Abstract
Background
Medication adherence is thought to be around 50% in the general and dialysis population. Reducing the pill burden (PB) reduces regime complexity and can improve adherence. Increased adherence should lead to improvement in treatment outcomes and patient quality of life. There is currently little published data on PB in CKD-5D across dialysis modalities.
Methods
This is a retrospective, single renal network study. All in-centre HD (MHD), peritoneal dialysis (PD) and home HD (HHD) patients were identified in the Greater Manchester East sector renal network. Information collected included age, sex, comorbidities, daily PB, dialysis vintage and adequacy. Data were retrieved from a customized renal database, clinic and discharge letters with cross validation from the general practitioner when needed.
Results
Two hundred and thirty-six prevalent dialysis patients were studied. HHD patients had a significantly lower PB (11 ± 7 pills/day) compared with PD and MHD (16 ± 7 pills/day). The HHD patients required fewer BP medications to meet the recommended target. HD setting was the only significant factor for reducing PB. For home therapies (HHD versus PD), weekly Kt/v and serum phosphate were significant factors influencing PB. When comparing all modalities, OR of PB ≥ 15/day for MHD versus HHD was 3.9 and PD versus HHD was 4.9. The influence of HHD is dominant above factors such as comorbidities or clinical variables in reducing PB for MHD. Higher clearances achieved by HHD could explain differences in PB with PD.
Conclusion
This is the first comparative study of PB across all dialysis modalities and factors that influence it. The PB advantage in HHD may result in greater adherence and might contribute to the outcome benefit often seen with this modality. Higher clearances achieved by HHD could explain differences in PB with PD but the precise reasons for lower PB remain speculative and deserve further research in larger settings.
Keywords: comparison, dialysis, home dialysis, pill burden
Introduction
Dialysis patients are likely to have a high pill burden (PB) due to complex chronic illness often associated with multiple comorbidities. One study found MHD patients had a median PB of 19 pills/day [1]. Medication adherence in the general population is typically around 50% [2] with similar reports in the haemodialysis population [3, 4]. Complex medication regimes with high PB have been shown to impair adherence in chronic conditions [2, 3, 5, 6]. Increasing adherence to medication regimens is important to achieve treatment outcomes [7]. Poor adherence has been reported as a cause of increased mortality, poor quality of life and increased healthcare costs [8–10]. There is currently an unmet need to identify measures to improve adherence in chronic diseases [7].
Within the CKD population, complex medication regimes have been shown to reduce adherence [11]. One study looking at patients taking phosphate binders found these contributed to 49 ± 19% of the daily PB [1]. Adherence to phosphate binders was only 38%, and patients prescribed more phosphate binders were less adherent.
A systematic review identified a number of studies looking at conversion from intermittent HD (IHD) to nocturnal HD (NHD) [12]. Within these studies there are conflicting results on the effect NHD has on the PB. In one study, where seven patients switched from IHD to NHD, there was a significant reduction in both serum phosphate and phosphate binder medication [13]. In another study, 12 NHD patients did not have a significant reduction in pre-dialysis phosphate or phosphate binder requirements [14]. The FHN trial group found a significant reduction in pre-dialysis phosphate for NHD compared with IHD but there was no available data on phosphate binders [15]. They did demonstrate a significant reduction in both systolic blood pressure (SBP) and number of prescribed blood pressure (BP) medications in the NHD population compared with conventional HD [15]. Conversion from IHD to NHD, reported by Chan et al. [16], showed significant reductions in BP-lowering medications and SBP. Rao et al. [17] showed a non-significant difference in haemoglobin (Hb) and epoetin requirements in NHD patients versus IHD. Fewer studies exist in peritoneal dialysis (PD) or across all prevalent modalities.
This study was seeking to identify if dialysis modality and setting has any impact on total daily PB in the CKD-5D population. To our knowledge this is the first study to compare PB across all dialysis modalities and factors that influence it.
Methods
This single renal network, retrospective study was conducted on prevalent centre based HD (MHD), Home HD (HHD) and PD patients. All patients were selected but were not eligible if they had been on dialysis <2 months, were a current inpatient or were undertaking HHD training. Due to no HHD patient >80 years, PD and MHD patients >80 years were excluded due to lack of a control group.
Demographic, clinical and medication information was collected from clinic and discharge letters and a customized electronic renal database (Clinical Vision 4, Clinical Computing, Bath Street Ipswich). Clinical information included comorbidities, BP, haemoglobin, serum adjusted calcium, phosphate and parathyroid hormone (PTH). The mean values of 3 months laboratory data were used. BP was measured pre-dialysis for HHD and MHD patients and random office BP for PD patients. Comorbidity score was calculated using the Liu score [18], which has been validated to correlate with mortality and hospital admissions using USRDS data.
Topical, injectable and inhaled medications were not included in PB but were recorded. Medications that were not prescribed daily were documented as a fraction. Any further clarification of medication was from the patient's general practitioner.
Patients were recruited from a single regional centre operating a network (Greater Manchester East Sector Renal Network) of centre-based dialysis units (70%) and substantial home dialysis population (30%). All in-centre patients were on thrice weekly high-flux HD (4-h session) and home patients were on high-flux HD with variable schedules, 25% conventional, 75% non-conventional regimen: 60% extended time or frequency and 15% short high frequency. The conventional regime was 4–5 h three times weekly, extended time and frequency included those dialysing >6 h four times weekly or 4–5 h four times weekly with those on short high-frequency dialysis, 3 h five to six times weekly. The medical, nursing, technical and pharmacy teams are common and shared between the modalities with standardized protocols and practice. Our protocol for secondary hyperparathyroidism includes oral alfacalcidol ± cinacalcet for all modalities.
Statistical analysis was carried out using Fisher's exact test, unpaired t test and binary logistical regression. Variables used in the regression included dialysis access, SpKt/V and standard weekly Kt/v, serum phosphate, adjusted calcium, PTH, haemoglobin, mean monthly epoetin doses, phosphate binder equivalence (PBE), dialysis vintage, dialysis modality, Liu comorbidity score, age and sex. The multivariate regression included a three-way analysis as well as a head-to-head comparison between the different modalities.
Results
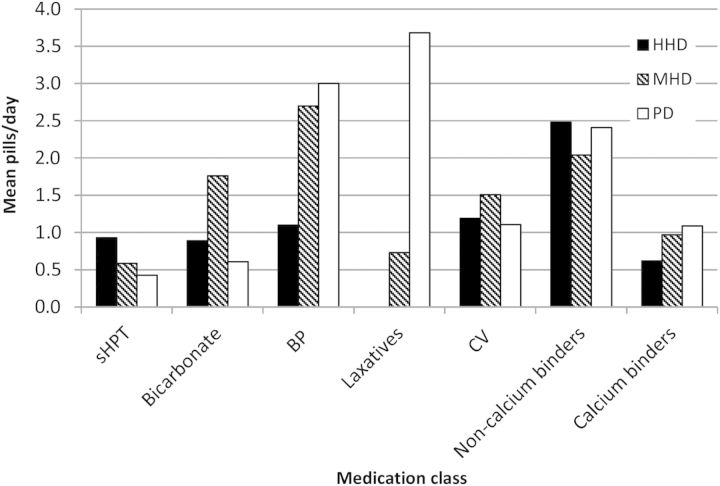
Two hundred and thirty-six dialysis patients were eligible to be studied. Demographic information is reported in Table 1. Twenty-six per cent of patients had a PB ≥ 20 pills/day and 50% had a PB of ≥15 pills/day. Mean daily PB was significantly lower (11) for HHD than MHD and PD (16) (P = 0.0001) (Table 2). Age-matched PB reduction persisted for HHD compared with both MHD and PD for age range 40–49 and 50–59 years. Other age groups (P = ns) were likely to have too few numbers of HHD patients to show significance. Figure 1 shows PB for different classes of medications across modalities.
Table 1.
Patient demographics
| HHD | MHD (P-value versus HHD) | PD (P-value versus HHD) | |
|---|---|---|---|
| N | 57 | 117 | 62 |
| Age, years | 51.1 ± 10.6 | 56.1 ± 13.7 (P = 0.016)* | 55.7 ± 16 (P = 0.0695) |
| Dialysis vintage, months | 61.3 ± 60.9 | 36.9 ± 36.1 (P = 0.001)* | 22.8 ± 20.4 (P < 0.0001)* |
| Liu score | 1.1 ± 1.4 | 2.5 ± 2.2 (P < 0.0001)* | 1.5 ± 1.7 (P = 0.1659) |
| Females % | 46 | 40 | 47 |
| Phosphate mmol/L | 1.52 ± 0.44 | 1.62 ± 0.52 (P = 0.2131) | 1.60 ± 0.43 (P = 0.3181) |
| Adjusted calcium mmol/L | 2.38 ± 0.2 | 2.48 ± 0.18 (P = 0.0011)* | 2.48 ± 0.15 (P = 0.0024)* |
| PTH pg/mL | 541 ± 368 | 420 ± 452 (P = 0.081) | 367 ± 221 (P = 0.002)* |
| Standard weekly Kt/v | 2.76 ± 0.45 | 2.31 ± 0.43 (P < 0.0001)* | 2.28 ± 0.62 (P < 0.0001)* |
PTH, parathyroid hormone; MHD, maintenance haemodialysis; HHD, home haemodialysis; PD, peritoneal dialysis.
*P = significant.
Table 2.
Mean daily pill burden for dialysis modality
| N | Mean pill burden (pills/day) | Significance versus HHD | |
|---|---|---|---|
| MHD | 117 | 16 ± 7 | P = 0.0001 |
| HHD | 57 | 11 ± 7 | |
| HHD ≥ 6 h/session | 15 | 10 ± 6 | |
| PD | 62 | 16 ± 7 | P = 0.0001 |
MHD, maintenance haemodialysis; HHD, home haemodialysis; PD, peritoneal dialysis.
Fig. 1.
Graph showing pill burden of different classes of medications across modalities. sHPT, secondary hyperparathyroidism medications including alfacalcidol and cinacalcet; BP, blood pressure medication including alpha-blockers, ACE Inhibitors, beta-blockers, calcium channel blockers and diuretics; CV, cardiovascular medication including anticoagulants, antiplatelets, antiarrythmics and cholesterol-lowering drugs. Non-calcium binders include sevelamer and lanthanum. Calcium binders include calcium acetate and calcium carbonate. HHD, home haemodialysis; MHD, maintenance haemodialysis; PD, peritoneal dialysis.
A significant reduction in antihypertensive medications was required for HHD to meet the pre-dialysis UK Renal Association (RA) SBP target of 120–160 mmHg and therefore required PD patients to meet a target of 130/80 mmHg (Table 3). The HHD population also had a higher per cent of patients meeting RA targets without the need for antihypertensives.
Table 3.
Blood pressure pill burden across dialysis modality
| Daily BP pill burden | Significance versus HHD | % meeting RA BP target | |
|---|---|---|---|
| MHD | 2.7 ± 2.6 | P = 0.0002 | 69 |
| HHD | 1.1 ± 1.5 | 76 | |
| HHD ≥ 6 h/session | 0.9 ± 0.7 | 93 | |
| PD | 3.0 ± 2.5 | P = 0.0001 | 66 |
MHD, maintenance haemodialysis; HHD, home haemodialysis; PD, peritoneal dialysis.
A trend for reduced phosphate binder requirements was seen for those patients on long hours of HHD. Sixty per cent of patients on longer hours of HHD met the RA phosphate targets of 1.1–1.7 mmol/L without any phosphate binder medication compared with 25% of all HHD patients, 28% MHD patients and 15% PD patients. The mean PB for phosphate binders was 3.1 ± 3.1, 3.1 ± 3.7 and 3.5 ± 2.5 for HHD, MHD and PD, respectively. We also looked at phosphate binder potency equivalence due to variability of phosphate binding capacity of binders [19]. However, there was still no reduction for HHD with binder potency equivalence being 2.7 ± 2.6, 2.2 ± 2.4 and 2.2 ± 1.7 for HHD, MHD and PD, respectively. For extended hours, HHD patients were on a mean 2.8 ± 3.6 phosphate binder pills with a binder equivalence of 1.9 ± 2.3.
The mean weekly Kt/V for HHD was 2.76 ± 0.45, which was significantly higher (P < 0.0001) than both MHD 2.31 ± 0.43, and PD 2.28 ± 0.62, respectively.
Epoetins were not included in PB but due to the high costs of therapy we compared requirements across modalities. Patients who dialysed for >6 h/sessions had a trend towards lower epoetin requirements. HHD for longer sessions had a mean darbopoetin® dose of 70 ± 78 mg/month. In HHD, MHD and PD, the mean monthly doses were 123 ± 126, 124 ± 127 and 109 ± 98 mg, respectively.
On multivariate logistical regression, the OR for having PB ≥ 15 pills/ day is shown in Table 4. The only significant factor that influenced PB was modality setting. When comparing PD and HHD, significant factors affecting PB were weekly Kt/v, P = 0.001 OR 0.6 (CI 0.5–0.8), and serum phosphate levels, P = 0.01 OR 0.2 (CI 0.1–0.7). When comparing HHD versus MHD the only significant factor was the modality setting, P = 0.001 OR 4.06 (CI 1.8–9.1). The Liu comorbidity score was not associated with an increase in PB.
Table 4.
Results from multivariate analysis when all dialysis modalities are compared
| OR ≥ 15 pills/day versus HHD | P-value | CI | |
|---|---|---|---|
| PD | 4.9 | <0.001 | 2.0–11.9 |
| MHD | 3.9 | 0.001 | 1.7–8.6 |
OR, odds ratio; CI, confidence interval; PD, peritoneal dialysis; MHD, maintenance haemodialysis; HHD, home haemodialysis.
Discussion
This study highlights a high total daily PB in dialysis patients with 26% of patients taking ≥20 pills/day, which is supported by other studies [1, 3]. Across all dialysis modalities, HHD patients had significantly reduced PB compared with MHD or PD. The impact of the HHD was dominant above comorbidities and other factors.
MHD patients were significantly older and had higher Liu comorbidity scores than those on HHD which is not unexpected. However age-matched PB across the modalities still showed a significant reduction of pills/day for HHD and in the multivariate regression, age did not come out as a significant factor affecting PB. Comorbidity (Liu score) was not associated with an increase in PB. For example, a patient with HIV would not score for comorbidity but may be on 4 pills/day. Further studies are required to determine links between PB and hard endpoint clinical outcomes, especially in the dialysis population.
HHD patients require significantly less antihypertensive medications. This has been described in two studies comparing NHD to IHD [15, 16]. Although the reason for this is not entirely clear, higher dialysis dose and clearance may be implicated. An increased frequency and/or duration of dialysis allows better removal of salt and solutes with a reduction in interdialytic and intradialytic volume shifts and improved water balance. Haemodialysis at home, with self-directed management of dry weight and ultrafiltration prescription, is often associated with improved salt and fluid balance; both may result in improvements in blood pressure, requiring less antihypertensive therapy.
There was a non-significant reduction in phosphate binder requirements between modalities; however, longer sessions of HHD showed a trend towards reduced requirements. Phosphate is mainly dialysed in the first 1–2 h HD with rebound after dialysis [20]. The use of more frequent and longer sessions of dialysis can improve phosphate clearance [13–15]. We did not evaluate nutritional status markers in this study. It is recognized that HHD patients tend to have an improved appetite, nutritional status [21] and thereby more liberal intake of phosphate, potentially offsetting the lower medication need. This phenomenon has been demonstrated in haemodiafiltration where despite higher phosphate clearances compared with conventional HD, phosphate binder requirements were no different 3 months post switch due to improvement in appetite and higher dietary phosphate intake. Enhanced phosphate clearances of the order seen in frequent nocturnal dialysis can override such dietary alterations to net result in hypophosphatemia and freedom from phosphate binders and some nocturnal dialysis patients in this study even required phosphate supplementation in their dialysate. Additionally there may be patients with poor nutritional status, more commonly in the conventional MHD, where phosphate binders may not be necessary.
The HHD study group has a significantly higher dialysis vintage compared with MHD and PD. Dialysis vintage is associated with increasing severity of hyperparathyroidism [22]. Mean PTH values were higher for HHD. This may lead to an increased need for phosphate binders, vitamin D and calcimimetics with a higher PB. The PB advantage seen in HHD patients despite higher vintage provides strong evidence of HHD superiority over other parallel dialysis modalities.
Small solute adequacy (Kt/v) was a significant factor in influencing PB when PD was compared with HHD. Lower weekly Kt/v for PD was associated with an increased PB. This suggests a direct relationship of uraemic burden to PB. The improved clearance of urea by HHD implies that clearance of other molecules and toxins would also be enhanced. This was supported by higher serum phosphate levels in PD patients being associated with an increase in PB.
The mean weekly Kt/v for MHD was significantly lower than HHD (P < 0.0001) but when other factors were considered it lost its significance. This suggests other advantages of HHD in addition to better clearances achieved.
Longer sessions of HHD showed a non-significant trend towards reduced epoetin requirements. The reason for this is unclear but potential enhanced removal of toxins may play a role. We were unable to collect data on iron requirements due to issues around subject recall and data validation, as many HHD patients collected their iron from their community pharmacy. Without information on IV iron use, another prominent therapy in renal anaemia management, it makes it difficult to draw conclusions as to whether other factors played a role in reduced epoetin requirements.
Metabolic acidosis is relatively common in patients undergoing HD [23]. Bicarbonate is present in dialysate but the effects may not last between sessions resulting in pre-dialysis metabolic acidosis. Because of the recognized benefits of correcting acidosis [23, 24] and recent concerns with using high bicarbonate dialysate [24] oral supplements may be prescribed. Increasing dialysis dose and frequency could reduce the metabolic acidosis and negate the use of sodium bicarbonate supplements additional to dialysis. MHD patients were taking the highest amount of supplementation which would be expected. Only the HHD patients on conventional regime (4–5 h three times weekly) were on oral bicarbonate supplements, all those on increased frequency and longer sessions took no supplementats. Other factors such as the use of diuretics and calcium-containing phosphate binders may also affect the level of metabolic acidosis but these were not considered in our analysis of bicarbonate supplementation.
The reasons for reduction in overall PB for the HHD population in this study have not been fully elucidated. Extended dialysis regimen with improved clearances, improved adherence in the context of self-management or perhaps active engagement of patients reporting non-adherence related to adverse effects or tolerability of their medications are possible factors to consider. Higher motivation, commitment and knowledge of medications are characteristics frequently seen in HHD patients and also key determinants of adherence [4, 25]. There is evidence of strong associations with fluid adherence and medication compliance [25] supporting the theory that those who are better at self-management of their condition are more likely to adhere.
The strong links of PB to adherence and outcomes in the general population suggest an important role in clinical practice. However, there are some limitations as exemplified in this study. The comparison of standard weekly Kt/v between PD and HD is difficult and may influence the results seen in this study. PD patients in the study had a high PB often related to laxative therapy, as routine practice to ensure regular bowel movements. This may account for, in part, the high PB in PD patients. Adherence was not evaluated in this study but remains a large problem in patients with chronic conditions, particularly those with a large PB. Adherence is difficult to measure conclusively and there are a variety of ways of assessing it, some of which include self-report, pill counts and pharmacy refills [26].
This study demonstrates differences in PB in dialysis clinical practice in favour of HHD. The PB advantage may have several implications in patient care contributing to improvement in medication adherence, clinical outcome, improved quality of life and overall treatment cost-effectiveness. PB studies in larger, multicentre settings to define its significance in the dialysis population merit further research.
Conflict of interest statement
We have had no involvements that might raise the question of bias in the work reported or in the conclusions, implications or opinions stated.
References
- 1.Chui YW, Teitelbaum I, Misra M, et al. Pill burden, adherence, hyperphosphataemia, and quality of life in haemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(Issue 2) doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Neri L, Martini A, Andreucci VE, et al. Regimen complexity and prescription adherence in dialysis patients. Am J Nephrol. 2011;34:71–76. doi: 10.1159/000328391. [DOI] [PubMed] [Google Scholar]
- 4.Schmid H, Hartmann B, Schiffl H. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literature. Eur J Med Res. 2009;14:185–190. doi: 10.1186/2047-783X-14-5-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31:213–224. doi: 10.1007/s10865-007-9147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–861. doi: 10.4065/80.7.856. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Health Adherence research network. www.hhs.gov. (January 2013, date last accessed)
- 8.Kaveh K, Kimmel PL. Compliance in hemodialysis patients; multidimensional measures in search of a gold standard. Am J Kidney Dis. 2001;37:244–266. doi: 10.1053/ajkd.2001.21286. [DOI] [PubMed] [Google Scholar]
- 9.Cleemput I, Kesteloot K, Vanrenterghem Y, et al. The economic implications of non-adherence after renal transplantation. Pharmacoeconomics. 2004;22:1217–1234. doi: 10.2165/00019053-200422180-00006. [DOI] [PubMed] [Google Scholar]
- 10.Esposito D, Bagchi AD, Verdier JM, et al. Medicaid beneficiaries with congestive heart failure: association of medication adherence with healthcare use and costs. Am J Manag Care. 2009;15:437–445. [PubMed] [Google Scholar]
- 11.Karamanidou C, Clatworthy J, Weinman J, et al. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol. 2008;9:2. doi: 10.1186/1471-2369-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh M, Culleton B, Tonelli M, et al. A systematic review of the effect of nocturnal haemodialysis on blood pressure, left ventricular hypertrophy, anaemia, mineral metabolism and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 13.Musci I, Hercz G, Uldall R, et al. Control of phosphate without any phosphate binders in patients treated with nocturnal haemodialysis. Kidney Int. 1998;53:1399–1404. doi: 10.1046/j.1523-1755.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay RM, Alhejaili F, Nesrallah G, et al. Calcium and phosphate balance with quotidian haemodialysis. Am J Kidney Dis. 2003;42:S24–S29. doi: 10.1016/s0272-6386(03)00534-1. [DOI] [PubMed] [Google Scholar]
- 15.Rocco MV, Lockridge RS, Beck GJ FHN trial group. The effects of frequent nocturnal home haemodialysis: the Frequent Haemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CT, Floras JS, Miller JA, et al. Regression of left ventricular hypertrophy after conversion to NHD. Kidney Int. 2002;61:2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 17.Rao M, Nuirhaed N, Klarenbach S, et al. Management of anaemia with quotidian dialysis. Am J Kidney Dis. 2003;42:18–22. doi: 10.1016/s0272-6386(03)00533-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Huang Z, Gilbertson DT, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 19.Daugirdas JT, Finn WF, Emmett M, et al. FHN trial group. The phosphate binder equivalent dose. Semin Dial. 2011;24:41–49. doi: 10.1111/j.1525-139X.2011.00849.x. [DOI] [PubMed] [Google Scholar]
- 20.Jindal K, Chan CT, Deziel C, et al. CSN haemodialysis clinical practise guideline: Chapter 3 Mineral metabolism. J Am Soc Nephrol. 2006;17:S1–S27. doi: 10.1681/ASN.2005121372. [DOI] [PubMed] [Google Scholar]
- 21.Sikkes ME, Kooistra MP, Weijs PJ. Improved nutrition after conversion to nocturnal home haemodialysis. J Ren Nutr. 2009;19:494–499. doi: 10.1053/j.jrn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Chertow GM, Plone M, Dillon MA, et al. Hyperparathyroidism and dialysis vintage. Clin Nephrol. 2000;54:295–300. [PubMed] [Google Scholar]
- 23.Soudan K, Ricanti ES, Leon JB, et al. Determinants of metabolic acidosis among hemodialysis patients. Hemodial Int. 2006;10:209–214. doi: 10.1111/j.1542-4758.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 24.Tentori F, Karaboyas A, Robinson B, et al. Association of dialysate bicarbonate concentration with mortality in the dialysis outcomes and practise patterns study (DOPPS) Am J Kidney Dis. 2013;62:738–746. doi: 10.1053/j.ajkd.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morduchowicz G, Sulkes J, Aizic S, et al. Compliance in haemodialysis patients: a multivariate regression analysis. Nephron. 1993;64:365–368. doi: 10.1159/000187355. [DOI] [PubMed] [Google Scholar]
- 26.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]