Abstract
Background
The lack of glomerulonephritis (GN) guidelines has historically contributed to substantial variability in the treatment of GN. We hypothesize that there are barriers to GN guideline implementation leading to incomplete translation of the 2012 KDIGO GN guidelines into patient care, and that current practice patterns deviate from guideline recommendations.
Methods
Adult nephrologists in Canada (N = 390) were surveyed using a web-based tool. The survey of 40 questions captured physician demographics, self-reported GN case load, treatment approaches and barriers to guideline implementation.
Results
The response rate was 44%. Physicians report seeing six (IQR 4,10) new cases of idiopathic GN every 6 months. The majority treat ANCA GN according to guidelines, but 9–37% treat nephrotic focal segmental glomerulosclerosis or membranous nephropathy with non-recommended immunosuppression and 6–9% do not treat with any immunotherapy, whereas 26% treat subnephrotic disease with immunosuppression. The majority indicated that standardized care tools would improve patient care, but they were only available to 25–44%. Patient education tools and nursing support are unavailable to 87 and 67%, respectively; insurance coverage for immune therapies is poorly accessible to 84%, yet 86% feel this would improve care and 96% of physicians support comparing their practice with benchmarks from provincial GN registries.
Conclusions
We show that 2 years after the publication of the KDIGO GN guidelines, 15–46% of Canadian nephrologists report treatment strategies not in keeping with guideline recommendations. We identify barriers to guideline implementation and widespread physician support for initiatives that address these barriers to improve patient care.
Keywords: care gap, glomerulonephritis, guideline implementation, KDIGO, knowledge translation
Introduction
There is substantial variability in the management of glomerulonephritis (GN) that may contribute to poor patient outcomes and high risk of end-stage renal disease. Paediatric patients with nephrotic syndrome are often under treated with steroids, increasing the risk of relapse or treatment resistance [1]. Over 75% of Canadians with IgA nephropathy (IgAN) in the modern era have proteinuria greater than 1 g/day, and yet only 33% get steroid treatment despite evidence suggesting this improves renal outcomes [2–4]. Furthermore, 19% of patients with membranous nephropathy (MN) and subnephrotic proteinuria are treated with immunosuppression in spite of a favourable long-term renal prognosis [5]. Of those with MN or focal segmental glomerulosclerosis (FSGS) and nephrotic-range proteinuria, 34–39% do not get immunosuppression in spite of potential benefit, and 26–33% receive non-recommended therapies [6–12]. While there are many factors that contribute to a physician's decision to treat a patient with GN, these results highlight the potential morbidity that may result from undertreatment with effective therapies, or over treatment resulting in unnecessary toxicity.
Part of this variability in treatment may be due to the historical lack of expert consensus regarding the best-practice management of glomerular diseases. The publication of the KDIGO GN clinical practice guidelines in 2012 represents a formidable achievement that addresses this deficit [13]. However, translation of guideline recommendations into clinical practice is often incomplete, resulting in underutilization of evidence-based interventions capable of improving patient outcomes [14, 15]. Patients in the USA may receive only 55% of the recommended care, resulting in a substantial difference between guideline-based recommended treatment and that received in clinical practice (which is termed a ‘care gap’) [16].
Reasons for care gaps are multifactorial and have been described in other non-GN areas of healthcare, and importantly include both physician- and health environment-related factors [17, 18]. However, knowledge translation of the KDIGO GN guidelines and barriers to guideline-based treatment in GN has not been investigated. We hypothesize that current uptake of the KDIGO GN guidelines into clinical practice is incomplete with significant variability in the treatment of GN that differs from guideline recommendations; that there are substantial barriers to guideline implementation related to the availability of local healthcare resources; and that there is support among nephrologists for initiatives capable of improving best-practice management in GN. To investigate these hypotheses, we undertook a survey of nephrologists across Canada to better characterize self-reported practice patterns and perceived barriers to guideline implementation.
Materials and methods
A cross-sectional web-accessible self-administered survey was conducted, with a sampling frame of all adult nephrologists practicing in Canada that were registered with the Canadian Society of Nephrology as of March 15th 2014 (N = 390). Distribution of the survey was through the Canadian Society of Nephrology, which is the national association for adult nephrologists in Canada. An email notification regarding the survey was distributed by the Canadian Society of Nephrologists in March 2014, with repeat email reminders in April 2014.
The survey consisted of 40 questions which are summarized in Table 1, and was designed to address the following dimensions of healthcare specific to glomerular diseases: physician characteristics, self-reported care gaps based on clinical vignettes and questions regarding patterns of practice, self-reported exposure to patients with different types of GN, access to resources that may facilitate management of patients with GN, opinions regarding potential initiatives to improve best-practice management of GN patients and support for provincial GN registries when used for different goals related to measuring the care and outcomes of GN patients. Questions were designed, so that answers were provided as either multiple choices, numerical data entry or a five-point Likert's scale, as appropriate (see Supplementary Appendix S1). The survey was developed using a focus group of research and non-academic nephrologists and statisticians with experience in survey design and methodology. A draft questionnaire was subsequently piloted on four nephrologists, modifications made and then adapted to a web-based format. All responses were anonymous, with options of ‘prefer not to answer’ available for potentially sensitive questions regarding physician demographics.
Table 1.
A summary of the questions in the web-based survey designed to address the uptake of the 2012 KDIGO GN guidelines into clinical practice.
| Survey questions | |
|---|---|
In regard to your nephrology practice:
|
What is your current availability of the following:
|
For ease of presentation the questions have been abbreviated, the full survey is available in the Supplementary Appendix S1.
Comparison between self-reported and potential GN caseload was performed in the subgroup of respondents from British Columbia in which there is a central pathology registry that captures all biopsy-proven cases of GN in the province. All nephrologists in the province are registered with the provincial renal agency (total of 65 adult nephrologists at the time of the survey). The average potential caseload was calculated as the number of total biopsy-proven GN cases divided by the number nephrologists. This comparison was not possible in other provinces due to the absence of central pathology registries.
Statistical analysis
Continuous variables were summarized as median (interquartile range, IQR) due to their skewed distribution, and categorical variables as count (frequency). For ease of presentation, some of the categorical variables were grouped according to clinical significance of the answers, or as the top two versus bottom three answers on a five-point Likert's scale. However, the complete distribution of the responses for all questions is available in the Supplementary Appendix S1. Comparisons across subgroups for statistical significance were made using the Kruskal–Wallis test for continuous variables and Fischer's exact test using Monte Carlo estimation of exact P-values for categorical variables. All tests were two sided, with P < 0.05 considered significant. All analyses were done using SAS version 9.3.
Results
Of the 390 nephrologists in Canada that were sent the survey, 175 responded. Three responses were excluded because the nephrologist only answered questions about physician characteristics. As a result, 172 responses were analysed (response rate 44%). Figure 1 describes the characteristics of the responders, including number of years in practice, working environment and province of practice.
Fig. 1.
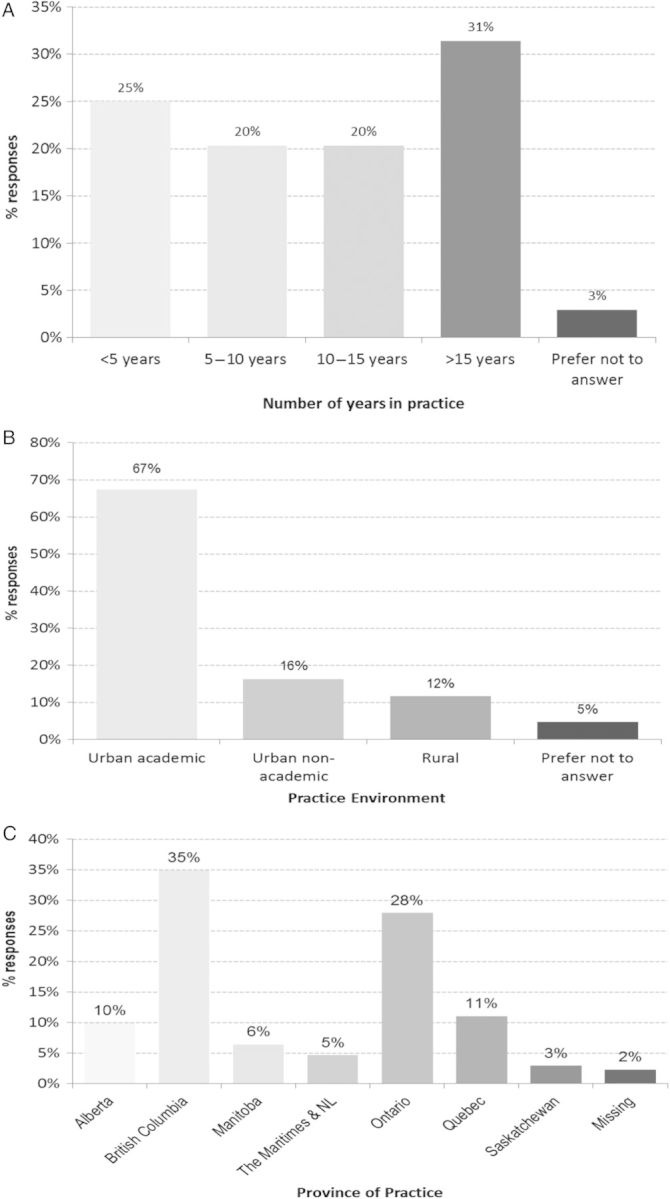
The number of years in practice, practice environment and province of the nephrologists who responded to the survey.
Self-reported GN caseload
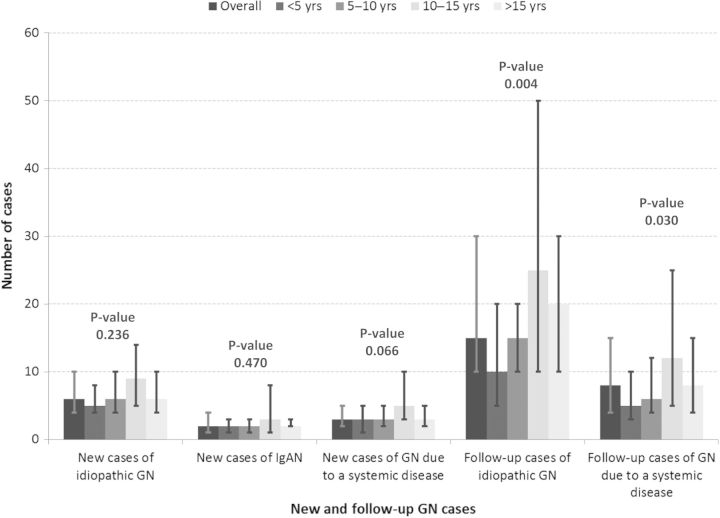
The median number of new patients with idiopathic GN with primary renal involvement (e.g. FSGS, MN or minimal change disease) that were reportedly seen in the previous 6 months was six (IQR 4, 10), including a median of two patients with IgAN (IQR 1, 4). The median number of additional new patients with GN due to a systemic disease that were reportedly seen in the previous 6 months (ex lupus, vasculitis) was three (IQR 2, 5). There was no statistical difference between practice environments in the per physician exposure to either new or follow-up cases of GN. Similarly, there was no significant difference in the number of new GN cases across categories of duration of clinical practice, but as expected there were more follow-up patients in physicians with a longer time in practice (see Figure 2).
Fig. 2.
Median number of self-reported patients seen in the previous 6 months with the following types of glomerular diseases. Whisker bars represent the interquartile range. P-values are for comparisons across categories of years in practice.
In the province of British Columbia in the year prior to the survey, there were 266 cases of biopsy-proven GN that were idiopathic or related to a systemic disease, which is similar to the 13-year historical average from 2000 to 2012 of 267 cases per year. This results in an average potential GN caseload in British Columbia of 4.1 biopsy-proven cases per nephrologist in the year prior to the survey. This is compared with a median self-reported rate of 24 cases per year (IQR 16, 32) among survey respondents from British Columbia.
Self-reported treatment of GN compared with guideline recommendations
Immune therapy for GN
The survey asked about self-reported management of different types of GN with varying clinical scenarios (after a period of conservative management with an ACE inhibitor if appropriate) in which patients had no known contraindication to any particular immune medication. Table 2 shows practice patterns in comparison with KDIGO recommendations. Results demonstrate that nephrologists in Canada are more likely to treat MN with cyclophosphamide (47%) compared with a calcineurin inhibitor (37%), whereas 15% treat MN with non-recommended or no immunotherapy. In patients with FSGS and proteinuria of 5 g/day, 53% of nephrologists would treat with prednisone, whereas 37% would use non-recommended first-line immune agents and 9% would not treat with any immune therapy. Conversely, 26% of nephrologists would treat FSGS and proteinuria of 2 g/day with an immunosuppressive medication such as prednisone or a calcineurin inhibitor. Most nephrologists would treat ANCA vasculitis and significant renal involvement with either intravenous (50%) or oral (44%) cyclophosphamide, both of which are KDIGO recommended therapies. Further, 3% would use rituximab and 18% would add plasma exchange. Variation in self-reported treatment from KDIGO guideline recommendations was not different based on physician practice type or duration of practice.
Table 2.
Self-reported first-line treatment choices for different clinical vignettes with idiopathic GN
| No immune therapy (%) | Prednisone alone (%) | Calcineurin inhibitor (%) | Cyclophosphamide (%) | Azathioprine or MMF (%) | |
|---|---|---|---|---|---|
| MN with 8 g/day | 6 | 5 | 37 | 47 | 4 |
| FSGS with 5 g/day | 9 | 53 | 28 | 8 | 1 |
| FSGS with 2 g/day | 74 | 15 | 9 | 1 | 1 |
All scenarios were presented as a 43-year-old man with the given type of GN and severity of proteinuria after 6 months of ACE inhibitor at the maximum tolerated dose, normal renal function and no contraindication to any immunosuppression medication. KDIGO first-line recommendations are shown in grey. Although cyclophosphamide is the recommended first-line therapy in MN, calcineurin inhibitors are also recommended in patients who refuse alkylating agents, and thus both are categorized as first-line therapies. Data presented as the percentage of respondents who selected each category.
Conservative management of GN
Table 3 demonstrates that all nephrologists would use an ACE inhibitor most or all of the time to control proteinuria in idiopathic GN, and 6% would use combination renin–angiotensin system blockade, such as an ACE inhibitor with an angiotensin blocker. Calcium and vitamin D were used by 91% of nephrologists most or all of the time for prevention of osteoporosis in patients on steroids, while there was substantial variability in the use of bisphosphonates for the same indication. Pneumocystis jiroveci pneumonia prophylaxis in patients on high-dose immunosuppression was used by 83% of nephrologists most or all of the time. There were no differences in these components of the conservative management of GN between physician characteristics (duration of clinical practice or practice environment).
Table 3.
Self-reported use of non-immune therapies in the management of patients with GN
| Never (%) | Rarely (%) | Some of the time (%) | Most of the time (%) | All of the time (%) | Plot | |
|---|---|---|---|---|---|---|
| ACEI to reduce proteinuria | 0 | 0 | 0 | 31 | 69 |  |
| Combination RASB to reduce proteinuria | 21 | 52 | 22 | 3 | 3 |  |
| Calcium/Vitamin D to prevent osteoporosis in those on steroids | 0 | 2 | 7 | 28 | 63 |  |
| Bisphosphonates to prevent osteoporosis in those on steroids | 11 | 16 | 30 | 30 | 13 |  |
| PCP prophylaxis in those on high-dose immunosuppression | 1 | 4 | 12 | 35 | 48 |  |
Data presented as the percentage of respondents who selected each category.
ACEI, ACE inhibitor; RASB, renin–angiotensin system blockade; PCP, pneumocystis carinii pneumonia (aka pneumocystis jiroveci pneumonia).
Barriers to the management of GN patients
To investigate potential barriers to the implementation of best-practice management of GN, the survey asked about the availability of different clinical resources and tools. Summary sheets of previously tried immunosuppression therapies and previous biopsy results were available to 31 and 23% of nephrologists, respectively, whereas standardized laboratory requisitions and immunosuppression protocols were available to 44 and 25%. As shown in Table 4, most physicians had access to other clinicians for a second opinion. However, the availability of patient-focused education tools and nursing support was either unknown or poor for 87 and 67% of nephrologists, respectively. Web-based decision support was readily accessible to only 43% of physicians. Despite Canada having universal healthcare, insurance coverage for immunosuppression medications for patients with GN was poorly accessible to 84% of physicians.
Table 4.
The availability of clinical support tools for the management of patients with GN, rated on a scale from 1 (not available) to 5 (very available).
| Don't Know (%) | 1 (Not available) (%) | 2 (%) | 3 (%) | 4 (%) | 5 (Very available) (%) | Plot | |
|---|---|---|---|---|---|---|---|
| Clinicians for a second opinion | 1 | 1 | 4 | 8 | 25 | 61 |  |
| Patient-focused educational tools | 9 | 26 | 26 | 26 | 8 | 5 |  |
| Web-based decision support | 14 | 11 | 13 | 19 | 19 | 24 |  |
| Nursing support | 4 | 24 | 14 | 25 | 21 | 12 |  |
| Insurance coverage for immunosuppression medications | 13 | 12 | 34 | 24 | 8 | 8 |  |
Data presented as the percentage of respondents who selected each category.
Compared with non-academic urban or rural physicians, those working in academic environments had better access to other clinicians for a second opinion (95 versus 63–69% P < 0.001), were less likely to have difficulty accessing insurance coverage for immunosuppression medications (77 versus 100% P < 0.001) and had better access to nursing support (40 versus 11–31% P = 0.017).
Initiatives to improve the implementation of best-practice management
The survey asked physicians to rate the perceived usefulness of several proposed initiatives designed to facilitate the best-practice management of GN patients (see Table 5). Most nephrologists would find it helpful to refer patients to a specialty GN clinic (64%) or to access phone consultations with a GN specialist (68%). The majority gave high ratings to the potential usefulness of standardized order sets for GN-specific immunosuppression medications (76%) and laboratory requisitions (68%), and access to immunosuppression medications through a centralized funding mechanism (86%). In addition, 96% of nephrologists consider it beneficial to compare the outcomes and management of their GN patients to benchmarks taken from the entire provincial GN population. Nephrologists with less than 5 years of experience were more likely than senior colleagues to view such initiatives as being helpful, although not all comparisons were significant. This included referring patients to a specialty GN clinic (79 versus 53–68% P = 0.11); access to a GN specialist for telephone consults (91 versus 57–63% P = 0.022); use of standardized order sets for GN-specific immunosuppression medications (88 versus 65–77% P = 0.13) and use of standardized laboratory requisitions (88 versus 59–65% P = 0.021). Rural nephrologists considered GN education rounds to be beneficial (95%) more often than those working in urban environments (65–77% P = 0.02).
Table 5.
Perceived usefulness of the following proposed initiatives targeting the care of GN patients, rated on a scale from 1 (not helpful) to 5 (most helpful).
| 1 (Not helpful) (%) | 2 (%) | 3 (%) | 4 (%) | 5 (Most helpful) | Plot | |
|---|---|---|---|---|---|---|
| Ability to refer patients to a specialty GN clinic | 3 | 14 | 19 | 27 | 37 |  |
| Access to phone consults with GN specialist | 2 | 9 | 22 | 28 | 40 |  |
| Access to nursing support | 2 | 8 | 27 | 31 | 31 |  |
| Standardized order sheets for immunosuppression | 5 | 5 | 14 | 34 | 42 |  |
| Standardized laboratory requisitions specific to GN | 6 | 10 | 16 | 26 | 42 |  |
| Access to immunosuppression medications through a centralized funding mechanism | 3 | 3 | 9 | 22 | 64 |  |
| Access to GN educational rounds | 5 | 7 | 16 | 41 | 32 |  |
Data presented as the percentage of respondents who selected each category.
Seventy-nine percentage of nephrologists felt that a provincial GN registry capable of reporting the incidence, prevalence and outcome of GN patients would be a useful resource. Further, the majority of nephrologists were willing to register their patients in such a registry for the purpose of advocating for resources (76 yes, 24% maybe), or to review provincial outcomes and/or resource utilization (76 yes, 24% maybe). Seventy-six percentage of nephrologists were supportive of enrolling their GN patients in therapeutic clinical trials, and 71% were supportive of enrolment in prospective observational studies. Support for a GN registry or for clinical trials was similar between physicians of different practice duration or practice environments.
Discussion
Using a national survey of nephrologists from across Canada conducted 2 years after the publication of the KDIGO GN guidelines, we demonstrate incomplete uptake of guideline recommendations into clinical practice with substantial variability in the self-reported management of glomerular disease. We also identify barriers to the implementation of GN guideline recommendations and widespread support among nephrologists for initiatives that may address these barriers and improve the best-practice management of GN (which are summarized in Table 6). This is the first published study, to the best of our knowledge, to investigate the need for and local barriers to the knowledge translation of the KDIGO Clinical Practice Guidelines for GN.
Table 6.
Some of the initiatives identified by the survey that are supported by nephrologists and may improve the uptake of the KDIGO GN guidelines into clinical practice.
| Standardized care pathways, immunosuppression order sets and GN-specific laboratory requisitions |
| Providing physicians with quality-of-care benchmarks based on regional population-level data |
| Patient engagement through education about GN |
| Improved patient access to GN immunosuppression medications |
| Development of regional GN registries to educate physicians about local incidence, prevalence, treatment and outcome of GN |
| Enhanced physician access to GN educational rounds |
Our results show that 15–46% of nephrologists in Canada report that they do not treat patients with FSGS or MN with guideline-based recommended treatment. This includes 6–9% of patients with severe disease who are not given any immunosuppression despite possible benefit, 26% of patients with mild disease who are overtreated with potentially toxic therapy and 9–37% of patients who receive either ineffective or non-recommended first-line immune therapies. All vignettes in the survey clearly stated there were no contraindications to any immune medications, thus it is not likely that these results reflect decisions to avoid toxicity in the context of specific patient circumstances, and instead represent true physician-level treatment variability. These results are consistent with observational studies of patients with FSGS or MN that use research registries, in which 19% of low-risk patients are treated with immune therapy, and 34–44% of high-risk patients do not get treated with immunosuppression while 26–33% get non-recommended therapies [5–8]. Our finding that nephrologists overestimate their exposure to new GN patients by 6-fold suggests that low patient volume per individual physician may contribute to the observed treatment variability.
In addition to describing care gaps in the management of GN, we attempted to identify barriers to the implementation of guideline recommendations in Canada and potential interventions that may address these deficiencies (see Table 6). Given that standardized care tools are proven to enhance guideline implementation, we were interested to find that 56–77% of nephrologists do not have access to such tools for GN (such as medication protocols or laboratory requisitions) [18]. The majority (68–76%) reported that they would value these resources in clinical practice, and were more clearly favoured in non-academic or rural environments and by those in practice for less than 5 years. Thus, our results suggest that standardized care pathways that target biopsy-proven cases of GN and that are based on KDIGO guideline recommendations may improve implementation, address an area of need and have substantial physician support. Furthermore, GN education tools for patients are unavailable or unknown to 87% of nephrologists. Because patient engagement has been shown to enhance knowledge translation, this finding identifies a potentially effective intervention that targets a current void [18, 19]. Providing physicians with achievable quality-of-care benchmarks using local patterns of practice is an effective method of improving guideline utilization [20]. Our study describes near universal support among nephrologists for such an initiative that focuses on the treatment and outcome of GN patients. Finally, 84% of nephrologists report that their patients have poor access to insurance coverage for GN immunosuppression medications, and 86% consider increased coverage as relevant to improving care. Canada is the only developed country with universal healthcare that does not include coverage for prescription medications, with the costs primarily borne by the patient. Thus, these findings identify glomerular diseases as being relatively underserviced and highlight the need for health policy to address this discrepancy by ensuring access to guideline-recommended therapies.
While our survey focused on the use of regional GN registries to provide quality-of-care and administrative benchmarks, we were also able to ascertain physician support for research. Research in glomerular diseases has been limited by the absence of large registries and poor recruitment into clinical trials [13, 21, 22]. However, our results suggest widespread physician support for GN registries, with 76% of nephrologists willing to enrol their patients into therapeutic clinical trials and 71% into observational studies. These findings imply that limitations to GN research are not due to a lack of physician enthusiasm, but instead may be the result of inadequate infrastructure capable of leveraging physician eagerness to participate in research. Currently, most research in glomerular diseases focuses on recruitment at academic tertiary referral centres. We show that there are a limited number of new GN cases seen yearly by individual nephrologists, and that those numbers are similar in rural, urban and academic environments. Therefore, recruitment of large numbers of GN patients into clinical trials should not be limited to academic institutions, but will depend on the development of more inclusive research infrastructure capable of enrolling patients from many different nephrologists practicing in all types of working environments.
There are several limitations to our study design that must be considered in the interpretation of our results. The survey likely underestimates the true magnitude of treatment variability because it does not address dosing or duration of therapy and is self-reported instead of actual practice which may overestimate guideline adherence by 27% [23]. This further highlights the importance of our results and the need to improve guideline implementation in Canada. The spectrum of respondents is representative of the provincial populations and the urban versus rural distribution of physicians, and thus our results are likely representative of nephrology in Canada. However, there may be limited ability to apply our findings to other countries with different healthcare environments. We were not able to compare self-reported GN caseload to physician-level biopsy data, and instead relied on provincial aggregate data. It is possible that physicians may not biopsy some GN patients, however, this is expected to have a small effect and the 6-fold difference between self-reported and potential GN caseload suggests substantial overestimation by nephrologists. The KDIGO GN guidelines were published in 2012, whereas the survey was conducted in 2014, and there are well-described time lags in the uptake of any guideline recommendations that may extend beyond 2 years. As such, our results should not be interpreted as a failure of guideline implementation, but instead highlight the variability in practice patterns and current care gaps as justification to improve knowledge translation initiatives. It is possible that our results may partially reflect limited physician support for the GN guidelines due to the fact that 78% of the recommendations are grade C or lower [13]. Finally, this survey is not a validated measurement tool to assess the implementation of GN guidelines: no literature on this specific topic is available and no such tool exists. However, we developed the survey based on robust principles of survey design, including domain dimensions and face validity. All of these are relative limitations and do not serve to detract from the main findings.
In conclusion, our study is the first to demonstrate at the national level the large variability in the management of GN and to quantify physician deviation from guideline-based recommendations. The findings in totality suggest a need for initiatives to improve the uptake of the KDIGO GN guidelines into clinical care. We identified barriers to guideline implementation that could form the basis of formal knowledge translation activities. We also describe widespread physician support for initiatives that address these barriers, including standardized clinical care tools, patient education tools, improved access to immunosuppression medications and the provision of achievable benchmark goals using local GN patterns of practice. This study is an important first step that identifies the need for resources focused on guideline implementation in glomerular disease. In this way, we would collectively improve the best-practice management of GN patients.
Supplementary data
Supplementary data is available online at http://ndt.oxfordjournals.org.
Funding
There was no funding support for this project.
Conflict of interest statement
None of the authors has financial disclosures related to this project. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Supplementary Material
References
- 1.Samuel S, Morgan C, Bitzan M, et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatr Nephrol. 2013;28:2289–2298. doi: 10.1007/s00467-013-2546-0. [DOI] [PubMed] [Google Scholar]
- 2.Manno C, Torres DD, Rossini M, et al. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694–3701. doi: 10.1093/ndt/gfp356. [DOI] [PubMed] [Google Scholar]
- 3.Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163. doi: 10.1097/01.asn.0000103869.08096.4f. [DOI] [PubMed] [Google Scholar]
- 4.Barbour SJ, Cattran DC, Kim SJ, et al. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int. 2013;84:1017–1024. doi: 10.1038/ki.2013.210. [DOI] [PubMed] [Google Scholar]
- 5.Hladunewich MA, Troyanov S, Calafati J, et al. The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol. 2009;4:1417–1422. doi: 10.2215/CJN.01330209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattran DC, Reich HN, Kim SJ, et al. Have we changed the outcome in membranous nephropathy? A propensity study on the role of immunosuppressive therapy. Clin J Am Soc Nephrol. 2011;6:1591–1598. doi: 10.2215/CJN.11001210. [DOI] [PubMed] [Google Scholar]
- 7.Troyanov S, Wall CA, Miller JA, et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 8.Sprangers B, Bomback AS, Cohen SD, et al. Idiopathic membranous nephropathy: clinical and histologic prognostic features and treatment patterns over time at a tertiary referral center. Am J Nephrol. 2012;36:78–89. doi: 10.1159/000339628. [DOI] [PubMed] [Google Scholar]
- 9.Cattran DC, Greenwood C, Ritchie S, et al. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int. 1995;47:1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 10.Cattran DC, Appel GB, Hebert LA, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 11.Jha V, Ganguli A, Saha TK, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18:1899–1904. doi: 10.1681/ASN.2007020166. [DOI] [PubMed] [Google Scholar]
- 12.Cattran DC, Appel GB, Hebert LA, et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 13.KDIGO clinical practice guidelines for glomerulonephritis. Kidney Int. 2012;S2:139–274. [Google Scholar]
- 14.Straus SE, Tetroe JM, Graham ID. Knowledge translation is the use of knowledge in health care decision making. J Clin Epidemiol. 2011;64:6–10. doi: 10.1016/j.jclinepi.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Straus SE, Tetroe J, Graham I. Defining knowledge translation. CMAJ. 2009;181:165–168. doi: 10.1503/cmaj.081229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 17.Lugtenberg M, Zegers-van Schaick JM, Westert GP, et al. Why don’t physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54. doi: 10.1186/1748-5908-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majumdar SR, McAlister FA, Furberg CD. From knowledge to practice in chronic cardiovascular disease: a long and winding road. J Am Coll Cardiol. 2004;43:1738–1742. doi: 10.1016/j.jacc.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA. 1999;281:1512–1519. doi: 10.1001/jama.281.16.1512. [DOI] [PubMed] [Google Scholar]
- 20.Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871–2879. doi: 10.1001/jama.285.22.2871. [DOI] [PubMed] [Google Scholar]
- 21.Leaf DE, Appel GB, Radhakrishnan J. Glomerular disease: why is there a dearth of high quality clinical trials? Kidney Int. 2010;78:337–342. doi: 10.1038/ki.2010.156. [DOI] [PubMed] [Google Scholar]
- 22.Pesce F, Schena FP. Worldwide distribution of glomerular diseases: the role of renal biopsy registries. Nephrol Dial Transplant. 2010;25:334–336. doi: 10.1093/ndt/gfp620. [DOI] [PubMed] [Google Scholar]
- 23.Adams AS, Soumerai SB, Lomas J, et al. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care. 1999;11:187–192. doi: 10.1093/intqhc/11.3.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.