Abstract
Chronic kidney disease (CKD) is one of the three causes of death that has had the highest increase in the last 20 years. The increasing CKD burden occurs in the context of lack of access of most of the world population to adequate healthcare and an incomplete understanding of the pathogenesis of CKD. However, CKD is not homogeneously distributed. CKD hotspots are defined as countries, region, communities or ethnicities with higher than average incidence of CKD. Analysis of CKD hotspots has the potential to provide valuable insights into the pathogenesis of kidney disease and to improve the life expectancy of the affected communities. Examples include ethnicities such as African Americans in the USA or Aboriginals in Australia, regions such as certain Balkan valleys or Central America and even groups of people sharing common activities or interests such as young women trying to lose weight in Belgium. The study of these CKD hotspots has identified underlying genetic factors, such as ApoL1 gene variants, environmental toxins, such as aristolochic acid and socioeconomic factors leading to nutritional deprivation and inflammation/infection. The CKD hotspots series of CKJ reviews will explore the epidemiology and causes in CKD hotspots, beginning with Australian Aboriginals in this issue. An online map of CKD hotspots around the world will feature the reviewed hotspots, highlighting known or suspected causes as well as ongoing projects to unravel the cause and providing a directory of public health officials, physicians and basic scientists involved in these efforts. Since the high prevalence of CKD in a particular region or population may only be known to local physicians, we encourage readers to propose further CKD hotspots to be reviewed.
Keywords: aristolochic acid, Balkan nephropathy, Chinese herbs, epidemiology, genetics, Mesoamerican nephropathy
Chronic kidney disease (CKD) is a worldwide problem, which overburdens healthcare systems and results in millions of deaths, years of life lost, years lived with disability and disability-adjusted life-years [1–4]. It is estimated that every year 3 200 000 people reach end-stage renal disease (ESRD) without initiating renal replacement therapy (RRT), while 440 000 do initiate RRT [5]. Indeed, according to the global burden of disease 2010 (GBD 2010) study, CKD was one of the three causes of death with the greatest increase from 1990 to 2010 among the top 20 killers [2]. The progressive ageing of the population in more developed countries, the emerging epidemic of obesity and diabetes in emerging countries and the identification of pockets of CKD in certain communities that either have appeared de novo or were previously unrecognized, contributed to the worldwide burden of CKD. In this regard, in Central and Andean Latin America, CKD is now the fifth most common cause of death [2, 6]. Mortality from CKD in central South America increased 350% in 20 years, from 1990 to 2010 [2, 6].
The increasing CKD burden occurs in the context of both a lack of access of most of the world population to adequate healthcare and an incomplete understanding of the pathogenesis of CKD that has prevented major advances in therapies to stop or reverse CKD progression in recent decades. Indeed, the mainstay of CKD therapy remains targeting the renin–angiotensin system with drugs first commercialized ∼40 years ago [7]. The analysis of CKD hotspots has the potential to both provide valuable insights into the pathogenesis of kidney disease and to improve the quality of life and life expectancy of the affected communities. CKD hotspots are defined as countries, region, communities or ethnicities with higher than average incidence of CKD when compared with the worldwide, country or regional rates. Examples include ethnicities such as African Americans in the USA or Aboriginals in Australia, regions such as certain Balkan valleys or Central America and even groups of people sharing common activities or interests such as young women trying to lose weight in Belgium [8–12]. As summarized below, the study of the epidemiology, clinical presentation and pathogenesis of these examples has already advanced our understanding of kidney disease and provides clues to decrease the burden of CKD. In this regard, genetic, environmental and socioeconomic factors have been identified as potential causative factors in diverse CKD hotspots around the world [9, 13, 14] Figure 1.
Fig. 1.
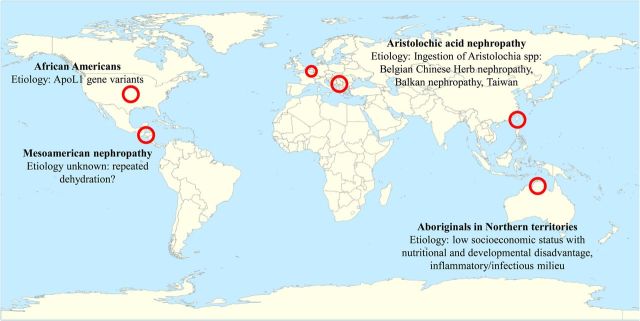
Some examples of CKD hotspots around the world and impact on our current understanding of CKD. By the end of the present CKJ review series, an interactive world map of CKD hotspots will be available at the CKJ website that will allow easy access to manuscripts in this series, and to information on ongoing research projects and epidemiological studies as well as contact information of physicians, researchers and health policy planners who wish to participate.
Genetics as an explanation for CKD hotspots
Adjusted incidence of ESRD has long been known to be roughly three times more frequent in Africans than in White Americans in the USA [8]. Indeed, adjusted incidence of hypertensive kidney disease in African Americans of sub-Saharan ancestry is 12-fold higher than in whites [15]. When the HIV epidemic struck, it became clear that the risk of ESRD in African Americans was not limited to traditional causes of CKD. Rather, there was a predisposition for kidney injury to progress for several unrelated causes of kidney injury. Thus, 24% of family members of HIV patients with ESRD had ESRD, even though they did not have HIV infection [16]. The initial tentative identification of MYH9 gene variants associated with a higher risk of CKD progression was followed by the identification of ApoL1 gene variants as the explanation for the higher sensitivity of African Americans to CKD progression [13]. MYH9 was a good candidate since mutations were known to cause a form of familiar nephropathy (Fechtner Syndrome) with deafness which could be confused with type IV collagen nephropathies (Alport syndrome) but that was additionally characterized by platelet abnormalities [17]. However, MYH9 variants initially associated with African American CKD were found to be in linkage disequilibrium with ApoL1 variants common in Western Africa, where they provided a genetic advantage by protecting from Trypanosoma brucei infection (sleeping sickness) [13]. Indeed, renal risk variants in APOL1 were associated with the higher rates of ESRD and progression of CKD observed in black patients as compared with white patients, regardless of diabetes status [13, 18]. ApoL1 is expressed by podocytes, tubular cells and vascular smooth muscle cells [19] and ongoing research will hopefully result in new therapeutic approaches to CKD of different aetiologies in African Americans. These studies, originally designed to solve a USA problem, may promote advances that benefit West African populations carrying these gene variants [20]. Furthermore, the discovery of ApoL1 variants linkage to CKD has shaken the concept of hypertensive nephropathy [21, 22]. If the most numerous group of hypertensive nephropathy patients in the USA has in fact a genetic predisposition to CKD, then how common is hypertensive nephropathy in non-Africans? Do cases of CKD attributed to hypertensive nephropathy indeed result from a primary kidney injury that promotes hypertension? And if this is the case, should blood pressure control be the mainstay of therapy in those patients or should we develop complementary therapeutic approaches?
Environmental factors underlying CKD hotspots
Balkan nephropathy had baffled researchers for decades. It was known to be an oligosymptomatic chronic nephropathy characterized by prominent tubulointerstitial damage, a need for RRT at around the sixth decade of life and a high incidence of upper urinary tract urothelial carcinoma [10]. Epidemiological studies have established the need for at least two decades of living in certain rural areas of certain valleys of Bosnia, Bulgaria, Croatia, Romania and Serbia. An environmental toxin was suspected and searched for, but not found. However, a breakthrough came from the study of a CKD hotspot among young Belgian women. These young women had in common a rapidly progressing CKD, reaching ESRD within months, with a renal biopsy characterized mainly by tubulointerstitial nephropathy and a high incidence of upper urinary tract urothelial carcinoma while on RRT [12]. There was also an unusual common feature between them: they were trying to lose weight by using Chinese herbs (hence the original term Chinese herb nephropathy). Plants of the Aristolochia family were components of these Chinese herbs and the toxin was identified as aristolochic acid [23]. Aristolochic acid now provides an animal model for acute kidney injury progressing to CKD [24]. The existence of an aristolochic acid exposure fingerprint, the presence of aristolactam-DNA adducts, provided the clue to the aetiology of Balkan nephropathy [25]. Renal biopsies from Balkan nephropathy patients displayed the characteristic aristolactam-DNA adducts. Contamination of harvests with Aristolochia weeds is now thought to lead to Balkan nephropathy. Thus, both Balkan nephropathy and Chinese herb nephropathy are now considered part of the clinical spectrum of aristolochic acid nephropathy [10]. Moreover, in Taiwan one-third of the population had been prescribed herbal remedies containing Aristolochia, and the recorded incidence of upper urinary tract cancers and the incidence of ESRD is the highest in the world [8, 25]. In this regard, the problem might also be present in mainland China, as supported by recent reports [26]. Interestingly Aristolochia spp are part of the traditional medicine armamentarium not only in Asia, but also in Mediterranean culture and South America. Aristolochia plants were used as a remedy for snake bites by the Romans and South American communities. Indeed, in 2004 Colombia outlawed the use of Aristolochia (commonly known in Spanish as ‘bejuco’), as a home remedy against rheumatism and snake bites [27]. To what extent the use of traditional medicine containing aristolochic acid or other toxins contributes to CKD in other cultures remains to be established.
Socioeconomic factors and CKD hotspots
The first review in this CKD hotspots series of CKJ reviews deals with CKD in the Australian Aboriginal population in the Northern Territories [9]. Wendy Hoy reports that with the high rate of CKD and ESRD in Aboriginals, albuminuria is used as a marker of CKD, and glomerulomegaly and focal glomerulosclerosis used as defining histologic features. In this regard, renin angiotensin system targeting, improved metabolic control and improved access to healthcare appear to be effective in decreasing ESRD incidence. Factors contributing to CKD may include low socioeconomic status with nutritional and developmental disadvantage, as well as an inflammatory/infectious milieu. The importance of socioeconomic factors cannot be downplayed in this and other CKD hotspots around the world. How exactly socioeconomic factors contribute to CKD should be explored in each different location. If overall socioeconomic conditions cannot be improved, and this may be the case when the global economy is in trouble, correction of specific factors may alleviate the CKD burden. Is it the lack of education and non-compliance with doctors recommendations? Lack of access to medical care? Lack of access to nephroprotective drugs? Excessive use of over-the-counter nephrotoxic drugs? Dietary or traditional medicine habits that promote kidney injury or developmental defects? Poor sanitation and contaminated drinking water? Infection-related kidney injury? Each specific problem may require a different intervention that may also differ for different economically deprived communities.
Mysteries still to be solved: is watering the answer?
Most recently, the existence of a nephropathy of unknown cause leading to ESRD has been identified in Central America and termed Mesoamerican nephropathy [11]. Mesoamerican nephropathy mainly affects, otherwise healthy, young men working in rural populations in Central America and is characterized by asymptomatic, progressive CKD, absent or sub-nephrotic proteinuria, the absence of haematuria and, typically, no evidence of diabetes or hypertension. Four key community-based studies in Nicaragua (three studies) and El Salvador (one study) have shed some light on the problem [28–31]. In Nicaragua the overall frequency of CKD, defined as eGFR < 60 mL/min/1.73 m2 was 20% in men and 8% in women in a study population with a mean age ∼35 years [28, 29]. These figures contrast with the 5% prevalence of CKD in the general population in US and European surveys. El Salvador has the highest overall mortality rate from CKD in the world, with 51.8 deaths per 100 000 inhabitants. [32]. Since 1989, increasing mortality rates from CKD have been reported in Guatemala, Costa Rica, Honduras and Panama, especially in Pacific coast communities. The prevalence was higher among agricultural workers, miners and fishermen in comparison with service-oriented workers. In particular, low altitude plantation workers, such as banana or sugarcane workers, are the most affected. There were no significant differences between populations regarding traditional risk factors for CKD, such as hypertension, diabetes, obesity and use of NSAIDs.
Analysis of the epidemiology of Mesoamerican nephropathy has rekindled the interest on the adverse effects of dehydration. The leading hypothesis suggests that Mesoamerican nephropathy results from repeated, unrecovered episodes of pre-renal acute kidney injury due to dehydration, volume depletion and rhabdomyolysis, caused by the severe heat stress and water and solute loss during agricultural work or mining [33]. In addition to this, nephrotoxic agents, such as non-steroidal anti-inflammatory drugs and other nephrotoxic medication use, inorganic arsenic, leptospirosis, or pesticides, are likely to aggravate kidney damage [34]. Animal studies suggest that recurrent dehydration may activate the local polyol pathway, resulting in the generation of endogenous fructose that might subsequently induce renal injury via metabolism by fructokinase [35]. In this regard, rehydration with solutions containing high fructose corn syrup, which also contains nephrotoxic compounds such as 3,4-dideoxyglucosone-3-ene (3,4-DGE) [36–38] may also be deleterious. Access to sufficient water during the dehydration period can protect mice from developing renal injury [35], suggesting a potential role of vasopressin. In this regard, vasopressin antagonists are protective in another form of progressive tubulointerstitial disease, autosomal dominant polycystic kidney disease [39]. Whether similar pathogenic events may contribute to the high prevalence of CKD in agricultural workers in Sri Lanka, Bangladesh and central Australia remains to be explored [33, 40, 41].
A call to action
In summary, the CKD hotspots series will devote reviews to countries, region, communities or ethnicities with higher than average incidence of CKD. These reviews will report on the epidemiology, underlying causes or efforts to unravel the causes if unknown, provide guidance on public policy and even be extrapolated to promote kidney health in other parts of the world. A final manuscript in the series will summarize the series and provide an online interactive map of CKD hotspots around the world, which highlights known or suspected causes as well as ongoing projects to unravel the cause. Overall, the series will provide a tool for healthcare authorities to plan public policy, for translational researchers with know-how and means to explore causes to identify CKD hotspots and contact the professionals fighting CKD in remote and not so remote regions of the world, and for these healthcare personnel to identify similarly minded professionals working on other CKD hotspot environments. The online tool will provide a directory of public health officials, on the ground physicians and interested basic scientists involved in these efforts, that will facilitate communication and foster collaborations in search of resources to pursue research and shape policies that help curtail this scourge.
A potential hurdle to be overcome is that much of the publicly available information refers to prevalence of ESRD in whole countries. Several factors may impact on incidence of ESRD that do not allow an easy extrapolation to prevalence of CKD [5]. Thus, policies for inclusion into an ESRD programme differ between countries and ESRD may be initiated at available facilities that may be far from the patient residence. Emerging information on CKD prevalence is also for the most part country- or region-based and efforts to compare prevalence between different regions in a country do not abound. In Colombia, the Cuenta del Alto Costo has generated detailed regional maps displaying differences in CKD prevalence (http://www.cuentadealtocosto.org/byblos/Docs/SITUACION_DE_LA_ENFERMEDAD_RENAL_CRONICA_2013.pdf). An ongoing European Renal Association-European Dialysis and Transplant Association Registry effort [Wanner C, presented at the 49th congress of the Spanish Society of Nephrology (SEN), Barcelona 2014] is combining and homogenizing several observational studies of CKD prevalence in European countries. This will allow the identification of European CKD hotspots. However, we realize that sometimes the high incidence of CKD in a particular country, region or even town may only be known to local authorities or healthcare personnel. We thus encourage readers to write to CKJ reporting such pockets of CKD. This may highlight the plea of that community, be the subject of a review in CKJ and facilitate the flow of resources to clarify the cause and identify potential solutions to the problem.
Acknowledgements
FIS PS09/00447, PI13/00047, ISCIII-RETIC REDinREN RD12/0021, Comunidad de Madrid S2010/BMD-2378, CYTED IBERERC. Programa Intensificación Actividad Investigadora (ISCIII) to A.O.
Conflict of interest statement. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Ortiz A, Covic A, Fliser D, et al. Board of the EURECA-m Working Group of ERA-EDTA. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 5.Anand S, Bitton A, Gaziano T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS One. 2013;8:e72860. doi: 10.1371/journal.pone.0072860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. http://www.healthmetricsandevaluation.org/gbd/visualizations/gbd-arrow-diagram. (30 July 2013, date last accessed)
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 8. http://www.usrds.org/2011/pdf/v2_ch01_11.pdf. (20 November 2013, date last accessed)
- 9.Hoy W. Kidney Disease in Aboriginal Australians: a perspective from the Northern Territory. Clin Kidney J. 2014;7:524–530. doi: 10.1093/ckj/sfu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gökmen MR, Cosyns JP, Arlt VM, et al. The epidemiology, diagnosis, and management of aristolochic acid nephropathy: a narrative review. Ann Intern Med. 2013;158:469–477. doi: 10.7326/0003-4819-158-6-201303190-00006. [DOI] [PubMed] [Google Scholar]
- 11.Correa-Rotter R, Wesseling C, Johnson RJ. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanherweghem J-L, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 13.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grollman AP, Shibutani S, Moriya M, et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci U S A. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. http://www.usrds.org/2010/pdf/V2_02.pdf. (20 November 2013, date last accessed)
- 16.Freedman BI, Soucie JM, Stone SM, et al. Familial clustering of end-stage renal disease in blacks with HIV-associated nephropathy. Am J Kidney Dis. 1999;34:254–258. doi: 10.1016/s0272-6386(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 17.Seri M, Cusano R, Gangarossa S, et al. Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. The May-Heggllin/Fechtner Syndrome Consortium. Nat Genet. 2000;26:103–105. doi: 10.1038/79063. [DOI] [PubMed] [Google Scholar]
- 18.Parsa A, Kao WH, Xie D, et al. AASK Study Investigators; CRIC Study Investigators. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madhavan SM, O'Toole JF, Konieczkowski M, et al. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman BI, Langefeld CD. The new era of APOL1-associated glomerulosclerosis. Nephrol Dial Transplant. 2012;27:1288–1291. doi: 10.1093/ndt/gfr812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman BI, Sedor JR. Hypertension-associated kidney disease: perhaps no more. J Am Soc Nephrol. 2008;19:2047–2051. doi: 10.1681/ASN.2008060621. [DOI] [PubMed] [Google Scholar]
- 23.Cosyns JP. Aristolochic acid and ‘Chinese herbs nephropathy’: a review of the evidence to date. Drug Saf. 2003;26:33–48. doi: 10.2165/00002018-200326010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grollman AP. Aristolochic acid nephropathy: harbinger of a global iatrogenic disease. Environ Mol Mutagen. 2013;54:1–7. doi: 10.1002/em.21756. [DOI] [PubMed] [Google Scholar]
- 26.Shaohua Z, Ananda S, Ruxia Y, et al. Fatal renal failure due to the Chinese herb ‘GuanMu Tong’ (Aristolochia manshuriensis): autopsy findings and review of literature. Forensic Sci Int. 2010;199:e5–e7. doi: 10.1016/j.forsciint.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 27. http://www.agenciadenoticias.unal.edu.co/nc/ndetalle/pag/5/article/aristolochia-the-forbidden-flower.html. (12 October 2013, date last accessed)
- 28.O'Donnell JK, Tobey M, Weiner DE, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26:2798–2805. doi: 10.1093/ndt/gfq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres C, Aragón A, González M, et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Wijkström J, Leiva R, Elinder CG, et al. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis. 2013;62:908–918. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Raines N, González M, Wyatt C, et al. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev. 2014;16:16–22. doi: 10.37757/MR2014.V16.N2.4. [DOI] [PubMed] [Google Scholar]
- 32. http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html. (12 October 2014, date last accessed)
- 33.Johnson RJ, Sánchez-Lozada LG. Chronic kidney disease: Mesoamerican nephropathy—new clues to the cause. Nat Rev Nephrol. 2013;9:560–561. doi: 10.1038/nrneph.2013.174. [DOI] [PubMed] [Google Scholar]
- 34.Wesseling C, Crowe J, Hogstedt C, et al. First International Research Workshop on the Mesoamerican Nephropathy. Resolving the enigma of the mesoamerican nephropathy: a research workshop summary. Am J Kidney Dis. 2014;63:396–404. doi: 10.1053/j.ajkd.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Roncal Jimenez CA, Ishimoto T, Lanaspa MA, et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014;86:294–302. doi: 10.1038/ki.2013.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Justo P, Sanz AB, Egido J, et al. 3,4-Dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes. 2005;54:2424–2429. doi: 10.2337/diabetes.54.8.2424. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Niño MD, Poveda J, Sanz AB, et al. 3,4-DGE is cytotoxic and decreases HSP27/HSPB1 in podocytes. Arch Toxicol. 2014;88:597–608. doi: 10.1007/s00204-013-1181-7. [DOI] [PubMed] [Google Scholar]
- 38.Gensberger S, Mittelmaier S, Glomb MA, et al. Identification and quantification of six major α-dicarbonyl process contaminants in high-fructose corn syrup. Anal Bioanal Chem. 2012;403:2923–2931. doi: 10.1007/s00216-012-5817-x. [DOI] [PubMed] [Google Scholar]
- 39.Torres VE, Chapman AB, Devuyst O, et al. TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanigasuriya K. Update on uncertain etiology of chronic kidney disease in Sri Lanka's north-central dry zone. MEDICC Rev. 2014;16:61–65. doi: 10.37757/MR2014.V16.N2.10. [DOI] [PubMed] [Google Scholar]
- 41.Jayatilake N, Mendis S, Maheepala P, et al. on behalf of the CKDu National Research Project Team. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]


