Abstract
Background
Pediatric patients with chronic kidney disease (CKD) are at increased risk of early cardiovascular disease and premature death. Abnormalities in microvascular structure and function may presage end-organ damage including vascular calcification and myocardial ischemia associated with disordered mineral metabolism. Early detection of microvascular rarefaction (reduced density of capillaries) may identify at-risk patients and prompt timely therapeutic interventions. Our objective was to study capillary rarefaction in pediatric hemodialysis (HD) patients and to determine possible associations with mineral metabolism and cardiac risk biomarkers.
Methods
Capillary density (CD) was measured by nailfold capillaroscopy in 19 pediatric HD patients and 20 healthy controls. Demographic and biochemical markers were collected at entry and 6-month follow-up.
Results
CD was significantly decreased in HD patients compared with controls with a deficit of 24 and 31% at baseline and subsequent follow-up. Maximal CD correlated significantly with intact parathyroid hormone (iPTH) (r = −0.45; P = 0.005), serum calcium (r = −0.38; P = 0.02) and 25(OH) vitamin D levels (r = +0.36; P = 0.03) in HD patients. Capillary functional measures were similar to controls. By multivariate analysis, the primary negative determinants of CD were African American race and hyperparathyroidism; whereas, glomerular disease had a positive influence on capillary rarefaction (R2 = 64.2% variance; P = 0.001).
Conclusion
Pediatric HD patients demonstrate a ‘structural deficit’ in CD but show preserved ‘functional integrity’. Capillary rarefaction, an early risk factor of incipient vascular calcification, was strongly associated with biomarkers of altered mineral metabolism. Further studies are warranted to determine the impact of optimizing blood pressure and metabolic control on changes in capillary rarefaction in young CKD patients.
Keywords: capillary rarefaction, microvascular disease, pediatric hemodialysis
Introduction
All-cause mortality in pediatric dialysis patients aged 0–19 years is at least 30 times that of the general pediatric population with cardiovascular disease (CVD) ranked as the leading cause of death in the pediatric dialysis population [1, 2]. Early markers of CVD risk include echocardiographic findings of abnormal cardiac geometry and large vessel anatomical changes related to hypertension and volume dysregulation. These are measured by increases in left ventricular mass, carotid intima-media thickness and large vessel stiffness (pulse wave velocity) [3–6].
Importantly, alterations in end-organ microvascular structure and function may preface the pathologic cascade into end-organ disease including diabetes, hypertension and kidney disease [7–9]. A recent focus on measuring tissue capillary rarefaction, defined as a decrease in the number of perfused capillaries in an area of tissue, has allowed an early assessment of microvascular function and tissue perfusion in various disease states including chronic kidney disease (CKD) [10–12]. Techniques developed to measure capillary rarefaction in the skin have been shown to accurately reflect central organ pathology including coronary artery disease and vascular calcification in dialysis patients [13–15]. Little is known of capillary rarefaction in hemodialysis (HD) patients and no studies have been published for pediatric patients [13, 16].
In this study, we sought to develop the technique of intravital nailfold capillaroscopy in pediatric HD patients and to assess its applicability as a non-invasive technique. The goal was to document capillary density (CD) in a pediatric dialysis population and its association with clinical and metabolic biomarkers of hypertension, cardiomyopathy and abnormal mineral metabolism.
Materials and methods
Study population and design
Nineteen pediatric patients with CKD stage 5 on chronic HD for >3 months and 20 healthy normotensive control subjects participated in this single-center, cross-sectional, 6-month longitudinal study. Since growth failure is prevalent in pediatric CKD patients, controls were closely matched for height-age, race and gender. The ‘height-age’, defined as the age equivalent to the 50th percentile for height and gender, is used to standardize physiologic and cardiac measurements in patients with growth failure. Of note, all subjects were either African American (AA) or Hispanic (H) which is consistent with the racial/ethnic demographic of the study site. In addition, control subjects had no significant prior or current medical illnesses or abnormal birth history such as prematurity or low birth weight. Patients were recruited from the pediatric dialysis unit and control subjects from the outpatient general pediatric clinics at Holtz Children's Hospital/Jackson Health System from April through December 2013. The study protocol was approved by the institutional review board of the University of Miami and informed consent was obtained from each subject. Patients were excluded if they had evidence of active vasculitis, congenital cardiac disease or were unable to sit quietly and allow the examination.
Clinical, demographic and biochemical data
Control subjects had sitting casual blood pressures measured at least twice in the clinic at the time of the nailfold capillaroscopy. Clinical history, demographic and body measurements were obtained at that encounter. Blood pressures were measured by an oscillometric method on the non-dominant and fistula-free arm (in HD patients) using a Dinamap® automated oscillometric device.
The primary renal disease in patients was classified as ‘glomerular’ when the diagnosis included predominant glomerular or vascular pathology such as nephrotic syndrome with focal glomerular sclerosis (FSGS), systemic lupus erythematosus (SLE), IgA nephropathy or vasculitis. ‘Non-glomerular’ disease included those with congenital obstructive uropathy, renal dysplasia or reflux nephropathy.
In the HD patients, body measurements [height, weight and body mass index (BMI) ] and blood pressures pre- and post-dialysis were obtained three times weekly. Laboratory assessments included at least monthly urea nitrogen, hemoglobin, ferritin, albumin, calcium, phosphorus and alkaline phosphatase. Multiple clinical and laboratory measurements were time-averaged over the 6-month study period. Intact parathyroid hormone (iPTH) and 25-hydroxy vitamin D (25(OH)D) were assayed every 3 months during the study period. Biomarkers specific to cardiovascular risk including high-sensitivity C-reactive protein (hsCRP) and pro-brain natriuretic peptide (Pro-BNP) were assayed once during the study period. Further, fibroblast growth factor 23 (FGF23), considered both a component of bone mineral metabolism and cardiac biomarker, was assayed using the C-terminal human FGF-23 ELISA (Immutopics, San Clemente, CA). Medications, including the weekly dose of activated vitamin D and the number and class of antihypertensive medications, were recorded. Dialysis efficiency (Kt/V) was calculated as the ratio of the urea clearance (K) during time on dialysis (t) over the volume of distribution (V).
Nailfold capillary microscopy
CD measurements were performed by nailfold microscopy of the fourth finger on the non-dominant hand unless the patient had a vascular access (fistula or graft) in that arm. The technique was a modification of that described by Serné et al. [17]. The fourth finger was selected as it was less likely to have had prior trauma. Measurements were conducted in a quiet, temperature-controlled room (23.4 ± 0.4°C) after resting for ∼15 min. Participants were fasting for 4–6 h and had abstained from caffeine-containing beverages for at least 24 h prior to the studies. HD patients took all their routine antihypertensive medications on the day of the exam, which was performed following the first or second dialysis session of the week.
Nailfold video capillaroscopy of the same visual field was performed in three phases: Phase 1 was a basal (unstimulated) measure; Phase 2 was after inducing reactive hyperemia by applying a blood pressure cuff to the upper arm and inflating to 20 mmHg above the systolic BP for 2 min and then releasing it; Phase 3 was after the patients were allowed to rest for 15 min, the blood pressure cuff was re-applied and inflated to 60 mmHg for 2 min to achieve venous occlusion. Video recording of the microscopic examination for at least 1 min during each phase was captured with the Dino-Lite Premier AM7013MZT4® digital handheld microscope (Taiwan) at a magnification of ×400 using DinoCapture® 2.0 software. The technique was modified for pediatric patients after the method of Serné et al. [17]. Additional incident lighting was provided by an LED light source for patients with darker skin tones. The images were stored digitally and the capillaries were counted off-line by the same observer (A.E.R.).
For each phase, the number of erythrocyte-perfused capillaries was counted in three 0.6 square millimeter (mm2) field clips of the stored images using Dinocapture® 2.0 software. CD for each phase of the examination was the highest capillary count of the three fields assessed, expressed as number (n) of capillaries per mm2. For verification of the counting methodology, two trained investigators counted the same fields in a blinded fashion from the preserved recordings. All readings were within 5% consistency between the two investigators (mean difference = 0.65 ± 2.3 capillaries/mm2; 95% confidence interval: −0.42 → 1.72).
Functional measures included capillary ‘recruitment’ and maximal ‘perfusion’ following stimulation maneuvers obtained during Phases 2 and 3. Percent (%) ‘recruitment’ was defined as the percent increase in perfused capillaries above basal CD during post-occlusive hyperemia (basal – post-hyperemia)/basal × 100). Percent (%) ‘perfusion’ was the post-hyperemia/maximal capillary density (MCD) post-venous occlusion × 100. The ‘structural’ component, in addition to absolute measures of CD, was assessed by the ‘% deficit in capillary density’ relative to controls. This was the difference in the average patient values compared with the control group expressed as a percent at each phase of stimulation and at baseline and 6 months [16].
Statistical analysis
All data sets were tested for normality with the D’Agostino and Pearson omnibus normality test. Continuous variables were expressed as the average ± SD when normally distributed or as the median and interquartile range for those that were not normally distributed. Intergroup comparisons were tested by one-way analysis of variance (ANOVA). Post-test comparisons for significance were performed by the Kruskal–Wallis test for non-parametric data and by the Bonferroni method for parametric data as appropriate. Differences between two groups were analyzed by Student's t-test. Proportional differences were tested with Fisher's exact test. Univariate correlations were performed with Pearson's correlation coefficient. Multivariate linear regression analysis was used to examine determinants of MCD based on P < 0.05 in univariate analysis and/or physiologic relevance. Graph Pad Prism version 6® for Windows (La Jolla, CA) and PAWS/SPSS® 18 (Chicago, IL) were the statistical programs used to perform the statistical analyses and to construct the graphs. A two-tailed P-value <0.05 was considered significant.
Results
Demographic and clinical characteristics
Table 1 provides the demographic parameters of the control subjects and HD patients at initial entry into the study. Although the chronological age of the HD patients was significantly greater than that of controls, the ‘height-age’ was comparable between the two groups. The race/ethnicity of the study population included Hispanics (N = 18) and African Americans (N = 21) with similar distribution in each group (P = 0.11), which reflects the predominantly urban population served by our hospital. Among the patients, the primary disease classification was ‘glomerular’ in 14 (7 FSGS; 4 SLE; 2 vasculitis and 1 nephrotic syndrome). The remaining five patients had ‘non-glomerular’ disease including renal dysplasia (4) and reflux nephropathy (1). The majority of HD patients required at least two antihypertensive agents, usually including both an angiotensin antagonist and a calcium channel blocker. There was no association between MCD and antihypertensive medication use (R2 = 0.005; P = 0.78). Except for the expected higher blood pressure measurements in the HD patients, all other parameters were comparable.
Table 1.
Demographic data at baseline
| Controls N = 20 | HD patients baseline N = 19 |
P value, controls versus patients | |
|---|---|---|---|
| Age (years) | 13.3 ± 3.3 | 16.6 ± 3.5 | <0.01 |
| Height age (years) | 13.3 ± 3.3 | 12.7 ± 2. 6 | 0.56 |
| Gender: N (%) | Female: 12 (60) | Female: 9 (47) | 0.53 |
| Race/ethnicity: N (%) | AA: 8 (40) | AA: 13 (68) | 0.11 |
| H: 12 (60) | H: 6 (32) | ||
| BMI (kg/m2) | 21 [20,25] | 21 [19,23] | 0.40 |
| BMI (SDS) | 1.6 ± 2.3 | −0.9 ± 1.3 | 0.02 |
| MAP (mmHg) | 80 ± 8 | 97 (92, 102) | <0.01 |
| SBP (mmHg) | 108 ± 8 | 129 (122, 139) | <0.01 |
| DBP (mmHg) | 68 ± 9 | 83 (74, 87) | <0.01 |
| Dialysis vintage (months) | – | 24.3 ± 17.3 | – |
Values are mean ± SD for parametric data or median (interquartile range) for non-parametric data.
AA, African American; H, Hispanic; SDS, standard deviation score; MAP, mean arterial pressure.
Laboratory and metabolic parameters
Table 2 shows the laboratory, cardiac and mineral biomarkers for the HD patients at study initiation and after 6 months. The patients demonstrated adequate measures of anemia control and dialysis efficiency (Kt/V). Levels of hsCRP and Pro-BNP were elevated above reference values.
Table 2.
Laboratory and metabolic parameters in HD patients
| Parameter | Reference values | Initial N = 19 |
6 months N = 18 |
|---|---|---|---|
| Hemoglobin (g/dL) | 12–14 | 11 ± 0.8 | 11 ± 0.6 |
| Kt/V ratio | >1.2 | 1.6 ± 0.2 | 1.6 ± 0.2 |
| Hs-CRP (mg/L) | <1.0 | 0.9 (0.35, 2.65) | – |
| Pro-BNP (pg/mL) | 0–125 | 1214 (539, 2270) | – |
| Total bicarbonate (mM/L) | 22–30 | 24 (22, 25) | 23 ± 2 |
| Albumin (g/dL) | 3.9–5.0 | 4.0 ± 0.4 | 3.9 ± 0.3 |
| Calcium (mg/dL) | 8.4–10.2 | 9.4 ± 0.6 | 9.3 ± 0.6 |
| Phosphorus (mg/dL) | 2.5–4.5 | 6.8 ± 2.0 | 6.6 ± 1.8 |
| Ca × P product (mg2/dL2) | <50 | 64 ± 20 | 62 ± 17 |
| 25(OH)D (ng/mL) | 30–100 | 31 ± 13 | 38 ± 19 |
| FGF-23 Log10 (RU/mL) | <2.4 | 3.96 (3.07, 4.78) | – |
| Intact PTH (pg/mL) | 15–65 | 483 ± 386 | 359 ± 202 |
The iPTH values were elevated at both time points, although within K/DOQI recommended ranges at 6 months [18]. The 25(OH)D levels were normal at both time points. As expected, FGF23 levels were markedly elevated in all HD patients.
Capillary microscopy
CD (capillaries/mm2) was significantly lower in HD patients compared with control subjects at each level of measurement including ‘basal’ (non-stimulated), post-occlusive hyperemia (recruitment) and post-venous occlusion (MCD) (Table 3 and Figure 1). Among the HD patients, only the MCD was significantly above basal levels at initial and 6-month measurements. Functional measurements including % recruitment and % perfusion were similar to control subjects (Table 3).
Table 3.
Capillary structure and function in pediatric HD patients
| Nailfold capillary density (capillaries/mm2) | ||||
|---|---|---|---|---|
| Basal | Post-hyperemia | Maximal post-venous occlusion | P values* | |
| HD patients—Initial | 19.2 ± 5.2 | 21.0 ± 5.6 | 25.1 ± 6.3 | <0.01 |
| —6 months | 18.3 ± 5.2 | 19.9 ± 6.0 | 22.8 ± 7.6 | <0.01 |
| Controls | 24.6 ± 7.8 | 26.6 ± 7.3 | 31.8 ± 8.4 | <0.01 |
| P-value** | <0.01 | <0.01 | <0.01 | <0.001 |
| Capillary function | ||||
| % Recruitment | % Perfusion | |||
| HD patients—Initial | 13.3 ± 16 | 84.4 ± 14 | ||
| —6 months | 10.1 ± 11 | 88.2 ± 11 | ||
| Controls | 11.6 ± 15 | 84.2 ± 11 | ||
| P-values** | 0.38 | 0.53 | ||
*Maximal versus basal and post-hyperemia.
**Controls versus HD patients.
Fig. 1.
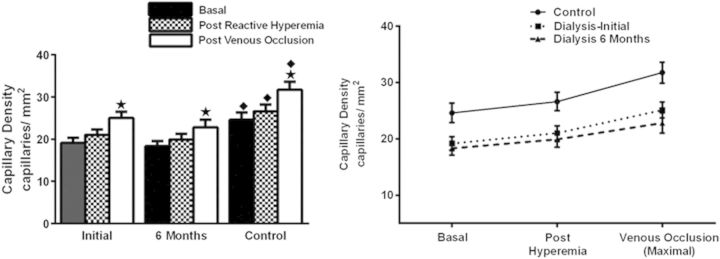
CD of HD patients is compared with controls at initiation and 6 months later. Response to stimulation from basal levels to post-arterial occlusion reactive hyperemia to maximal stimulation with venous occlusion is shown. Stars: significantly greater than basal within each group, P < 0.01. Closed diamonds: significantly greater than HD patients at each level from basal to maximal stimulation, P < 0.01
The capillary ‘deficit’ as shown in Table 3 and Figure 1 was significant in the HD patients compared with controls at each measure from basal to maximal stimulation. Moreover, the deficit in MCD increased substantially in the HD patients between both time points from 24 to 31%, a near-significant difference (P = 0.065).
Capillary rarefaction, mineral metabolism and cardiovascular risk markers
By univariate analysis, capillary rarefaction displayed an inverse association with both iPTH (r = −0.45; P = 0.005) and serum calcium (−0.39; P = 0.02) and a positive association with 25(OH)D levels (r = +0.36; P = 0.02). Neither serum phosphorus nor FGF23 associated with capillary rarefaction. Similarly, cardiac and inflammatory markers (hsCRP and Pro-BNP) did not associate with CD and were not analyzed further.
The only measure of systemic blood pressure in the study population that correlated significantly with capillary rarefaction was the mean arterial pressure (MAP) (r = 0.30; r = 0.02). However, in stepwise multivariate regression involving only the HD patients at baseline, MAP was not significantly associated with MCD and was not included in the final model. Similarly, interdialytic weight gain, as an indirect measure of volume status, did not reach significance when correlated to MCD (r = 0.32; P = 0.051).
Capillary rarefaction, demography and metabolic bone
Table 4 provides the linear and multivariate regression analyses comparing the demographic characteristics of the study population and their relative impact on the primary determinants of capillary rarefaction. Age, BMI, birth weight, dialysis vintage and duration of CKD were excluded due to lack of significant correlation with MCD with a P-value >0.1 in the initial analyses. Similarly, calcium, phosphorus and their product were also excluded. The remaining demographic variables that were significantly associated with capillary rarefaction were female gender (r = +0.46; P = 0.02); African American race (−0.50; P = 0.02) and glomerular disease (+0.59; P = 0.004). Additional variables included in this model were the mineral biomarkers, iPTH and 25(OH)D. This model showed that the primary negative determinants of capillary rarefaction in young HD patients were AA race and hyperparathyroidism; whereas, glomerular disease was a positive determinant. The R2 of this model explained 64.2% of the variance; P = 0.001 (N = 19).
Table 4.
Demographic and bone mineral determinants of MCD in pediatric HD patients
| Linear regression matrix |
Multiple regression |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson r |
MCD = 28 − 0.01 (iPTH) − 6.1 (AA race) + 6.1 (glomerular disease) |
|||||||||
| Parameter | MCD | Female | AA race | Glomerular disease | iPTH | 25(OH) VitD | B-coefficient | β-coefficient | P value | 95% confidence interval |
| MCD (n/mm2) | 1.00 | 28.3 | − | <0.001* | 21.3 → 35.3 | |||||
| Female | +0.46* | 1.00 | ||||||||
| AA race | −0.50* | −0.19 | 1.00 | −6.07 | −0.46 | 0.01* | −10.5 → −1.7 | |||
| Glomerular disease | +0.59* | +0.39* | −0.15 | 1.00 | +6.06 | +0.44 | 0.02* | +1.3 → +10.8 | ||
| iPTH (pg/mL) | −0.42* | +0.03 | −0.09 | −0.24 | 1.00 | −0.01 | −0.36 | 0.03* | −0.01 → −0.000 | |
| 25 (OH)D (ng/mL) | +0.53* | +0.06 | −0.33 | +0.41* | −0.44* | 1.00 | ||||
| Mean ± SD | 24.0 ± 7 | 9/19 (47%) | 13/19 (68%) | 14/19 (74%) | 426 ± 265 | 34 ± 16 | F = 5.9 | 0.001* | R2 = 64.2% | |
Linear and multiple regression analysis.
MCD, maximal capillary density [capillaries (n)/square millimeter (mm2)]; iPTH, intact parathyroid hormone; AA, African American; 25(OH)VitD, 25 hydroxy vitamin D level.
*Significance <0.05.
Mean ± SD or number/total patients with percent (%) is given for all parameters in the units specified.
R2 of the multivariate model explains 64.2% of the variance; P = 0.001. B is the ‘unstandardized coefficient’, which denotes the change in MCD (n/mm2) for every 1 unit increment in the specified parameter. The standardized β (beta) coefficient reflects the strength of the effect change of a given parameter on MCD relative to all other independent parameters in the model.
In further analysis by a simple t-test, the difference in MCD between those with congenital non-glomerular disease was 19 ± 1 versus 27 ± 2; P < 0.01. Those with glomerular disease were primarily female (8/14 = 57%); whereas, those with non-glomerular disease were primarily male and African American (80%). Although there was no correlation between MCD and duration of disease, the average duration of illness for non-glomerular disease patients was 15 ± 2 years which was significantly longer than an average duration of illness of 8 ± 1 years for those with glomerular disease (P = 0.01).
Discussion
This study, to our knowledge, is the first in pediatric HD patients to demonstrate structural abnormalities in microvascular architecture utilizing the non-invasive methodology of intra-vital capillaroscopy. These structural abnormalities of capillary rarefaction appeared to be independent of blood pressure elevations and were strongly related to the degree of hyperparathyroidism. Disturbances of mineral metabolism including hyperphosphatemia, secondary hyperparathyroidism and vitamin D deficiency have been identified as non-classical risk factors for CVD and mortality in patients with CKD [2, 19].
Hyperphosphatemia is commonly found in patients with advanced CKD and has been implicated in the markedly higher incidence of cardiovascular mortality, as well as of visceral and peripheral vascular calcification in these patients [20]. In this study, our HD patients with capillary rarefaction displayed significantly higher serum phosphorus concentrations above the normal range. Our findings support and expand a previous study in adults with advanced CKD in which high phosphorus levels were correlated with increased capillary rarefaction [16].
Our data also revealed significant associations of capillary rarefaction with other components of mineral metabolism. Serum calcium concentrations and iPTH levels correlated inversely with MCD, suggesting a possible detrimental effect of elevated calcium and hyperparathyroidism on vascular integrity. Although we did not investigate coronary changes in our patients, it is conceivable that the observed capillary rarefaction may reflect the presence of calcification in other vascular networks including the coronaries [21]. Further studies are required to demonstrate this association. As would be predicted, elevations of calcium and parathyroid hormone had a negative impact on rarefaction; whereas, therapeutic levels of 25(OH)D improved capillary rarefaction. Circulating 25(OH)D levels were above the level of 30 ng/mL defining insufficiency and improved on sequential measurements. Most patients were receiving supplements of vitamin D as well as an activated vitamin D (calcitriol) or one of its analogs (paricalcitol) following standard guidelines as part of their routine clinical management [18]. The levels of 25(OH)D correlated positively and significantly with the MCD, indicating improved capillary rarefaction with higher levels. Treatment with vitamin D may have exerted an attenuating effect on the capillaroscopic findings, because improving hyperparathyroidism may reduce the release of skeletal muscle minerals and reduce their participation in the process of vascular and coronary calcification [22].
Finally, despite exponentially elevated levels of the phosphaturic hormone FGF23 in our HD patients, we did not observe an association with capillary rarefaction. While cardiovascular morbidities including cardiac hypertrophy have been associated with escalating concentrations of FGF23 [23], this hormone does not appear to be directly involved in vascular calcification [24].
Microvascular rarefaction can be distinguished into both ‘structural’ and ‘functional’ components. ‘Functional’ rarefaction is defined as a decrease in the number of perfused vessels without reduction of the number of vessels anatomically present; whereas, ‘structural’ rarefaction refers to an actual reduction in the number of anatomically present vessels in the tissue [10]. Importantly, they can co-exist and ‘structural rarefaction’ may progress to ‘functional rarefaction’. It remains unclear at which point ‘structural rarefaction’ can be reversed and/or whether preservation of microvascular function may prevent end-organ damage. These are important potential targets for therapeutic interventions at all stages of CKD [11, 12].
In this study, the HD patients demonstrated a significant structural deficit in CD at each level of measurement including basal (non-stimulated), post-occlusion hyperemia and maximal stimulation with venous occlusion. The initial maximally stimulated deficit was 24%. After 6 months, it increased to 31%, although this did not reach significance. These findings are consistent with previous studies in patients with hypertension and normal renal function [25, 26] and CKD patients [16]. Importantly, the ‘functional measures’ of recruitment and perfusion were similar in patients compared with controls, suggesting a preservation of microvascular function. This could be due to the young age of the patients and/or the effect of the antihypertensive medications they were receiving including angiotensin antagonists believed to protect the microvasculature [27].
The traditional strong association of capillary rarefaction with systemic blood pressure was not apparent in our HD patients who were clearly hypertensive. This may be due to the small numbers of patients, but, may also highlight the minimal effect of blood pressure on capillary rarefaction in this group of CKD patients who were on longstanding antihypertensive medications. The role of endothelial dysfunction should also be addressed because post-occlusion hyperemia may be an indirect measure of endothelial function and was clearly lower in our patients compared with controls [28]. Further longitudinal studies with attention to parameters of vascular stiffness and endothelial function are required.
Of interest, in our cohort of dialysis patients, the trend was for those with primary glomerular disease to have less capillary rarefaction than those with obstructive uropathy and/or renal dysplasia. Although the classification of glomerular disease paradoxically included those with a primary diagnosis of vasculitis, they also, by virtue of having acquired disease, had shorter lifetime exposure to CKD. In contrast, those with congenital disease had had exposure since birth. Moreover, those with glomerular disease were primarily female while those with congenital disease were primarily male and African American. This suggests potential genetic and/or epigenetic influences on capillary rarefaction in children with CKD that may influence their progression to ESRD [11, 29].
This study has a number of limitations. First, although it appeared that the capillary deficit was increasing over time, our observation period was too short to determine any true difference. Second, the issue of AA race and female gender could not be clearly determined in this study. Our South Florida demographic is predominantly of Hispanic and African ethnicity and, consequently, our study subjects did not include any of non-Hispanic white race/ethnicity. Similarly, female gender was associated with increased CD as reported previously [17]. The difference in the measurements in the AA subjects does not appear to be a technical limitation because our results are similar to other observations in which blacks were specifically excluded [30] or included [31].
In conclusion, this study demonstrates significant structural abnormalities in capillary rarefaction in young HD patients with some evidence for preserved microvascular function. The strong association with disordered mineral metabolism and poorly controlled hyperparathyroidism is ominous for impending microvascular calcification. Current screening practices focus on identifying cardiac and large vessel disease; whereas, early recognition and treatment of microvascular rarefaction may have more potential for improving long-term prognosis in these vulnerable patients. Hence, there is a need to standardize these techniques with a goal towards incorporating the measurement of capillary rarefaction into clinical practice.
Acknowledgements
We acknowledge the kind support and encouragement of Dr Carlos Cuervo, Audrey Ofir and Stephanie White as well as the assistance of our Nurse Liaison, Teresa Cano, R.N. and the Pediatric Dialysis Nurses and Kathy Parks, our Unit Secretary at the Holtz Children's Hospital.
Funding. This study was funded in part by the Children's Medical Services, Florida Department of Health.
Conflict of interest statement. The authors have no competing financial interests to declare. The results appearing in this manuscript have not been previously published in whole or part, except in abstract form.
References
- 1.Mitsnefes MM, Laskin BL, Dahhou M, et al. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA. 2013;309:1921–1929. doi: 10.1001/jama.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol. 2012;23:578–585. doi: 10.1681/ASN.2011111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnstone LM, Jones CL, Grigg LE, et al. Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int. 1996;50:998–1006. doi: 10.1038/ki.1996.401. [DOI] [PubMed] [Google Scholar]
- 4.Litwin M, Wühl E, Jourdan C, et al. Evolution of large-vessel arteriopathy in paediatric patients with chronic kidney disease. Nephrol Dial Transplant. 2008;23:2552–2557. doi: 10.1093/ndt/gfn083. [DOI] [PubMed] [Google Scholar]
- 5.Brady TM, Schneider MF, Flynn JT, et al. Carotid intima-media thickness in children with CKD: results from the CKiD study. Clin J Am Soc Nephrol. 2012;7:1930–1937. doi: 10.2215/CJN.03130312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groothoff JW, Gruppen MP, Offringa M, et al. Increased arterial stiffness in young adults with end-stage renal disease since childhood. J Am Soc Nephrol. 2002;13:2953–2961. doi: 10.1097/01.asn.0000037677.16961.df. [DOI] [PubMed] [Google Scholar]
- 7.Groen BB, Hamer HM, Snijders T, et al. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985) 2014;116:998–1005. doi: 10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 8.Secomb TW, Pries AR. The microcirculation: physiology at the mesoscale. J Physiol. 2011;589:1047–1052. doi: 10.1113/jphysiol.2010.201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang DH, Joly AH, Oh SW, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 10.Chade AR. Renal vascular structure and rarefaction. Compr Physiol. 2013;3:817–831. doi: 10.1002/cphy.c120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014;29:333–342. doi: 10.1007/s00467-013-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pries AR. Microcirculation in hypertension and cardiovascular disease. Eur Heart J Suppl. 2014;16(Suppl A):A28–A29. doi: 10.1093/eurheartj/ehm448. [DOI] [PubMed] [Google Scholar]
- 13.Niizuma S, Takiuchi S, Okada S, et al. Decreased coronary flow reserve in haemodialysis patients. Nephrol Dial Transplant. 2008;23:2324–2328. doi: 10.1093/ndt/gfm954. [DOI] [PubMed] [Google Scholar]
- 14.Antonios TF, Kaski JC, Hasan KM, et al. Rarefaction of skin capillaries in patients with anginal chest pain and normal coronary arteriograms. Eur Heart J. 2001;22:1144–1148. doi: 10.1053/euhj.2000.2442. [DOI] [PubMed] [Google Scholar]
- 15.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 16.Thang OH, Serné EH, Grooteman MP, et al. Capillary rarefaction in advanced chronic kidney disease is associated with high phosphorus and bicarbonate levels. Nephrol Dial Transplant. 2011;26:3529–3536. doi: 10.1093/ndt/gfr089. [DOI] [PubMed] [Google Scholar]
- 17.Serné EH, Gans RO, ter Maaten JC, et al. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension. 2001;38:238–242. doi: 10.1161/01.hyp.38.2.238. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis. 2005;46:S1–1219. [PubMed] [Google Scholar]
- 19.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 20.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 21.Kruger A, Stewart J, Sahityani R, et al. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 2006;70:157–164. doi: 10.1038/sj.ki.5001511. [DOI] [PubMed] [Google Scholar]
- 22.Hruska KA, Saab G, Mathew S, et al. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Semin Dial. 2007;20:309–315. doi: 10.1111/j.1525-139X.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 23.Seeherunvong W, Abitbol CL, Chandar J, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol. 2012;27:2129–2136. doi: 10.1007/s00467-012-2224-7. [DOI] [PubMed] [Google Scholar]
- 24.Scialla JJ, Lau WL, Reilly MP, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C, Diamond JJ, Falkner B. Functional capillary rarefaction in mild blood pressure elevation. Clin Transl Sci. 2008;1:75–79. doi: 10.1111/j.1752-8062.2008.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng C, Daskalakis C, Falkner B. Capillary rarefaction in treated and untreated hypertensive subjects. Ther Adv Cardiovasc Dis. 2008;2:79–88. doi: 10.1177/1753944708089696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munk VC, Sanchez de Miguel L, Petrimpol M, et al. Angiotensin II induces angiogenesis in the hypoxic adult mouse heart in vitro through an AT2-B2 receptor pathway. Hypertension. 2007;49:1178–1185. doi: 10.1161/HYPERTENSIONAHA.106.080242. [DOI] [PubMed] [Google Scholar]
- 28.Philpott A, Anderson TJ. Reactive hyperemia and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2007;27:2065–2067. doi: 10.1161/ATVBAHA.107.149740. [DOI] [PubMed] [Google Scholar]
- 29.Mayer G. Chronic kidney disease: who is affected, who is at risk and who cares? Nephrol Dial Transplant. 2014;29:937–941. doi: 10.1093/ndt/gft475. [DOI] [PubMed] [Google Scholar]
- 30.Penna GL, Garbero Rde F, Neves MF, et al. Treatment of essential hypertension does not normalize capillary rarefaction. Clinics (Sao Paulo) 2008;63:613–618. doi: 10.1590/S1807-59322008000500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng C, Daskalakis C, Falkner B. Alterations in capillary morphology are found in mild blood pressure elevation. J Hypertens. 2010;28:2258–2266. doi: 10.1097/HJH.0b013e32833e113b. [DOI] [PMC free article] [PubMed] [Google Scholar]


