Abstract
Background
To date, research has neglected the patient's psychosocial and cognitive conditions as contributing factors to dialysis modality decision-making. Hence, the Choice of Renal Replacement Therapy (CORETH) study aims to examine these conditions with regard to their impact on the choice. Here we describe the design of the multicentre study, which is supported by a grant from the German Ministry for Education and Research.
Methods
Two groups of patients will be compared after having chosen peritoneal or haemodialysis as permanent treatment. About 1200 participants from 50 dialysis centres all over Germany will be questioned. The questionnaire addresses social, psychological and shared decision-making aspects. Furthermore, cognitive functioning will be tested. For an economic evaluation direct and indirect costs of treatment will be calculated. Changes will be examined through a one-year follow-up.
Conclusions
The results will enlighten the treatment choice under the German healthcare system. They will provide further insight regarding the discussion on patient autonomy. From the patients' perspective, the results will help to strengthen their participation in the individual process of health-related decision-making.
Keywords: haemodialysis, kidney failure chronic, peritoneal dialysis, psychosocial factors, shared decision-making
Introduction
Both end-stage renal disease (ESRD) itself as well as the restrictions compromising everyday life can provoke depression, social drawback and decreased mental capability. More than a third of ESRD-affected people could choose between haemodialysis (HD) and peritoneal dialysis (PD) [1]. However, surprisingly, decisions in favour of PD only occur in 5% of all cases in Germany, even though both modalities are regarded as equivalent concerning mortality [2, 3].
Besides analyses of equivalence, most comparative studies have been dedicated to the impact of dialysis modality on health-related quality of life (hrQoL) [4–14]. Summarizing the results, researchers were struggling with contradictory findings regarding differences in hrQoL between groups. A meta-analysis of 52 studies, including more than 36 000 participants, yielded no significant differences between dialysis modalities with respect to the SF36 dimensions [13]. However, these studies are likely biased by factors influencing the choice of treatment leading to extremely different patient populations. Approaches intending randomized comparison of both modalities failed [9] because the majority of patients refused randomization once they were educated about the treatment procedures.
Furthermore, several comparative studies focus on psychological (depression [15]) or cognitive (memory, executive functioning, attention [16–20]) impairment, satisfaction with treatment [21] or health beliefs of dialysis patients [14]. Additionally, socio-economic differences [21, 22] and costs of care [23] have been examined. In these approaches, dialysis modality was implicitly considered as a fixed factor affecting diverse clinical outcomes.
However, dialysis patients often differ from other populations because they have a choice with regard to their treatment [24]. Research concerning dialysis modality decision-making has neglected the role of psychosocial and cognitive conditions so far. The question, how factors such as the need for autonomy, depression or the social network determine the choice or the shared decision-making between physicians and patients, has not yet been addressed.
A recent narrative paper from the viewpoint of an ESRD patient describes some psychosocial challenges at the point of decision-making. For example, with regard to PD, relevant aspects were aesthetics, spatial and temporal feasibility, self-determination, safety and social acceptance [25]. A qualitative analysis (N = 398) showed that marginalized groups (here African Americans) receive poorer information at the assignment to dialysis treatment [26]. Findings suggested that the initiation of HD often occurs as an urgent lifesaving action or as a bridging treatment while waiting for transplantation. In addition, many patients learn about PD just because healthcare professionals or other patients who are not involved in their decision told them about it. A recent smaller interview study (N = 13) addressed the question of what special issues older dialysis patients (65+ years) have to cope with when being confronted with the choice [27]. It became apparent that the decision is determined by an increasing uncertainty with respect to the possibility of death in the elderly. On the one hand, the study benefits from the fact that it has been conducted prior to the initiation of dialysis treatment. The authors have good reason to criticize previous trials, which are often retrospective and can be contaminated with memory artefacts. On the other hand, smaller studies like the aforementioned possibly lack generalizability.
The gap of knowledge regarding psychosocial factors at the decision-making process may be due to multiple methodological challenges. These include inter alia the necessity of patient group matching to ensure valid comparative conclusions and the difficulties of time-consuming conduction of longitudinal studies [7, 8, 24]. As pointed out earlier, randomization turned out to be nearly impossible in these patients [10]. Because both modalities are very intrusive, the patients wanted to make their own decision and refused to leave it to chance. If such quasi-experimentally formed patient groups lack a sophisticated matching (e.g. using age and gender) results can be heterogeneous.
Another challenge relates to the measurement of subjective data. When patients are to estimate their present health status, their perception often differs from the objective assessment [24]. Differences were found in other medical areas with respect to the assessment of comorbidity [28]. Comorbidity is sometimes raised as a confounder [29] and can lead to contradictory findings because of discrepancies between expert- and self-assessments. Moreover, people sometimes tend to respond with a self-serving bias showing their real situation in a favourable light [30]. Provided that a relevant number of ESRD patients are severely depressed, their self-evaluations can be negatively distorted, too.
Regarding the prevalence of depression in dialysis patients, the literature is remarkably divergent (0–100%) [24]. The heterogeneity may be due to frequently used but inadequate screening tests [15] which can overestimate symptoms in the chronically ill because they are designed for normal populations. For instance, typical symptoms of depression such as sleep disorders, fatigue or loss of appetite are of limited validity in dialysis patients because they can be caused by renal failure itself.
Although the connection between the development of depressive symptoms and social inclusion is well documented in ESRD patients [15], the exact influence of the social situation on the choice of dialysis modality remains unclear. In particular, a paradox with regard to the impact of the family structure has been described: dialysis patients with a strong family structure have a poorer probability to survive than their less supported counterparts [24]. It has been considered that well-integrated families with a strong cohesion tend to persevere in attempts to cope by their own without calling on professional help. Consequently, strong families may perceive a more internal locus of control than do weaker families and thus, adversely affect the adherence of the sick dependant. There is evidence that the family plays a central role in reducing cognitive dissonance following the choice [26]. However, the question remains unanswered which persons contribute significantly to the decision-making process and why.
Studies investigating the effects of gender disagree on psychosocial consequences of dialysis treatment. For instance, Bakewell and colleagues [7] conclude on an empirical basis that ‘[…] men find it more difficult to adapt to changes brought about by chronic illness. The perceived role of the male as the head of the household may still exist […]. In his role […], a man who becomes unemployed as a result of ESRD may feel a greater burden of illness than a female.’ In contrast, Sensky [24] explains on the basis of reviewed findings: ‘[…] It has been suggested that the overall role demands on women are more stressful than on men, and become much harder to satisfy when a woman falls ill […]. Equally, it may be that, because the nurturant [female] role is acknowledged as flexible, there is often an expectation that this role will be sustained despite illness, whereas it is easier to sacrifice a fixed role (such as ‘breadwinner’) to illness.’ Apart from the argument of role expectations, differences in the tendency of socially desirable responding in males and females are rarely emphasized [31]. The inadequate control of gender effects can also lead to inconsistent results with respect to the influence of social support on the adherence in dialysis patients [24].
Likewise, research on cognitive impairment in ESRD patients has to take several confounders into account. The time of testing (prior to or following dialysis session), psychopharmacological medication and the underestimation of the true prevalence because of bias towards testing the less affected are important variables to control [19]. Some recent papers stress the development of disease-specific screening methods because existing assessments suffer from insufficient sensitivity [16, 32]. Nonetheless, the explanatory approaches of cognitive impairment in ESRD patients prosper: it is associated with inflammation, oxidative stress, vascular calcification, haemodynamic factors, genetic predispositions, cerebral perfusion, etc. However, no consensus exists regarding the cause–effect relationship of dialysis treatment and cognitive impairment. For example, Sarnak et al. [20] showed impairment of the executive functions in ESRD patients and emphasized the causal role of vascular factors. In light of the cause–effect–conflict, it would be desirable to particularly focus on the onset and the effect of cognitive performance deficits in ESRD patients and compare them with healthy controls to avoid misjudgements. So far, it is not known whether PD patients show less cognitive impairment in contrast to their HD matching parts, even if there are hints that this might be the case [26]. The influence of cognitive impairment on the choice of dialysis modality is not yet understood in detail.
Materials and methods
Study objectives
The main objective of the study is to provide answers to the question how psychosocial and cognitive conditions of ESRD patients influence the choice between HD and PD as renal replacement therapy (RRT). Thereby, it is of interest how differences between groups emerge at baseline and after 1 year.
The specific research questions of the project are, to what extend does the psychological status (i), the social situation (ii) and the cognitive status (iii) of the patients contribute to the decision-making process? In order to operationalize the ‘decision-making process’ we will raise via self-assessment: the main reason for the choice, the shared decision-making between physicians and patients, and the satisfaction with the choice. We will control medical, comorbidity-related and socio-demographic confounders.
Along with the psychosocial focus of the study, we will conduct an economic evaluation. The key issues in this context are differences between treatment groups in Germany regarding direct and indirect costs (e.g. medication, incapacity to work), as well as quality adjusted life years (QALYs). So far, in the German healthcare system the bundled reimbursement for both modalities is nearly identical. However, it is currently unresolved whether there is an economic equivalence after involvement of further cost factors.
Study organization
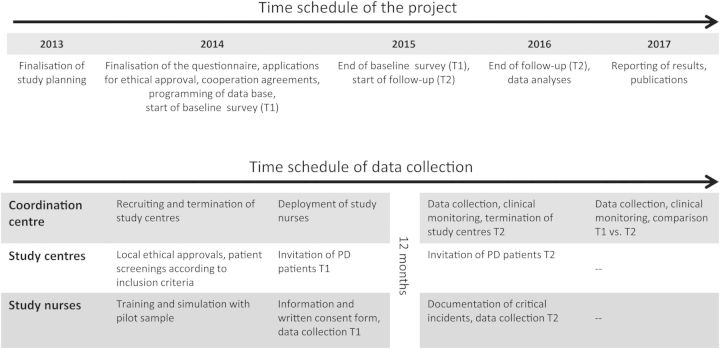
Patients will be recruited in more than 50 nephrological centres across Germany. The centres are recruited from all levels of outpatient care including private practice, non-profit dialysis providers and hospital-associated dialysis centres. Two study nurses will perform the patient interviews. The coordination, data management and statistical analyses will be realized within the framework of the Choice of Renal Replacement Therapy (CORETH) project group (see Appendix). The scheduling is shown in Figure 1.
Fig. 1.
Scheduling of the study.
Study population and sampling
A randomized prospective approach cannot be done because intensely informed patients want to make their own decisions regarding treatment options [10]. Thus, the information should be gained from carefully selected and characterized patient groups in whom the decision is already made. A non-randomized study is chosen because it allows for a comparison of patients using one of these treatments without interfering with the therapy. The time of study entry is set at 6–24 months (T1) after initiation of chronic RRT. At this time, the patients still remember the circumstances of modality choice and already have enough experience with their treatment to have a stable opinion on it. The time criterion also ensures the absence of any acute complications or adaptation problems during the early phase of RRT. Patients are being interviewed by using standardized questionnaires at T1 and after 1 year (T2). Inclusion and exclusion criteria are shown in Table 1.
Table 1.
Inclusion and exclusion criteria in the CORETH study
| Inclusion criteria |
| ESRD (ICD10:N18.5) of any cause |
| RRT for 6–24 months |
| Age 18 years and older |
| Absence of acute psychiatric symptoms |
| Able to read and write German |
| Informed written consent |
| Exclusion criteria |
| Unable or unwilling to provide consent |
The calculation of sample size is based on the SF36 subscale ‘Social Functioning’. With respect to our main hypothesis, it is assumed that the extent of social functioning differs between HD and PD patients. According to the SF36 manual, a difference of five points (on a 100 points scale) is considered clinically relevant. On this basis and the standard deviation derived from the norm population for chronic kidney disease (SD = 24.51), the estimates were made for an effect size of d = 0.20. Thus, a total sample size (α = 0.05; 1–β = 0.80) with N = 758 (n = 379 per group) should be sufficient to prove differences. As the main hypothesis will be assessed within a matched propensity score (PS) analysis with a 1:1 matching ratio [33], the sample size in the HD group will be doubled to find enough matches for each PD patient. Finally, n = 379 PD patients and n = 758 HD patients are needed at baseline.
Study design and outcomes
The study consists of a baseline evaluation and a one-year follow-up. The baseline evaluation will answer the questions on social, psychological and cognitive conditions. A specific part of the questionnaire about the decision-making process will be used in addition. The medical situation and socio-economic characteristics will also be measured. The order of questionnaire responding and cognitive testing will be randomized for each dialysis centre in order to control sequence effects (i.e. fatigue). During the follow-up, all major adverse events leading to either unplanned use of healthcare resources, hospitalization or death will be recorded. In addition, direct treatment-associated resource consumption will be recorded. Further, patients will be asked to disclose their employment status, times of work disability or pension. The follow-up approach will provide unique data on the time dependent development of these parameters. During the observation time, the treatment of patients will not be influenced by the study, however, documented changes due to the wish of the patient or medical reasons are possible at any time. All instruments (see Table 2) are reliable and tested in different populations. The ‘Network Generator’ [38, 39] maps the extent of social inclusion by the number of significant persons from the patient's viewpoint. For characterizing the structure and quality of the relationships, the following aspects will be answered for each named person in the second step: age, gender, kind of relationship, geographic distance, frequency of contact and satisfaction with relationship.
Table 2.
Study outcomes
| Outcome | Operationalization | Instrument | Data source |
|---|---|---|---|
| Psychological situation | Autonomy preference | API | Questionnaire |
| Psychological wellbeing | SF12—psychological subscale | Questionnaire | |
| Depression | HADS | Questionnaire | |
| Social situation | Social support | BSSS | Questionnaire |
| Social functioning | SF36—subscale ‘Social Functioning (SF)’ | Questionnaire | |
| Social network | NWG | Questionnaire | |
| Cognitive situation | Cognitive functioning | KDQOL-SF™-subscale ‘Cognitive Function (CF)’ | Questionnaire |
| Task switching | TMT-B | Cognitive testing | |
| Selective attention | d2-R | Cognitive testing | |
| Decision-making process | Main reason for choice | Generic (nine items) | Questionnaire |
| Shared decision-making | PEF | Questionnaire | |
| Satisfaction with dialysis modality | Generic (four items) | Questionnaire | |
| Economy | Quality of life | SF36, EQ-5D | Questionnaire |
| Quality adjusted life years | QALYs | Economic calculation | |
| Inpatient stays, outpatient operations, medical consultations, medical applications, absenteeism, duration of stay and logistics in the dialysis centre | Generic (11 items) | Questionnaire | |
| Diagnostic list, medication | Medical report | Medical report | |
| Medical situation | Physical wellbeing | SF12—physical subscale | Questionnaire |
| Target weight, body height, onset of dialysis treatment | Generic (seven items) | Questionnaire | |
| Comorbidity | Expert-assessed | CCI | Medical expert |
| Self-assessed | SCQ | Questionnaire | |
| Socio-demographic characteristics | Family status, children, partnership, education, job status, housing situation, household size, household income | Generic (13 items) | Questionnaire |
German versions: API, Autonomy Preference Index [34]; SF, Short Form of Health Survey [35]; HADS, Hospital Anxiety and Depression Scale [36]; BSSS, Berlin Social Support Scales [37]; NWG, Network Generator [38, 39]; KDQOL-SF™, Kidney Disease Quality of Life Short Form [40]; TMT-B, Trail Making Test-Part B [41]; d2-R, Test d2-Revision [42]; PEF (SDM-Q-9), ‘Fragebogen zur Partizipativen Entscheidungsfindung’ (Shared Decision-Making Questionnaire) [43]; EQ-5D, EuoQoL-5D [44]; CCI, Charlson Comorbidity Index [45]; SCQ, Self-Administered Comorbidity Questionnaire [46].
Data protection
The study protocol will be approved by local ethics committee and written informed consent is required from every patient. Data safety will be guaranteed by the ‘Coordination Centre for Clinical Studies’ (KKS, see Appendix). All study procedures will comply with the standards set by the Declaration of Helsinki. The data base will be located at the KKS. Data protection measures analogue to GCP regulations are in place to prevent misuse of the data. The data will be owned by the University Halle-Wittenberg. Access to pseudonymized data will be possible only by permission of the study steering committee.
Statistical considerations
With respect to the PS modelling, we will use an optimal matching algorithm with a calliper width of 0.2 times the standard deviation of the logit of the observed PS to arrive at a 1:1 matching of HD and PD patients. The model will be estimated by a logistic regression model including the variables gender, age, education, employment, marital status, physical functioning, comorbidity and duration of treatment as covariates. The analysis will be based on matched pairs. As the sample size in the HD group is twice as large as that in the PD group, a sensitivity analysis with a variable matching ratio will also be performed. Prospective cost and quality of life (QoL) analyses (comparing costs and QALYs) will be performed.
Quality assurance
All procedures will be described in standard operating procedures (SOPs) and included in a quality manual. The study nurses will be educated on the basis of these SOPs. The patient interviews will be supervised by the study team at the beginning and at least twice during the data acquisition. Therefore, the study nurses will be accompanied by a team member. Observations during this supervision will be discussed at regular study staff meetings. The database will be programmed according to KKS standards and GCP regulations. A pseudonymization procedure will be established allocating a unique data code to each patient. Personal identification data of the individual patient will remain at the study sites and connection to the data base will be prevented.
Discussion
The CORETH study will focus on the previously understudied field of psychosocial and cognitive conditions in ESRD patients. The results are intended to be used for improvement of patient education material. Therefore, the main results will be made publicly available via the Internet besides scientific publications. We will assist institutions that already offer decision support tools by providing guidance for the development of such materials. In doing so, the decision-making for or against one dialysis modality will be facilitated and optimized.
Recruitment of the dialysis centres might be contaminated with selection bias, because data collection is limited to partner sites that are voluntarily participating. As we ensure recruitment throughout Germany, however, we can reflect the sample composition to data from the German dialysis quality assurance system providing statistics at the state level. Therefore, it is possible to control how representative our sample is of the entire population of dialysis patients in Germany. As the main hypothesis is assessed in a non-randomized setting, the statistical analysis will account for structural differences between groups. The matched propensity method, which is used here, has several advantages. The most important advantage is the possibility of making existing or missing overlap between treatment groups explicit. Because RRT is subject to strict quality control measures in Germany with mandatory communication of parameters such as treatment frequency, urea clearance, and haemoglobin levels to the authorities, the quality of treatment will likely not be very diverse among the centres.
At the level of participants, a selection bias has to be discussed. Particularly, only ESRD patients who already have a conducive psychosocial or cognitive situation may give their consent. Indeed, it is even likely that more persons with negative conditions die before the second trial [16, 20]. Since we will recruit PD patients during their typical consultation times and select HD patients according to the inclusion and exclusion criteria randomly, there will only be persons included who are in this moment in the dialysis centre. Thus, representativeness with respect to the particular group can be assumed at the participant level. Moreover, many characteristics such as willingness to respond or socially desirable responce are approximately normally distributed in large samples and hence representative. Since the mobile study team will be extensively trained prior to data collection, we assume its objectivity. This criterion is ensured especially by the questionnaire format with standardized instructions and the fact that some scores, e.g. the CCI, are calculated on behalf of medical experts.
We will cope with the initially mentioned methodological challenges in different ways. First, we will perform a longitudinal study [7, 8, 24] and compare data sets from two measuring points with a one-year interval. Since there are several statistical tests executed with the same sample, we have to take an inflation of the probability of error into account. To still achieve accurate results, the ex-ante probability of error will be Bonferroni-adjusted. Second, with regard to comorbidity differences between expert and self-assessments (physicians versus patients) [28], plausibility and consistency of data will be controlled. Third, in order to deduct valid statements for the chronically ill population, we will measure depression as a psychological decision determinant with a recommended instrument for the sector of somatic medicine (Hospital Anxiety and Depression Scale, HADS). The HADS features high-level practicability, acceptance and especially convergent validity. The correlation of HADS scores and expert assessment of depression thus amounts to r = 0.70 [36]. Fourth, in order to explain the influence of social parameters, we will apply a detailed instrument (NWG) which allows a differentiated mapping of the social network of patients [38]. With the addition of these data, we will be able to answer the questions which persons in the patients' environment explicitly impact the choice of the dialysis modality and how satisfied patients are with these relationships. In this way, CORETH will contribute to the design and address the information materials according to a dialysis modality more personally and be more network specific. It should be pointed out that this study, unlike others in the field, is entirely supported by a grant from the German government thus excluding influences by dialysis providers or industry. Finally, as to control effects of the time of testing and of psychopharmacological medication, the time of cognitive testing and the patients' medication lists will be documented carefully.
In conclusion, the CORETH study will draw a detailed picture of the psychological, social and cognitive conditions in German ESRD patients. The project strives to give solutions for shared decision-making in order to optimize the individual consultation and to enhance the QoL of the chronically ill. By means of our economic analysis, we will be able to further illuminate gaps in the German healthcare system and generate possibilities to make health services more effective.
Acknowledgements
The CORETH project is supported by grants from the German Federal Ministry of Education and Research (VERSSTUD 12-2-047; grant number 01GY1324). The nursing staffs from the different dialysis units, who already confirmed their willingness to participate in this study, are gratefully acknowledged for their cooperation.
Appendix: CORETH Project Group
Project coordination
Medical Faculty of the Martin-Luther-University Halle-Wittenberg, Department of Internal Medicine II
Prof Matthias Girndt, MD
Medical Faculty of the Martin-Luther-University Halle-Wittenberg, Institute for Rehabilitation Medicine
Prof Wilfried Mau, MD
Executive coordination centre
Medical Faculty of the Martin-Luther-University Halle-Wittenberg, Institute for Rehabilitation Medicine
Dr Maxi Robinski
Economic analyses
Hannover Medical School, Institute of Epidemiology, Social Medicine and Health Care System Research
PD Dr Christian Krauth
Data management and clinical monitoring
Coordination Centre for Clinical Studies Halle (KKS)
Dr Joerg Steighardt
Statistical consulting
Medical Faculty of the Martin-Luther-University Halle-Wittenberg, Institute of Medical Epidemiology, Biostatistics, and Informatics
Prof Andreas Wienke
Conflict of interest statement
None declared.
References
- 1.Jassal SV, Krishna G, Mallick NP, et al. Attitudes of British Isles nephrologists towards dialysis modality selection: a questionnaire study. Nephrol Dial Transplant. 2002;17:474–477. doi: 10.1093/ndt/17.3.474. [DOI] [PubMed] [Google Scholar]
- 2.Vonesh EF, Moran J. Mortality in end-stage renal disease: a reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 1999;10:354–365. doi: 10.1681/ASN.V102354. [DOI] [PubMed] [Google Scholar]
- 3.Yeates K, Zhu N, Vonesh EF, et al. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant. 2012;27:3568–3575. doi: 10.1093/ndt/gfr674. [DOI] [PubMed] [Google Scholar]
- 4.Steele TE, Baltimore D, Finkelstein SH, et al. Quality of life in peritoneal dialysis patients. J Nerv Ment Dis. 1996;184:368–374. doi: 10.1097/00005053-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael P, Popoola J, John I, et al. Assessment of quality of life in a single center dialysis population using the KDQOL-SFTM questionnaire. Qual Life Res. 2000;9:195–205. doi: 10.1023/a:1008933621829. [DOI] [PubMed] [Google Scholar]
- 6.Majkowicz M, Afeltowicz Z, Lichodziejewska-Niemierko M, et al. Comparison of the quality of life in hemodialysed (HD) and peritoneally dialysed (CAPD) patients using the EORTC QLQ-C30 questionnaire. Int J Artif Organs. 2000;23:423–428. [PubMed] [Google Scholar]
- 7.Bakewell AB, Higgins RM, Edmunds ME. Quality of life in peritoneal dialysis patients: decline over time and association with clinical outcomes. Kidney Int. 2002;61:239–248. doi: 10.1046/j.1523-1755.2002.00096.x. [DOI] [PubMed] [Google Scholar]
- 8.Harris SA, Lamping DL, Brown EA, et al. Clinical outcomes and quality of life in elderly patients on peritoneal dialysis versus hemodialysis. Perit Dial Int. 2002;22:463–470. [PubMed] [Google Scholar]
- 9.Fukuhara S, Lopes AA, Bragg-Gresham JL, et al. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;64:1903–1910. doi: 10.1046/j.1523-1755.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 10.Korevaar JC, Feith GW, Dekker FW, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int. 2003;64:2222–2228. doi: 10.1046/j.1523-1755.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu AW, Fink NE, Marsh-Manzi JV, et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol. 2004;15:743–753. doi: 10.1097/01.asn.0000113315.81448.ca. [DOI] [PubMed] [Google Scholar]
- 12.Lew SQ, Piraino B. Psychosocial factors in patients with chronic kidney disease: quality of life and psychological issues in peritoneal dialysis patients. Semin Dialysis. 2005;18:119–123. doi: 10.1111/j.1525-139X.2005.18215.x. [DOI] [PubMed] [Google Scholar]
- 13.Liem YS, Bosch JL, Arends LR, et al. Quality of life assessed with the medical outcomes study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2007;10:390–397. doi: 10.1111/j.1524-4733.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 14.Ginieri-Coccossis M, Theofilou P, Synodinou C, et al. Quality of life, mental health and health beliefs in haemodialysis and peritoneal dialysis patients: investigating differences in early and later years of current treatment. BMC Nephrol. 2008;9:14. doi: 10.1186/1471-2369-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zalai D, Szeifert L, Novak M. Psychological distress and depression in patients with chronic kidney disease. Semin Dialysis. 2012;25:428–438. doi: 10.1111/j.1525-139X.2012.01100.x. [DOI] [PubMed] [Google Scholar]
- 16.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 17.Madero M, Gul A, Sarnak MJ. Review: cognitive function in chronic kidney disease. Semin Dialysis. 2008;21:29–37. doi: 10.1111/j.1525-139X.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 18.Lux S, Mirzazade S, Kuzmanovic B, et al. Differential activation of memory-relevant brain regions during a dialysis cycle. Kidney Int. 2010;78:794–802. doi: 10.1038/ki.2010.253. [DOI] [PubMed] [Google Scholar]
- 19.Tamura MK, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol. 2010;5:1429–1438. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013;80:471–480. doi: 10.1212/WNL.0b013e31827f0f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin HR, Fink NE, Plantinga LC, et al. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA. 2004;291:697–703. doi: 10.1001/jama.291.6.697. [DOI] [PubMed] [Google Scholar]
- 22.Zhang AH, Bargman JM, Lok CE, et al. Dialysis modality choices among chronic kidney disease patients: identifying the gaps to support patients on home-based therapies. Int J Nephrol Urol. 2010;42:759–764. doi: 10.1007/s11255-010-9793-9. [DOI] [PubMed] [Google Scholar]
- 23.Sennfalt K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis—a cost-utility analysis. Perit Dial Int. 2002;22:39–47. [PubMed] [Google Scholar]
- 24.Sensky T. Psychosomatic aspects of end-stage renal failure. Psychother Psychosom. 1993;59:56–68. doi: 10.1159/000288649. [DOI] [PubMed] [Google Scholar]
- 25.Hope J. A patient perspective on the barriers to home dialysis. J Ren Care. 2013;39:3–8. doi: 10.1111/j.1755-6686.2013.00333.x. [DOI] [PubMed] [Google Scholar]
- 26.Sheu J, Ephraim PL, Powe NR, et al. African American and non-African American patients’ and families’ decision making about renal replacement therapies. Qual Health Res. 2012;22:997–1006. doi: 10.1177/1049732312443427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harwood L, Clark AM. Dialysis modality decision-making for older adults with chronic kidney disease. J Clin Nurs. 2014;3:1–13. doi: 10.1111/jocn.12582. [DOI] [PubMed] [Google Scholar]
- 28.Sangha O, Stucki G, Liang MH, et al. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthrit Care Res. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 29.Miskulin DC, Meyer KB, Athienites NV, et al. Comorbidity and other factors associated with modality selection in incident dialysis patients: The CHOICE study. Am J Kidney Dis. 2002;39:324–336. doi: 10.1053/ajkd.2002.30552. [DOI] [PubMed] [Google Scholar]
- 30.Sherrill M. Self-serving bias. Am Psychol. 2007;41:954–969. [Google Scholar]
- 31.Hebert JR, Ma Y, Clemow L, et al. Gender differences in social desirability and social approval bias in dietary self-report. Am J Epidemiol. 1997;146:1046–1055. doi: 10.1093/oxfordjournals.aje.a009233. [DOI] [PubMed] [Google Scholar]
- 32.Tamura MK, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int. 2011;79:14–22. doi: 10.1038/ki.2010.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1993;70:41–55. [Google Scholar]
- 34.Simon D, Kriston L, Haerter M. Die deutsche modifizierte Fassung des Autonomie-Praeferenz-Index (API-Dm) Klin Diag Eval. 2011;4:5–14. [Google Scholar]
- 35.Bullinger M, Kirchberger I, Ware J. Der deutsche SF-36 Health Survey: Uebersetzung und psychometrische Testung eines krankheitsuebergreifenden Instruments zur Erfassung der gesundheitsbezogenen Lebensqualität. Z Gesundh Wiss. 1995;3:21–36. [Google Scholar]
- 36.Herrmann-Lingen C, Buss U, Snaith R. HADS-D: Hospital Anxiety and Depression Scale—Deutsche Version, 2nd edn. Bern, Swiss: Huber; 2005. [Google Scholar]
- 37.Schulz U, Schwarzer R. Soziale Unterstützung bei der Krankheitsbewältigung: Die Berliner Social Support Skalen (BSSS) Diagnostica. 2003;49:73–82. [Google Scholar]
- 38.Lamprecht J, Thyrolf A, Rennert D, et al. Deutsche Rentenversicherung Bund, ed. 22. Rehabilitationswissenschaftliches Kolloquium. Mainz, Germany: 2013. Struktur und Einfluss des sozialen Netzwerkes auf die sportliche Aktivitaet bei Frauen drei Monate nach Ende einer orthopaedischen oder onkologischen Rehabilitation; pp. 365–367. Teilhabe 2.0—Reha neu denken? [Google Scholar]
- 39.Rennert D, Mau W, Lamprecht J. Die nahestehende Person als Koproduzent des Rehabilitationserfolgs am Beispiel der Sportaktivitaet. Phys Rehab Kur Med. 2013;23:292–300. [Google Scholar]
- 40.Hays RD, Kallich J, Mapes DL, et al. Santa Monica, CA: Rand; 1997. Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3: A Manual for Use and Scoring. [Google Scholar]
- 41.Tischler L, Petermann F. Trail making test (TMT) Z Psychiatr Psychol Psychother. 2010;58:79–81. [Google Scholar]
- 42.Brickenkamp R, Schmidt-Atzert L, Liepmann D. Test d2—Revision: Aufmerksamkeits- und Konzentrationstest. Goettingen, Germany: Hogrefe; 2010. [Google Scholar]
- 43.Kriston L, Scholl I, Hoelzel L, et al. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. 2010;80:94–99. doi: 10.1016/j.pec.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 44.Rabin R, deCharro FD. EQ-SD: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 45.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 46.Streibelt M, Schmidt C, Bruenger M, et al. Komorbiditaet im Patientenurteil—geht das? Orthopade. 2012;41:303–310. doi: 10.1007/s00132-012-1901-3. [DOI] [PubMed] [Google Scholar]