Abstract
Background
Total joint arthroplasty (TJA) is a common procedure with demand for arthroplasties expected to increase exponentially. Incidence of acute kidney injury (AKI) following TJA is reportedly low, with most studies finding an incidence of <2%, increasing to 9% when emergency orthopaedic patients are included.
Methods
Retrospective medical record review of consecutive primary, elective TJA procedures was undertaken at a large tertiary hospital (Alfred). Demographic, peri-operative and post-operative data were recorded. Factors associated with AKI (based on RIFLE criteria) were determined using multiple logistic regression.
Results
Between January 2011 and June 2013, 425 patients underwent TJA; 252 total knee replacements (TKR) and 173 total hip replacements (THR). Sixty-seven patients (14.8%) developed AKI, including 51 TKR. Factors associated with AKI (adjusting for known confounders) include increasing body mass index [adjusted odds ratio (AOR) 1.14; 95% CI: 1.07, 1.21], older age (AOR 1.07; 95% CI 1.02, 1.13) and lower pre-operative glomerular filtration rate (AOR 0.97; 95% CI 0.96, 0.99) and taking angiotensin-converting enzyme inhibitors (AOR 2.70; 95% CI 1.12, 6.48) and angiotensin-II receptor blockers (AOR 2.64; 95% CI 1.18, 5.93). In most patients, AKI resolved by discharge, however, only 62% of patients had renal function tests after discharge.
Conclusions
This study showed a rate of AKI of nearly 15% in our TJA population, substantially higher than previously reported. Given that AKI and long-term complications are associated, prospective research is needed to further understand the associated factors and predict those at risk of AKI. There may be opportunities to maximize the pre-operative medical management and mitigate risk.
Keywords: acute kidney injury, elective surgery, orthopaedics
Introduction
Total joint arthroplasty (TJA) is a commonly performed procedure and the demand for primary and revision arthroplasties is expected to increase exponentially with time [1]. In Australia, there were over 27 000 total hip replacements (THR) and nearly 40 000 total knee replacements (TKR) performed in 2013. This represents an increase by 40 and 60%, respectively, over the preceding decade (https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2013. Accessed 5 February 2014).
Acute kidney injury (AKI) following both elective and emergency orthopaedic surgical procedures has been reported to complicate up to 9% [2] of cases. The incidence is greater in patients requiring emergency procedures for hip fracture fixation [3, 4] while the incidence of AKI following elective joint replacements is lower, with most studies reporting an incidence of <2% [5–7]. A number of risk factors for AKI post-TJA have been identified in previous studies including age, diabetes, increased body mass index (BMI), pre-operative renal impairment, positive history of chronic obstructive airways disease, liver disease, congestive heart failure, hypertension, peripheral vascular disease, need for vasopressors, medications including angiotensin-converting enzyme (ACEi) inhibitors, angiotensin-II receptor blockers (ARBs), non-steroidal anti-inflammatory drugs (NSAIDs), nephrotoxic antibiotics and diuretics [2, 4, 8]. However, studies which are isolated to elective TJA are scarce.
Post-operative AKI is an independent predictor of mortality after both cardiac [9] and non-cardiac surgery [10, 11]. The increased long-term mortality risk persists irrespective of recovery of renal function by the time of discharge from hospital [9]. Furthermore, acute renal complications are associated with prolonged length of hospital stay [8, 11] and a more complicated peri-operative course [9, 11].
Patients undergoing elective procedures commonly have access to pre-operative assessments with a view to minimize post-operative complications and reducing associated risks being one of the primary goals. These consultations represent opportunities to transiently optimize medications and the management of comorbidities for the peri-procedural period, however, this requires a framework of appropriate characteristics to target.
The aim of our study is to retrospectively review the elective, primary TJA performed at a single tertiary centre, to ascertain the incidence of post-operative AKI and determine the factors associated with this complication in our population, which may be amenable to pre-operative identification and targeted intervention.
Methods
Design and subjects
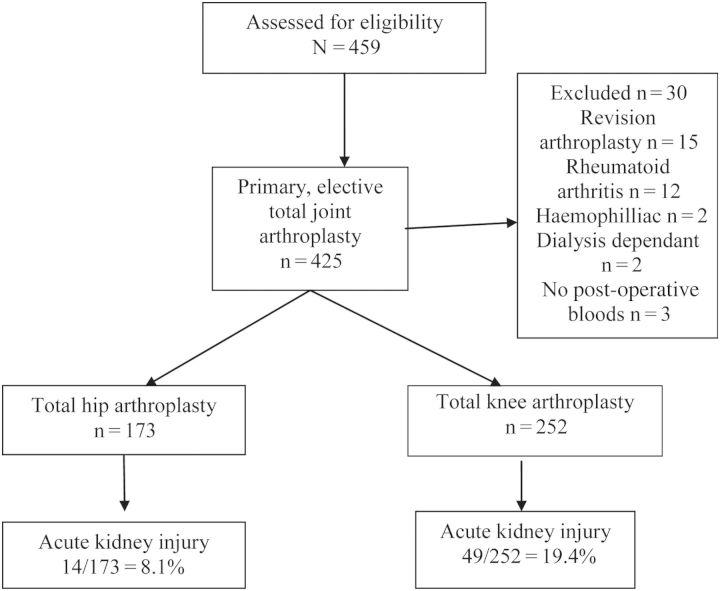
The charts of 459 consecutive patients who underwent a hip or knee arthroplasty between January 2011 and June 2013 inclusive were retrospectively reviewed at our institution, a large tertiary teaching hospital in Melbourne, Australia. These patients were identified from a database, which commenced in 2011. Patients with rheumatoid arthritis or revision arthroplasty were excluded for the purposes of this data collection, leaving 425 patients with primary, elective joint arthroplasty for analysis (Figure 1). The study was approved by The Alfred Hospital Research and Ethics Committee.
Fig. 1.
Patient characteristics and flow.
Data collection
Demographic data, comorbidities and pre-operative renal function [measured by serum creatinine and estimated glomerular filtration rate (eGFR) by modification of diet in renal disease calculation] were collected and tabulated (Table 1). Data on medications including ACEi, ARBs, diuretics and NSAIDs were also obtained from the medication charts. Operative data including anaesthetic medications given peri-operatively and post-operatively, operation type and times and length of stay were also collected (Table 1).
Table 1.
Factors associated with the development of AKI post-TJA (univariate association)
| Descriptor | No post-operative AKI, n = 362 (85.28%) | Post-operative AKI, n = 63 (14.8%) | Total, n = 425 | P-value |
|---|---|---|---|---|
| Age (median, 25/75 percentile) (years) | 70.1 (62.7, 76.2) | 75.2 (70.3, 79.7) | 71.1 (63.8, 76.6) | <0.001 |
| Gender | ||||
| Male | 119 (32.9) | 25 (39.7) | 144 (33.9) | 0.292 |
| Female | 243 (67.1) | 38 (60.3) | 281 (66.1) | |
| Joint | ||||
| Hip | 159 (43.9) | 14 (22.2) | 173 (40.7) | 0.001 |
| Knee | 203 (56.1) | 49 (77.8) | 252 (59.3) | |
| BMI (median, 25/75 percentile) | 30.8 (26.7, 35.6) | 35.1 (30.8, 39.6) | 31.2 (27.3, 36.5) | <0.001 |
| Pre-operative ASA score | ||||
| 1 | 12 (3.4) | 0 (0) | 12 (2.8) | 0.03 |
| 2 | 202 (57.6) | 26 (42.3) | 228 (53.6) | |
| ≥3 | 137 (38) | 37 (58.7) | 174 (40.9) | |
| Pre-operative NSAIDs | ||||
| Yes | 87 (24) | 16 (25.4) | 103 (24.2) | 0.816 |
| No | 275 (76) | 47 (74.6) | 322 (75.8) | |
| Pre-operative eGFR (median, 25/75 percentile) | 85.5 (72.3, 97.4) | 74.3 (58.8, 87.8) | 83.6 (70.2, 96.4) | <0.001 |
| Pre-operative creatinine (median, 25/75 percentile) | 69 (62, 79) | 75 (69, 99) | 70 (63, 80) | <0.001 |
| ARBs | ||||
| Yes | 111 (30.7) | 35 (56.6) | 146 (35.4) | <0.001 |
| No | 251 (69.3) | 28 (44.4) | 279 (65.6) | |
| ACEi | ||||
| Yes | 87 (21.3) | 22 (34.9) | 109 (25.6) | 0.068 |
| No | 285 (78.7) | 41 (65.1) | 316 (74.4) | |
| Diuretic | ||||
| Yes | 87 (24) | 18 (28.6) | 105 (24.8) | 0.441 |
| No | 275 (76) | 45 (71.4) | 320 (75.2) | |
| Diabetes | ||||
| Yes | 72 (19.9) | 25 (39.7) | 97 (22.8) | 0.001 |
| No | 290 (81.1) | 38 (60.3) | 328 (77.2) | |
| Cardiac comorbidities | ||||
| 0 | 291 (80.4) | 41 (65.1) | 332 (78.1) | 0.061 |
| 1 | 44 (12.2) | 14 (22.2) | 58 (13.6) | |
| 2 | 21 (5.8) | 5 (7.9) | 26 (6.1) | |
| ≥3 | 6 (1.7) | 3 (4.8) | 9 (2.1) | |
| Total comorbidities | ||||
| 0 | 101 (27.9) | 3 (4.7) | 104 (24.4) | P < 0.001 |
| 1 | 121 (33.4) | 16 (25.4) | 137 (32.2) | |
| 2 | 82 (22.7) | 27 (42.9) | 109 (25.6) | |
| 3 | 37 (10.2) | 12 (19.0) | 49 (11.5) | |
| ≥4 | 21 (5.8) | 5 (7.9) | 26 (6.1) | |
| Operation time (median, 25/75 percentile) (minutes) | 118 (105, 135) | 125 (108, 135) | 119 (105.5, 135) | 0.337 |
| Anaesthetic type | ||||
| General anaesthetic | 213 (59) | 41 (65.1) | 255 (60) | 0.659 |
| Spinal and general | 6 (1.7) | 1 (1.6) | 7 (1.6) | |
| Spinal anaesthetic | 142 (39.3) | 21 (33.3) | 163 (38.4) | |
| Tourniquet (TKR only) AKI = 49 | ||||
| Yes | 188 (92.6) | 41 (83.6) | 229 (90.9) | 0.051 |
| No | 15 (7.4) | 8 (16.4) | 23 (9.1) | |
| LIA with ketorolac | ||||
| Yes | 123 (34) | 29 (46) | 152 (35.8) | 0.065 |
| No | 239 (66) | 34 (54) | 273 (64.2) | |
| Blood transfusion | ||||
| Yes | 103 (28.5) | 32 (50.8) | 135 (31.8) | <0.001 |
| No | 259 (71.5) | 31 (49.2) | 290 (68.2) | |
| NSAIDs | ||||
| Yes | 106 (29.2) | 6 (9.5) | 112 (26.4) | 0.001 |
| No | 256 (70.8) | 57 (90.5) | 313 (73.6) | |
| Cox inhibitor | ||||
| Yes | 186 (54.4) | 32 (50.8) | 218 (51.3) | 0.870 |
| No | 176 (45.6) | 31 (49.2) | 207 (48.7) | |
| Length of stay (median, 25/75 percentile) days | ||||
| Acute | 5 (4, 7) | 7 (5, 10) | 6 (5, 7) | <0.001 |
| Acute and rehabilitation | 8 (5, 20) | 17 (7, 26) | 9 (5, 20) | <0.001 |
Numbers is n (%) unless otherwise stated.
BMI, body mass index; ASA, American Society of Anaesthesiologists; NSAIDs, non-steroidal anti-inflammatory drugs (COX 1 class only); eGFR, estimated glomerular filtration rate; ARBs, angiotensin-11 receptor antagonists; ACEi, angiotensin-converting enzyme inhibitors; TKR, total knee replacement; LIA, local anaesthetic infiltration.
The primary outcome was incidence of AKI (as measured by the biochemical markers of the RIFLE criteria) [12]. The RIFLE criteria classify patients according to stages including (i) risk: eGFR decrease >25%, serum creatinine increased 1.5 times or urine production of <0.5 mL/kg/h for 6 h; (ii) injury: eGFR decrease >50%, doubling of creatinine or urine production <0.5 mL/kg/h for 12 h; (iii) failure: eGFR decrease >75%, tripling of creatinine or creatinine >355 µmol/L (with a rise of >44) (>4 mg/dL) OR urine output below 0.3 mL/kg/h for 24 h or anuria for 12 h; (iv) loss: persistent AKI or complete loss of kidney function for more than 4 weeks and (v) end-stage renal disease: need for renal replacement therapy for >3 months. We based the diagnosis of AKI on reduction in eGFR or increase in serum creatinine given that urine output was not available in the data set. Length of stay in the acute hospital and with the addition of inpatient rehabilitation, was also collected as was follow-up renal function tests (if available) to determine if resolution of AKI had occurred.
Statistical analysis
Descriptive parametric analysis was used to characterize the profile of the patients identified (Table 1). Continuous variables were expressed by median and interquartile range and categorical variables in percentage terms.
A multivariate ordinal logistic regression was performed to determine those factors associated with an increasing RIFLE score indicating post-operative AKI, and their adjusted odds, of this outcome (Table 2). These included age, gender, BMI, comorbidities, American Society of Anaesthesiologists (ASA) score [13], pre-operative/peri-operative/post-operative medications, pre-operative eGFR, operation type and tourniquet use. The ASA score provides a six-category physical status classification system for assessing a patient before surgery with grading from normal healthy patient (1) to brain-dead (6). These factors were all previously cited in the literature as being potential predictors of AKI [2, 4, 8].
Table 2.
Factors associated with AKI following TJA (multivariate analyses)
| Descriptor | AOR (95% CI) | P-value |
|---|---|---|
| Age | 1.07 (1.02, 1.13) | 0.006 |
| Body mass index | 1.14 (1.07, 1.21) | <0.001 |
| eGFR pre-operatively | 0.97 (0.96, 0.99) | 0.004 |
| Gender | ||
| Female (reference) | 1 | 0.067 |
| Male | 1.88 (0.96, 3,68) | |
| Cardiac comorbidities | ||
| None (reference) | 1 | 0.373 |
| 1 | 1.42 (0.59, 3.43) | |
| 2 | 1.23 (0.36, 4.21) | |
| 3 | 3.80 (0.54, 26.70) | |
| Diabetes | ||
| No | 1 | 0.055 |
| Yes | 2.03 (0.98, 4.21) | |
| Chronic lung disease | ||
| No | 1 | 0.308 |
| Yes | 0.61 (0.23, 1.59) | |
| Cerebrovascular disease | ||
| No | 1 | 0.126 |
| Yes | 2.84 (0.75, 10.88) | |
| Peripheral vascular disease | ||
| No | 1 | 0.742 |
| Yes | 0.78 (0.18, 3.43) | |
| Anaesthetic type | ||
| General (reference) | 1 | 0.136 |
| Spinal | 0.76 (0.54, 1.09) | |
| LIA with ketorolac | ||
| No | 1 | 0.217 |
| Yes | 1.65 (0.74, 3.67) | |
| NSAIDs | ||
| No (reference) | 1 | 0.008 |
| Yes | 0.22 (0.07, 0.67) | |
| ACEi | ||
| No (reference) | 1 | 0.026 |
| Yes | 2.70 (1.12, 6.48) | |
| ARBs | ||
| No (reference) | 1 | 0.018 |
| Yes | 2.64 (1.18, 5.93) | |
| Cox 2 inhibitors | ||
| No (reference) | 1 | 0.216 |
| Yes | 1.56 (0.77, 3.15) | |
| Gentamycin | ||
| No (reference) | 1 | 0.907 |
| Yes | 0.95 (0.39, 2.29) | |
| Blood transfusion | ||
| No (reference) | 1 | 0.027 |
| Yes | 2.24 (1. 10, 4.57) | |
AOR, adjusted odds ratio; eGFR, estimated glomerular filtration rate; LIA, local infiltration of anaesthetic; NSAIDs, non-steroidal anti-inflammatory drugs (COX 1 class only); ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin-11 receptor antagonists.
A P-value of 0.05 was used to determine statistical significance. All analyses were completed using STATA Version 11.2 (StataCorp, College Station, TX, USA).
Results
The characteristics of the population are shown in Figure 1 and Table 1. The median age of the cohort was 71 years (range 33–91 years) with one-third of the population being male. A total of 63 patients (14.8%) had a post-operative complication of AKI with 41, 18 and 4 in the risk, injury and failure categories, respectively. Demographic data, pre-operative patient information and operative data/post-operative data comparing those who did and did not develop post-operative AKI can be found in Table 1.
Factors associated with AKI
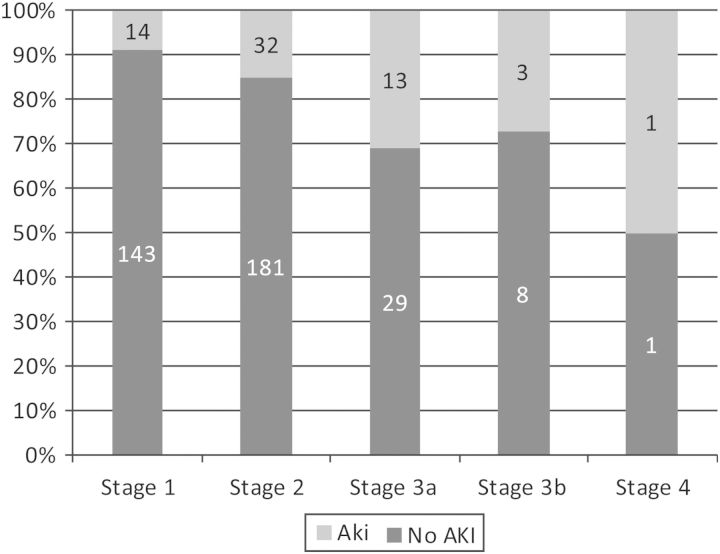
Each year increase of age was associated with a 7% higher risk AKI [adjusted odds ratio (AOR) 1.07 (95% CI 1.02, 1.13), P-value = 0.006]. For each kg/m2 increase in BMI, there was a 14% increased risk of AKI developing [OR 1.14 95% CI (1.07, 1.22), P-value < 0.001]. Pre-operative renal impairment identified by a reduced eGFR was also strongly associated with AKI, with the adjusted odds of AKI increasing by 2.5% per 1 mL/min reduction of eGFR. This was also clearly demonstrated by the proportion of patients developing AKI across different stages of chronic kidney disease (Figure 2). Taking ACEi or ARBs or having a post-operative blood transfusion increased the adjusted odds of post-operative AKI 2-fold while post-operative NSAIDs (COX 1 inhibitors only) appeared to have a protective effect.
Fig. 2.
Acute kidney injury according to stages of chronic kidney disease. Stage 1 = eGFR > 90; Stage 2 = eGFR 60–89; Stage 3a = eGFR 45–59; Stage 3b = eGFR 30–45; Stage 4 = eGFR 15–29.
There was a trend towards males being more likely to develop AKI but this association was not statistically significant (P = 0.067). Comorbidities, ASA score and use of gentamicin were also not shown to be associated with AKI in our population.
When considering the TKA group alone, the factors associated were the same as the whole group, with tourniquet use (specific for TKA surgery) not significantly associated with AKI.
Length of stay was significantly increased in patients with AKI, with an average of two extra days of acute hospital stay and a total inpatient length of stay (incorporating acute and rehabilitation periods) increased by 9 days (Table 1). The occurrence of AKI was associated with the adjusted odds of an increased acute length of stay increasing approximately 2-fold.
Biochemical follow-up was available on 33 out of the 63 patients with AKI (62%), demonstrating an average decrease of 7.7% in eGFR from pre-operative renal function tests to time of follow-up. The median follow-up time was 285 days post-surgery (range 23–1023 days). No patients required renal replacement therapy and there were no deaths recorded.
Discussion
A paradigm shift has occurred in the diagnosis of acute renal failure with standardization of diagnostic criteria and the recognition that even subtle degrees of renal injury are important [14]. Given the appropriate identification of relevant, modifiable risk factors, the elective surgical environment represents a unique opportunity to screen for and potentially alter peri-operative characteristics in order to mitigate risk in the lead up to the procedure.
This study demonstrates a 14.8% incidence of AKI when objective criteria are applied to a group of elective, primary joint arthroplasty patients. The incidence of AKI in our study was considerably higher than reported in other studies of elective joint arthroplasty patients [7, 8]. The lack of institutional follow-up data in 40% of this cohort may further underestimate the magnitude of the problem. The published experience comprises registry analyses, the largest in 17 938 patients [8], and has reported an incidence of AKI between 0.1 and 0.55% [5, 6]. The criteria used to define AKI in these reports were generally not described or lack a common reference point—differing from the objective RIFLE criteria applied in this cohort. Kateros et al. [2] reported that AKI occurred in 8.9% of their peri-operative orthopaedic population (using the strict acute kidney injury network criteria); however, these data may be confounded by the inclusion of emergency orthopaedic admissions with an expected higher risk of AKI compared with an elective surgical population.
Risk factors for the development of AKI include older age, higher BMI, having pre-operative renal insufficiency, having a post-operative blood transfusion and taking ACEi or ARBs. Increasing BMI was the most significant factor associated with AKI. The median BMI of our population was over 31 (with a BMI >30 kg/m2 classified as obese) [15]. Obesity is an established risk factor with a 65% increase in odds of developing AKI within 30 days of admission to hospital when compared with non-obese patients [16]. The degree of risk excess generally remains unaltered through higher degrees of multivariate modelling, which is consistent with the findings in our population. In line with previous reports [2, 8, 10], increasing age and the presence of pre-operative kidney dysfunction were also significantly associated with AKI and represent non-modifiable markers in the peri-operative context.
The continued prescription of either an ACEi or ARB for the treatment of hypertension is significantly associated with the development of AKI, with patients taking these drugs having twice the odds of post-operative AKI than those who did not. Consistent with the findings of existing retrospective TJA analysis [7] and with experience in the cardiac surgery population [17], this represents an important and potentially modifiable risk factor, which may be identified early in the work-up phase. Paradoxically, post-operative use of NSAIDs appeared to be protective in our population. This observation persisted through adjustment for age, comorbidities and pre-operative eGFR in our multiple logistic regression analysis though may reflect underlying type I error in that five patients with pre-operative eGFR <60 were taking these drugs (9%), compared with 107 patients without pre-operative renal insufficiency (29%). Given that NSAIDs may counteract the response to ACEi and ARBs with an opposing effect on systolic blood pressure [18, 19], the mechanisms of any potential benefit conferred by NSAIDs in terms of renal function may be counterintuitive, suggesting the role of sodium and fluid retention with secondary support of circulating volume and renal blood flow an attractive avenue of speculation. NSAIDs and ACEi/ARBs exert their renal effects on microvascular perfusion of the glomerulus at the level of the afferent and efferent arterioles, respectively, to impair autoregulation of flow. Chronic treatment with an ACEi in particular is associated with intensified hypotensive effects of anaesthesia [20–22]. In the acute surgical context, it may be that the relative volume state and support for renal blood flow partially explain the apparent protective effect of NSAIDs though this warrants a suitably powered prospective approach for confirmation. A prospective methodology would at least include the rigorous assessment of blood pressure as a surrogate for renal perfusion in addition to a standardized approach to intravenous fluid repletion and circulating volume assessment.
In the context that any episode of AKI is associated with increased mortality, longer hospitalization and an increase in the odds of both further episodes of AKI and the later development of chronic kidney disease [11], an AKI rate of nearly 15% is worrying. While the median eGFR pre-operatively and at discharge remained fairly similar (83 mL/min compared with 86 mL/min across the entire study population), the group that developed AKI had not returned to baseline by discharge, with an average 7.7% reduction in eGFR compared with pre-operatively. The longer-term implications of AKI events are potentially more severe in this subgroup given the diminished renal reserve whereby any reductions occur from a lower baseline of function.
These data suggest that the incidence of significant AKI might be much higher than previously recognized. The reasons for this are likely to be multifactorial. Accepting limitations inherent to retrospective observations and mindful of attributing association rather than causation, the strength of this study and point of difference compared with the published experience is the objective assessment of AKI by RIFLE criteria on a complete peri-operative data set. Potential ‘centre biases’ in terms of patient complexity may partially explain the observations in this group. The mean age of our patients (71 years) appears to be at the older end of the aggregated range of mean ages of patients undergoing joint replacement in Australia and Europe (63–73 years depending on the individual centre) [23]. The average BMI in our group was over 30 kg/m2, which is higher than that recorded in other studies in which a relationship between increasing BMI and complications are described [6, 24]. Furthermore, the average number of comorbidities in our patient group is 1.5 and may potentially reflect the more complex casemix of a large public, tertiary referral centre.
Recognition of potentially modifiable mediators for peri-operative AKI in an at-risk population confers both an opportunity and responsibility to abrogate those factors prior to surgery. Armed with the knowledge that the role of medications is important and likely mediated through a renal perfusive mechanism, a cautious approach to short-term peri-operative discontinuation and staged reintroduction on the basis of blood pressure and biochemistry seems appropriate, particularly in the setting of TKR procedures. Clearly, the presence of non-modifiable risk factors such as age, BMI and pre-existing renal insufficiency should serve as triggers to exercise greater caution around the time of surgery.
This study has the limitations that are inherent in all retrospective studies. The urine output, peri-operative hypotensive events, role of intravenous hydration and severity of dehydration could not be assessed in this study due to limitations of documentation in medical records. We also only have adequate longer-term follow-up data on 62% of our AKI population so we are unable to confidently report on the resolution or otherwise of the AKI.
The strengths of our study include the collection of a variety of pre- and post-operative factors and the relatively small amount of missing data for the patients during their hospital stay.
Conclusion
This study shows that AKI rates of nearly 15% may occur around the time of elective orthopaedic procedures. Such rates appear to be substantially higher than previously reported, likely driven by greater capture through the rigid application of strict definitions of AKI.
Given the important association of AKI with post-operative outcomes in both the short- and long-term, dedicated prospective research is needed to help predict those at risk of developing AKI after elective orthopaedic surgery with a view to mitigating this risk through targeted interventions. It is important that these risk factors are acknowledged and identified as they represent important opportunities to preemptively support elective patients through these increasingly common procedures.
Conflict of interest statement
The authors have no conflict of interests or financial disclosures to declare.
References
- 1.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Kateros K, Doulgerakis C, Galanakos SP, et al. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101. doi: 10.1186/1471-2369-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White SM, Rashid N, Chakladar A. An analysis of renal dysfunction in 1511 patients with fractured neck of femur: the implications for peri-operative analgesia. Anaesthesia. 2009;64:1061–1065. doi: 10.1111/j.1365-2044.2009.06012.x. [DOI] [PubMed] [Google Scholar]
- 4.Bennet SJ, Berry OM, Goddard J, et al. Acute renal dysfunction following hip fracture. Injury. 2010;41:335–338. doi: 10.1016/j.injury.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(Suppl 1)):139–145. doi: 10.1016/j.arth.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Belmont PJ, Goodman GP, Waterman BR, et al. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96:20–26. doi: 10.2106/JBJS.M.00018. [DOI] [PubMed] [Google Scholar]
- 7.Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth. 2012;59:1111–1118. doi: 10.1007/s12630-012-9797-2. [DOI] [PubMed] [Google Scholar]
- 8.Jafari SM, Huang R, Joshi A, et al. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty. 2010;25(Suppl 6)):49–53. doi: 10.1016/j.arth.2010.04.008. 53.e41–42. [DOI] [PubMed] [Google Scholar]
- 9.Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 10.Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892–902. doi: 10.1097/01.anes.0000290588.29668.38. [DOI] [PubMed] [Google Scholar]
- 11.Coca S, Yusuf B, Shlipak M, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellomo R, Kellum J, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33:409–413. doi: 10.1007/s00134-006-0478-x. [DOI] [PubMed] [Google Scholar]
- 13.Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classification: a study of consistency of ratings. Anaesthesiology. 1978;49:239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kellum A, Bellomo R, Ronco C. Definition and classification of acute kidney injury. Nephron Clin Pract. 2008;109:182–187. doi: 10.1159/000142926. [DOI] [PubMed] [Google Scholar]
- 15.Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 16.Kelz RR, Reinke CE, Zubizarreta JR, et al. Acute kidney injury, renal function, and the elderly obese surgical patient: a matched case–control study. Ann Surg. 2013;258:359–363. doi: 10.1097/SLA.0b013e31829654f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora P, Rajagopalam S, Ranjan R. et al. Preoperative use of angiotensin-converting enzyme/angiotensin receptor blockers is associated with increased risk of acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3:1266–1273. doi: 10.2215/CJN.05271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sturrock ND, Struthers AD. Non-steroidal anti-inflammatory drugs and angiotensin converting enzyme inhibitors: a commonly prescribed combination with variable effects on renal function. Br J Clin Pharmacol. 1993;35:343–348. doi: 10.1111/j.1365-2125.1993.tb04149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier J-P, Sommet A, Bourrel R, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) and hypertension treatment intensification: a population-based cohort study. Eur J Clin Pharmacol. 2012;68:1533–1540. doi: 10.1007/s00228-012-1283-9. [DOI] [PubMed] [Google Scholar]
- 20.Colson P, Saussine M, Seguin JR, et al. Hemodynamic effects of anesthesia in patients chronically treated with angiotensin-converting enzyme inhibitors. Anesth Analg. 1992;74:805–808. doi: 10.1213/00000539-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology. 1994;81:299–307. doi: 10.1097/00000542-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand M, Godet G, Meersschaert K, et al. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg. 2001;92:26–30. doi: 10.1097/00000539-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ackerman IN, Dieppe PA, March LM, et al. Variation in age and physical status prior to total knee and hip replacement surgery: a comparison of centers in Australia and Europe. Arthritis Care Res. 2009;61:166–173. doi: 10.1002/art.24215. [DOI] [PubMed] [Google Scholar]
- 24.Patel AD, Albrizio M. Relationship of body mass index to early complications in hip replacement surgery. Int Orthop. 2007;31:439–443. doi: 10.1007/s00264-006-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]