and postoperative complication rates. The long-term functional outcome was measured evaluating the changes in the nutritional parameters during the 1 year after surgery and the postoperative follow-up endoscopic findings at 1 year after surgery.
7. Statistical analysis
All variables are expressed as the mean and the standard deviation. One-way analysis of variance (ANOVA) was used to evaluate continuous variables among the three groups and chi-square or Fisher exact test was used to evaluate categorical variables. The changes in postoperative nutritional parameters during the 1 year postoperatively, such as body weight, serum albumin level, and serum hemoglobin level were compared using one-way ANOVA among the three reconstruction methods. Statistical analysis was performed using the PASW ver. 18.0 for Windows (IBM Co., Armonk, NY, USA). A P-value of less than 0.05 was considered statistically significant.
Results
The clinicopathological characteristics of the patients in each group are shown in Table 1. B-I, B-II, and RY were performed in 165 (23.7%), 371 (53.2%), and 161 patients (23.1%), respectively. Gender, body mass index, and stage of gastric cancer were similar across all groups, although, there were significant differences in age, comorbidity, previous operation history, and tumor location among the groups. RY reconstruction was performed more frequently in younger patients (55.9±10.9, P<0.001). The tumors were located significantly more at the distal-third of stomach in the B-I group (P<0.001). The short-term surgical outcomes are listed in Table 2. Operation time was significantly shorter in B-I (173.4±44.7 minute, P<0.001) and the amount of blood loss was significantly lower in RY (87.1±65.9 ml, P<0.001) than in the other procedures. Time to first flatus (2.8±0.8 days, P=0.009), time to first soft diet (4.3±1.0 days, P<0.001), and postoperative hospital stay (7.7±4.0 days, P=0.004) were shorter in the B-I group.
Table 1. Clinicopathologic characteristics of patients (n=697).
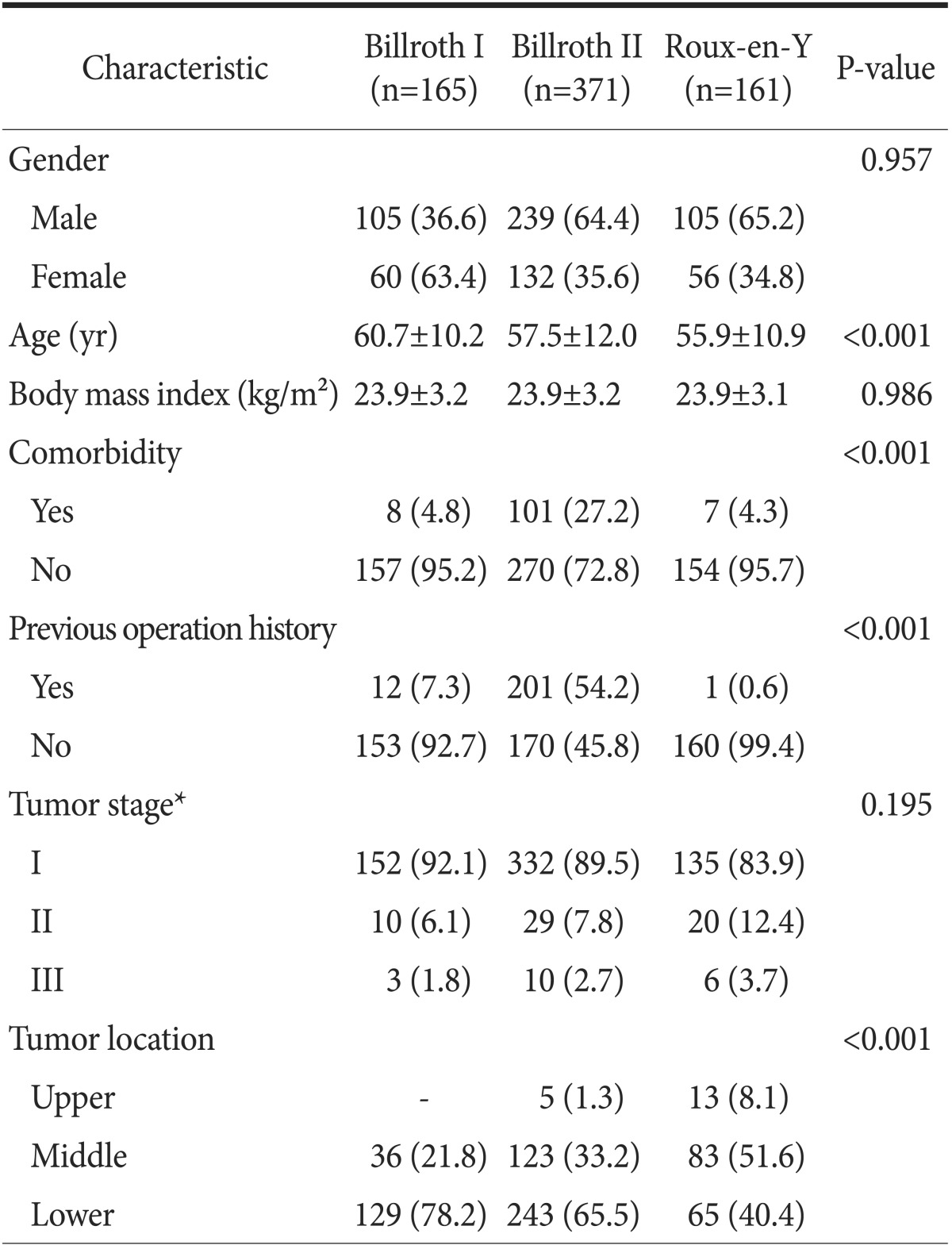
Values are presented as number (%) or mean±standard deviation. *Pathological stage according to the seventh edition of the American Joint Committee on Cancer Staging System.
Table 2. Comparisons of perioperative short-term surgical outcomes.
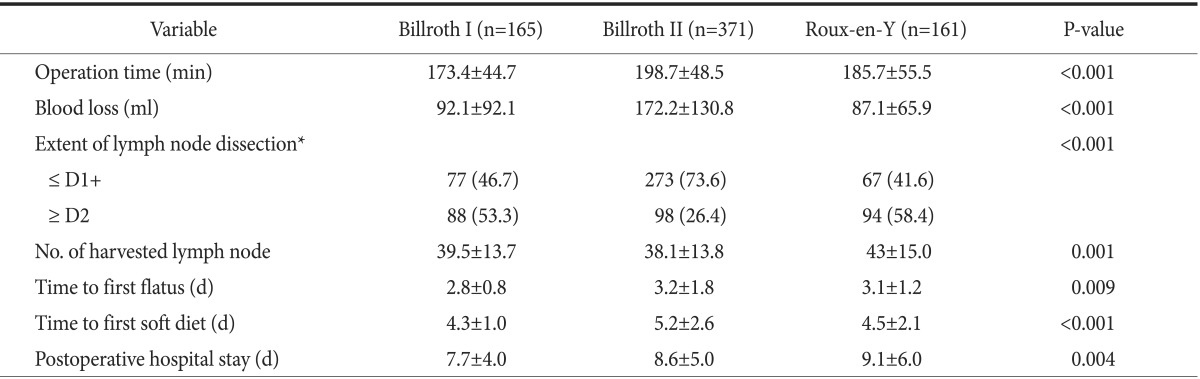
Values are presented as mean±standard deviation or number (%). *Extent of lymph node dissection following the Japanese Gastric Cancer Treatment Guidelines 2010.
Postoperative complications are shown in Table 3. Postoperative complications higher than Clavien-Dindo grade III occurred in 61 patients (8.8%). Reoperation was required in 15 patients (2.2%). Although the postoperative complication rate was not different among groups (P=0.797), the nature of the complications was slightly different. Duodenal stump leakage occurred more frequently in the B-II group (8 cases, 2.2%), and internal hernia occurred more frequently in RY patients (4 cases, 2.5%). However, there were no statistically significant differences in the numbers of such complications among groups.
Table 3. Postoperative complications higher than grade III in the Clavien-Dindo grading system.
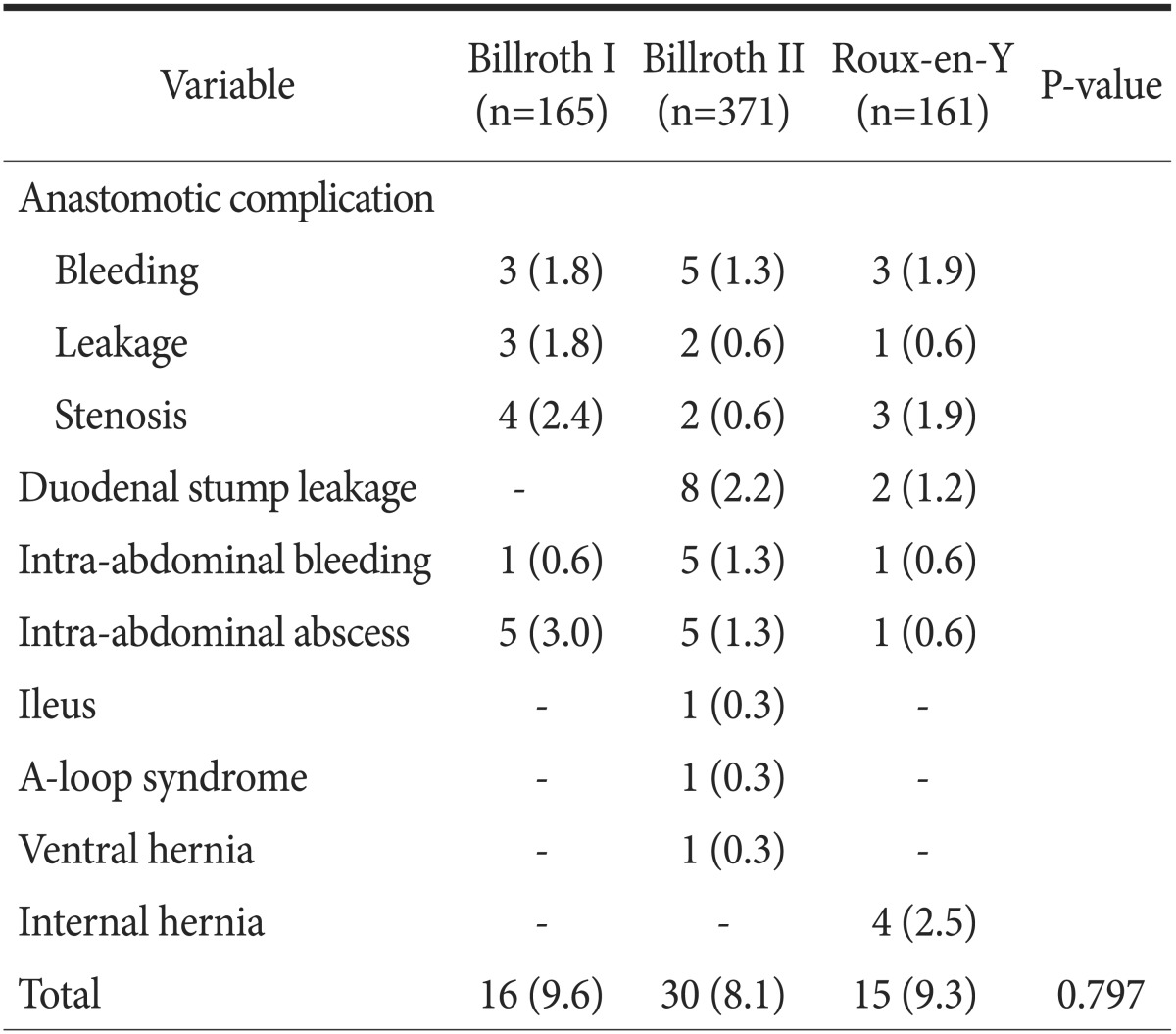
Values are presented as number (%).
Four cases of internal hernia, 3 cases of duodenal stump leakage; 3 cases of anastomosis site leakage; and 1 case each of duodenal stump leakage, anastomosis site bleeding, adhesive ileus, Aloop syndrome, and ventral hernia were treated by operation; and all other cases with complications recovered well with conservative management.
The postoperative body weight change was significantly different between groups (P=0.021), but there were no differences in postoperative albumin or hemoglobin change (Table 4). Postoperative endoscopic findings on follow-up showed that the amount of food residue (P<0.001), the degree of remnant gastritis (P<0.001), bile reflux into the remnant stomach (P<0.001), and reflux esophagitis (P=0.009) were significantly less in the RY group (Table 5).
Table 4. Nutritional parameters after surgery.
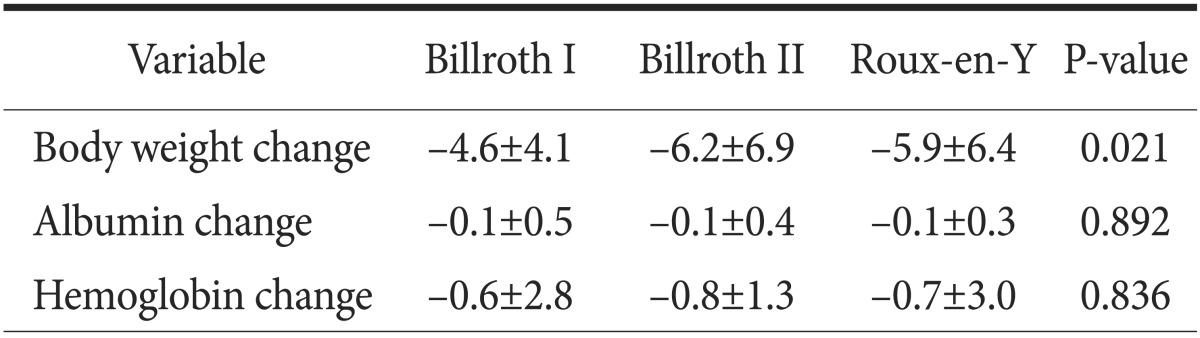
Values are presented as mean±standard deviation.
Table 5. Classification of endoscopic findings according to the RGB and LA classification at 1 year postoperatively year.
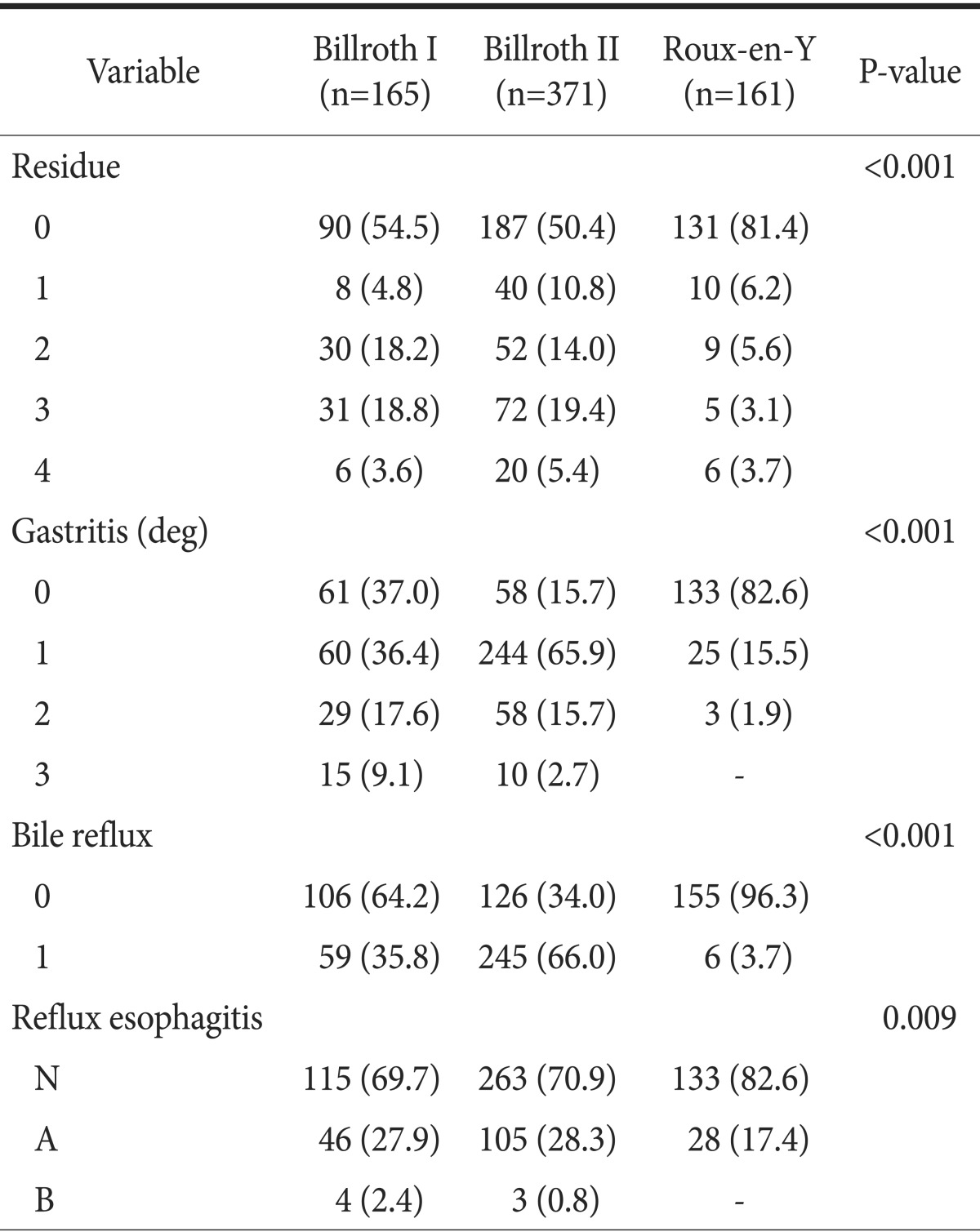
Values are presented as number (%). RGB = residue, gastritis, bile classification; LA = Los Angeles classification system.
Discussion
In this study, we retrospectively compared the outcomes of 3 reconstruction methods in 697 patients who underwent LDG. To the best of authors' knowledge, this is the largest series evaluated for this type of study. In particular, we focused on the comparison of the long-term functional outcomes of each reconstruction method. Long-term functional outcomes have an impact on patient quality of life, as overall survival of gastric cancer patients is prolonged due to increased early detection, especially in Korea.
Inokuchi et al.23 reported that RY results in less food residue (P=0.027), less gastritis (P<0.001), less bile reflux (P<0.001) and esophagitis (P<0.001) in follow-up endoscopic findings, compared with the B-I group. However, relative body weight (P=0.50), relative values of serum albumin, and total cholesterol (P=0.56 and P=0.34, respectively) were similar among groups. Lee et al.24 reported significant differences in bile reflux among groups. RY had less bile reflux than B-II (P<0.003). However, reflux esophagitis was not significantly different among groups at 12 months follow-up (P=0.211). A meta-analysis comparing B-I, B-II, and RY demonstrated that RY was definitely effective in the prevention of gastroesophageal reflux and enterogastric reflux as compared with B-I or B-II.15 In our study, RY showed a lower degree of gastritis (P<0.001), less bile reflux (P<0.001) and esophagitis (P<0.001) than either the B-I or B-II group in follow-up endoscopy findings at 1 year postoperatively (Table 5); RY was the preferred reconstruction method in younger patients over B-I or B-II (Table 1). These results support the findings of other studies indicating that RY is superior to B-I or B-II in the prevention of enterogastric reflux and gastroesophageal reflux, and thus may present more advantages in terms of prevention of remnant gastric carcinogenesis, as well as improvements in quality of life, especially in young-aged gastric cancer patients. Nevertheless, the amount of food residue in RY was significantly less than that in B-I or B-II in our study. This finding differs from those of other reports showing that the greatest amount of residual food occurs in RY.10,15 This may be because RY in our series contained numerous uncut RY cases (82.5%), which is known to be effective in the prevention of delayed gastric empting.25,26 Regarding postoperative nutritional parameters, several studies have reported no differences in nutritional parameters among different reconstruction methods.27,28 On the contrary, we observed some differences in body weight change in our study. There was less body weight loss in B-I than in B-II (P=0.021)
In terms of short-term surgical outcomes, B-I showed superior results compared with either B-II or RY in our study. Bowel function recovery was faster and postoperative hospital stay was shorter in B-I as shown in Table 2. We believe that this may be due to the shorter operation time necessary for B-I reconstruction. Several recent studies have reported similar results to ours in terms of short-term surgical outcomes including shorter operation time, shorter time to first flatus and to first soft diet in B-I, compared with the other reconstruction techniques.27,29 In our study, B-I was preferred when the tumor was located at the distal-third of the stomach and in older patients (Table 1). Overall, B-I can guarantee better short-term surgical outcomes than the other reconstruction methods when the tumor is located at distal-third of the stomach in older patients. However, we must be very cautious in drawing such conclusions because of the non-homogeneity of our patient population. There were more patients with a history of previous operation and with comorbidities in the B-II group than in either the B-I or RY groups (Table 1). The longest operation time and the largest amount of estimated blood loss in B-II treated cases may be due to this inhomogeneity among the patient population within groups. We feel that this is the main limitation of this study, which originates from its retrospective nature, despite its large scale. However, there were no differences in the clinicopathological characteristics between the B-I and RY groups, so we can cautiously recommend the B-I technique when the tumor is located at the distal-third of stomach. Future large-scale prospective randomized trials are needed in order to draw a firm conclusion.
Several studies have shown different postoperative complications according to the reconstruction methods used. Anastomotic stenosis and bleeding have been reported to be more frequent in B-I, duodenal stump leakage more frequent in B-II, and internal hernia more frequent in RY.13,29,30 In the present study, postoperative complications were classified according to the Clavien-Dindo classification and those complications higher than grade III were collected. The overall complication rate was not statistically different among the groups. An anastomotic complication was more frequent in B-I (6%), duodenal stump leakage was more frequent in B-II (2.2%), and internal hernia was more common in the RY group (2.5%), as in other studies, but the differences were not statistically significant. Reducing the tension of the anastomosis site and suturing the anastomotic stoma are recommended to decrease anastomotic complications in B-I;31 Braun anastomosis or reinforcement of the staple line is recommended to decreased duodenal stump leakage in B-II;32 and repair of mesenteric and Petersen's defect is recommended to decrease internal hernia in RY.33
As discussed above, RY showed excellent long-term functional outcomes compared with B-I or B-II in our study. However, our measurement of the long-term functional outcome was performed only using endoscopic findings that were taken 1 year postoperatively. We did not investigate any postgastrectomy symptoms such as delayed gastric empting, and dumping syndrome or postgastrectomy parameters such as volume of ingested food, appetite or performance status. A more comprehensive understanding of the long-term functional outcomes of the 3 reconstruction methods after LDG will be obtained after performing these various measurements.
In conclusion, RY reconstruction after LDG for middle-third gastric cancer, especially in younger patients, is more beneficial with regards to long-term functional outcome, whereas B-I reconstruction for distal-third gastric cancer is more beneficial for short-term surgical outcome and postoperative weight change.
Acknowledgments
This study was presented at the Korea International Gastric Cancer Week 2014 65th Annual Congress of the Korean Surgical Society, held in Daejeon, Korea.
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46:109–123. doi: 10.4143/crt.2014.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JP. Current status of surgical treatment of gastric cancer. J Surg Oncol. 2002;79:79–80. doi: 10.1002/jso.10050. [DOI] [PubMed] [Google Scholar]
- 4.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 5.Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69–77. doi: 10.5230/jgc.2011.11.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 7.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 9.Uyama I, Suda K, Satoh S. Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer. 2013;13:19–25. doi: 10.5230/jgc.2013.13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirao M, Takiguchi S, Imamura H, Yamamoto K, Kurokawa Y, Fujita J, et al. Osaka University Clinical Research Group for Gastroenterological Study. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591–1597. doi: 10.1245/s10434-012-2704-9. [DOI] [PubMed] [Google Scholar]
- 11.Katai H, Nunobe S, Saka M, Fukagawa T, Sano T. Reconstruction after distal gastrectomy. Nihon Geka Gakkai Zasshi. 2008;109:264–268. [PubMed] [Google Scholar]
- 12.Kobori O, Shimizu T, Maeda M, Atomi Y, Watanabe J, Shoji M, et al. Enhancing effect of bile and bile acid on stomach tumorigenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. J Natl Cancer Inst. 1984;73:853–861. [PubMed] [Google Scholar]
- 13.Kang KC, Cho GS, Han SU, Kim W, Kim HH, Kim MC, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Comparison of Billroth I and Billroth II reconstructions after laparoscopy-assisted distal gastrectomy: a retrospective analysis of large-scale multicenter results from Korea. Surg Endosc. 2011;25:1953–1961. doi: 10.1007/s00464-010-1493-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol. 2008;15:2692–2700. doi: 10.1245/s10434-008-0075-z. [DOI] [PubMed] [Google Scholar]
- 15.Zong L, Chen P. Billroth I vs. Billroth II vs. Roux-en-Y following distal gastrectomy: a meta-analysis based on 15 studies. Hepatogastroenterology. 2011;58:1413–1424. doi: 10.5754/hge10567. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A 3th, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 17.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Song KY, Chin HM, Kim W, Jeon HM, Park CH, et al. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. 2008;22:436–442. doi: 10.1007/s00464-007-9446-y. [DOI] [PubMed] [Google Scholar]
- 19.Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Kim JG, et al. Long-term outcomes and survival after laparoscopy-assisted distal gastrectomy for gastric cancer: three-year survival analysis of a single-center experience in Korea. J Surg Oncol. 2011;104:511–515. doi: 10.1002/jso.21982. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. 2002;5:83–89. doi: 10.1007/s101200200014. [DOI] [PubMed] [Google Scholar]
- 22.Miwa H, Yokoyama T, Hori K, Sakagami T, Oshima T, Tomita T, et al. Interobserver agreement in endoscopic evaluation of reflux esophagitis using a modified Los Angeles classification incorporating grades N and M: a validation study in a cohort of Japanese endoscopists. Dis Esophagus. 2008;21:355–363. doi: 10.1111/j.1442-2050.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 23.Inokuchi M, Kojima K, Yamada H, Kato K, Hayashi M, Motoyama K, et al. Long-term outcomes of Roux-en-Y and Billroth-I reconstruction after laparoscopic distal gastrectomy. Gastric Cancer. 2013;16:67–73. doi: 10.1007/s10120-012-0154-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee MS, Ahn SH, Lee JH, Park do J, Lee HJ, Kim HH, et al. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539–1547. doi: 10.1007/s00464-011-2064-8. [DOI] [PubMed] [Google Scholar]
- 25.Mathias JR, Fernandez A, Sninsky CA, Clench MH, Davis RH. Nausea, vomiting, and abdominal pain after Roux-en-Y anastomosis: motility of the jejunal limb. Gastroenterology. 1985;88:101–107. doi: 10.1016/s0016-5085(85)80140-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009;39:647–651. doi: 10.1007/s00595-009-3964-2. [DOI] [PubMed] [Google Scholar]
- 27.Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. A comparison of Roux-en-Y and Billroth-I reconstruction after laparoscopy-assisted distal gastrectomy. Ann Surg. 2008;247:962–967. doi: 10.1097/SLA.0b013e31816d9526. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa M, Kitayama J, Kaizaki S, Nakayama H, Ishigami H, Fujii S, et al. Prospective randomized trial comparing Billroth I and Roux-en-Y procedures after distal gastrectomy for gastric carcinoma. World J Surg. 2005;29:1415–1420. doi: 10.1007/s00268-005-7830-0. discussion 1421. [DOI] [PubMed] [Google Scholar]
- 29.Xiong JJ, Altaf K, Javed MA, Nunes QM, Huang W, Mai G, et al. Roux-en-Y versus BillrothIreconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19:1124–1134. doi: 10.3748/wjg.v19.i7.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumagai K, Hiki N, Nunobe S, Jiang X, Kubota T, Aikou S, et al. Different features of complications with Billroth-I and Roux-en-Y reconstruction after laparoscopy-assisted distal gastrectomy. J Gastrointest Surg. 2011;15:2145–2152. doi: 10.1007/s11605-011-1683-7. [DOI] [PubMed] [Google Scholar]
- 31.Bo T, Zhihong P, Peiwu Y, Feng Q, Ziqiang W, Yan S, et al. General complications following laparoscopic-assisted gastrectomy and analysis of techniques to manage them. Surg Endosc. 2009;23:1860–1865. doi: 10.1007/s00464-008-0312-3. [DOI] [PubMed] [Google Scholar]
- 32.Pugliese R, Maggioni D, Sansonna F, Scandroglio I, Ferrari GC, Di Lernia S, et al. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc. 2007;21:21–27. doi: 10.1007/s00464-005-0409-x. [DOI] [PubMed] [Google Scholar]
- 33.Kojima K, Inokuchi M, Kato K, Motoyama K, Sugihara K. Petersen's hernia after laparoscopic distal gastrectomy with Roux-en-Y reconstruction for gastric cancer. Gastric Cancer. 2014;17:146–151. doi: 10.1007/s10120-013-0256-8. [DOI] [PubMed] [Google Scholar]


