Abstract
Background:
Left ventricular (LV) twist is due to oppositely directed apical and basal rotation and has been proposed as a sensitive marker of LV function. We sought to assess the impact of chronic pure mitral regurgitation (MR) on the torsional mechanics of the left human ventricle using tissue Doppler imaging.
Methods:
Nineteen severe MR patients with a normal LV ejection fraction and 16 non-MR controls underwent conventional echocardiography and apical and basal short-axis color Doppler myocardial imaging (CDMI). LV rotation at the apical and basal short-axis levels was calculated from the averaged tangential velocities of the septal and lateral regions, corrected for the LV radius over time. LV twist was defined as the difference in LV rotation between the two levels, and the LV twist and twisting/untwisting rate profiles were analyzed throughout the cardiac cycle.
Results:
LV twist and LV torsion were significantly lower in the MR group than in the non-MR group (10.38° ± 4.04° vs. 13.95° ± 4.27°; p value = 0.020; and 1.29 ± 0.54 °/cm vs. 1.76 ± 0.56 °/cm; p value = 0.021, respectively), both suggesting incipient LV dysfunction in the MR group. Similarly, the untwisting rate was lower in the MR group (−79.74 ± 35.97 °/s vs.−110.96 ± 34.65 °/s; p value = 0.020), but there was statistically no significant difference in the LV twist rate.
Conclusion:
The evaluation of LV torsional parameters in MR patients with a normal LV ejection fraction suggests the potential role of these sensitive variables in assessing the early signs of ventricular dysfunction in asymptomatic patients.
Keywords: Elasticity imaging techniques, Heart ventricles, Mitral valve insufficiency
Introduction
Chronic primary mitral regurgitation (MR) due to myxomatous mitral valve disease is an important cause of morbidity and mortality.1 It causes progressive left ventricular (LV) dilatation and irreversible myocardial damage and is associated with eccentric hypertrophy and complex LV adaptive remodeling.2 This remodeling includes an increase in LV end-diastolic diameter, LV end-diastolic volume, LV end-systolic volume index, and LV mass index.3 In MR patients with reduced afterload, a greater proportion of the contractile energy of the myocardium is expended in shortening than in tension development and the LV adapts to the load imposed by MR and, therefore, exhibits elevation in the ejection phase indices of myocardial contractility such as ejection fraction (EF), fractional fiber shortening, and the velocity of circumferential fiber shortening despite impaired myocardial function.4
LV torsion has recently attracted a great deal of attention to LV mechanics and is believed to be a sensitive indicator of LV systolic and diastolic performance and a marker of incipient LV dysfunction. Torsion of the LV is the wringing motion of the heart around its long axis,5 determined by contracting myofibers in the LV wall arranged in opposite directions between the subendocardial and subepicardial layers.6–9 This motion is essential for regulating LV systolic and diastolic functions.10, 11 Different methods have been utilized for the assessment of LV torsion such as cineangiographic markers,12 rotational optical devices,13 echocardiography,8, 14 and tagged myocardial resonance imaging (MRI). The latter is the gold standard, but its low availability, low temporal resolution, and cost have remained its major limitations.8, 14, 15 Echocardiography is a widely available method inasmuch as it is more feasible for bedside assessment and is a practical technique in patients with implantable cardioverter-defibrillators (ICD) and cardiac resynchronization devices (CRT).2 This should explain the increasing interest in the development of different techniques in the assessment of LV twist and torsion via echocardiography.
The existing literature contains only a few studies on the impact of chronic pure MR on the left human ventricle twist and torsional mechanics.1, 3, 16 The aim of the present study was to evaluate the impact of severe MR due to myxomatous mitral valve (MV) on LV twist, LV torsion, and such related parameters as the twisting rate and the untwisting rate using tissue Doppler imaging (TDI) and to test the hypothesis that in chronic MR patients, global LV torsion in the subclinical phase of LV systolic dysfunction is lower than that in controls, before the other indices of LV systolic function (e.g. left ventricular ejection fraction [LVEF]) are impaired.
Methods
This case-control study evaluated all asymptomatic patients with severe MR due to myxomatous MV disease referred to Rajaei Cardiovascular, Medical and Research Center in Tehran between June 2012 and November 2012. Based on our inclusion and exclusion criteria, we included 19 patients. The control group (16 participants) comprised adults with no history of cardiovascular disease and normal physical examination, electrocardiography (ECG), and resting echocardiography; however, if the member was older than 40 years or had risk factor(s) for coronary artery disease, he/she was included if stress echocardiography was normal. The exclusion criteria in both patient and control groups were atrial fibrillation (AF) rhythm, previous cardiac surgery, LVEF < 60% in the MR group, ischemic heart disease, diabetes, and bundle branch block (BBB). The study was approved by the institutional Ethics Committee on Human Research, and informed consent was obtained from the all the participants.
Two-dimensional (2D) conventional, pulse, and tissue Doppler transthoracic echocardiography was performed with commercial GE Vivid 7 system (Horten, Norway), equipped with an M3S multi-frequency harmonic phased array transducer. Images were acquired with the subjects at rest, lying in the lateral supine position at the end of expiration, and 2D ECG was superimposed on the images and end-diastole was considered at the peak R-wave of the ECG.
MR severity was determined in accordance with the recommendations of the American Society of Echocardiography (ASE) using quantitative parameters such as vena contracta (VC > 7 mm) and effective regurgitant orifice area (EROA ≥ 0.4 cm2). If there was a discrepancy between the findings, regurgitant volume (RV > 65 ml) was calculated.17 LV global systolic function was evaluated using the modified biplane Simpson method for calculating LVEF by measuring end-diastolic and endsystolic volumes in the 2D images.
Using standard parasternal short-axis views in two base and apical levels, color Doppler myocardial imaging (CDMI) was recorded throughout three cardiac cycles according to the guidelines of the ASE. An appropriate velocity scale was chosen to avoid CDMI data aliasing, and the sector angle was adjusted to ensure the highest possible sampling frequency. Meticulous attention was paid so as to maintain the anterior and posterior LV segments perpendicular to the ultrasound beam and keep it aligned at as near zero degrees to the radial motion as possible, and the images were stored digitally in cine-loop format in the memory of the scanner.
The digitally stored CDMI data sets were processed off-line using the Echo Pac quantitative analysis software, equipped to obtain regional myocardial velocity. The tissue velocity imaging analysis with 8-mm volume samples was performed from the anterior and posterior segments of the LV walls to extract radial velocity-time curves and the lateral and septal segments to extract the tangential component of velocity in both basal and apical short-axis levels.
The velocity-time data of each sample throughout the cardiac cycle were saved on compact disc (CD) using the system CD writer and were transferred to a spreadsheet Excel 2003 program for the basal and apical rotations, LV twist, twist rate, and torsion calculations. All the calculations were averaged for at least three consecutive heart beats.
Tangential velocity (cm/s) was converted into angular velocity (degree/s) by estimating the time-varying radius of the LV [R(t)] both at the basal and apical levels using the anterior and posterior velocity data sets:18
where Va and Vp were the myocardial velocity at the anterior and posterior regions and R(0) was the end-diastolic radius.
From the lateral and septal velocity data sets, LV rotational velocity was estimated from the averaged tangential velocity, corrected with R(t), as follows:
where Vl and Vs were myocardial velocity at the lateral and septal regions and Vrot(t) was LV rotational velocity (degree/s) both at the basal and apical levels.
The LV twist rate (degrees/s) was calculated as follows:
where Apical Vrot(t) and Basal Vrot(t) were rotational velocity (degree/s) at the apical and basal levels, respectively.
LV rotation (degrees) at the basal and apical levels was calculated as follows:
LV twist (degrees) was calculated as follows:
The peak systolic twist, peak twisting, and peak untwisting rates were measured as is demonstrated in Figures 1 and 2. LV torsion was calculated as LV twist divided by LV diastolic longitudinal length. In addition, the peak twisting and untwistingrates were normalized through their division by LV diastolic longitudinal length.
Figure 1.
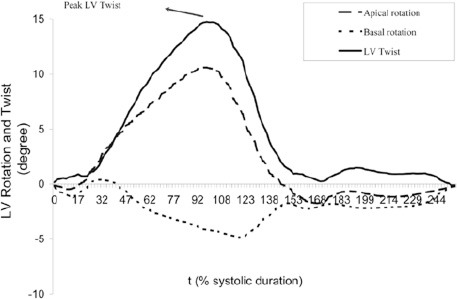
Left ventricular (LV) rotation at the basal and apical levels and left ventricular twist and untwisting throughout one cardiac cycle. End-diastolic drift was compensated by assuming constant estimation error over the cardiac cycle
Figure 2.
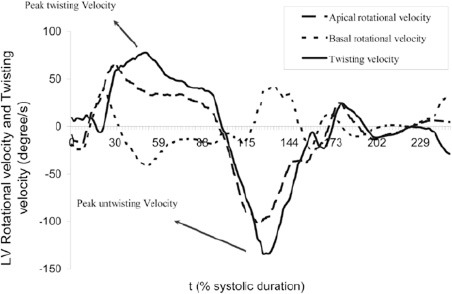
Left ventricular (LV) rotational velocities at the basal and apical levels and left ventricular twisting and untwisting velocities throughout one cardiac cycle
The data were tested for normal distribution via the D’Agostino-Pearson test and graphical methods. All the continuous variables with a normal distribution are presented as mean ± standard deviation (SD) and were compared between the two study groups by independent-samples t-test. The Mann-Whitney test was used to test the significance of the difference between two independent samples when the distribution of the samples was not normal. The categorical variables were compared using the chi-squared test. A p value < 0.05 was considered statistically significant. All the statistical analyses were conducted using SPSS 13 (SPSS Inc. Chicago, IL, USA) and Med Calc 9.2 software package (Med Calc Inc. Maria Kerke, Belgium).
Results
A total of 19 asymptomatic patients (mean age = 49.5 ± 14.1 years; 11 men and 8 women) with severe MR due to myxomatous MV disease and 16 non-MR controls (mean age = 41.1 ± 12.8 years; 6 men and 10 women) were enrolled. The clinical characteristics and echocardiographic data of the two groups are summarized in Table 1 and Table 2, respectively. The mean age, heart rate, systolic blood pressure, diastolic blood pressure, and body surface area of the two groups were not statistically significant. Maximal LA volume, volume index, LV end-diastolic diameter (LVEDD), EDD index, LV end-systolic diameter (LVESD), ESD index, and LVEF were significantly greater in the patients with MR than in the non-MR group.
Table 1.
Demographic and hemodynamic characteristics of the study participants*
| Variable | MR Group (n=19) | Non-MR Group (n=16) | P value |
|---|---|---|---|
| Gender (Male/Female) | 11/8 | 6/10 | 0.229 |
| BSA (m2) | 1.8 (1.7–1.9) | 1.8 (1.7–1.8) | 0.337 |
| Age (y) | 49.5±14.1 | 41.1±12.8 | 0.077 |
| Heart rate (beats/min) | 83.8±11.8 | 77.1±15.4 | 0.164 |
| SBP (mmHg) | 120.4±12.7 | 124.9±15.7 | 0.355 |
| DBP (mmHg) | 89.3±27.4 | 78.9±14.2 | 0.162 |
Data are presented as proportion, mean±SD, or Median (interquartile range [IQR])
MR, Mitral regurgitation; BSA, Body surface area; SBP, Systolic blood pressure; DBP, Diastolic blood pressure
Table 2.
Resting echocardiographic characteristics of the study participants
| Variable | MR Group (n=19) | Non-MR Group (n=16) | P Value |
|---|---|---|---|
| LA volume (ml) | 111.1±34.0 | 45.0±7.8 | < 0.001 |
| LA volume/BSA (ml/m2) | 60.9±20.9 | 25.5±4.1 | < 0.001 |
| LVEDD (cm) | 5.8±0.5 | 4.6±0.3 | < 0.001 |
| LVEDD/BSA (cm/m2) | 3.2±0.3 | 2.6±0.2 | < 0.001 |
| LVESD (cm) | 3.7±0.5 | 3.2±0.4 | 0.003 |
| LVESD/BSA (cm/m2) | 2.1±0.3 | 1.8±0.3 | 0.014 |
| LVEF (%) | 69.4±6.1 | 59.2±4.2 | < 0.001 |
Data are presented as mean±SD
MR, Mitral regurgitation; LA, Left atrium; BSA, Body surface area; LVEDD, Left ventricular end-diastolic diameter; LVESD, Left ventricular end-systolic diameter; LVEF, Left ventricular ejection fraction
Peak LV twist was significantly lower in the MR group than in the non-MR group (10.38° ± 4.04° vs. 13.95° ± 4.27°; p value = 0.020). Also, LV peak systolic torsion was significantly lower in the MR group (1.29 ± 0.54 °/cm vs. 1.76 ± 0.56 °/cm; p value = 0.021), both suggesting incipient LV dysfunction in the MR group.
The peak LV untwisting rate was significantly lower in the MR group than in the non-MR group (−79.74 ± 35.97 °/s vs. −110.96 ± 34.65 °/s; p value = 0.020), but there was no significant difference in the LV twisting rate (79.53 ± 33.07 °/s vs. 95.79 ± 24.87 °/s; p value = 0.111). When the peak untwisting rate was normalized by the LV length, there was a significant difference between the study groups (−9.85 ± 4.49 °/s/cm in the MR group vs. −14.02 ± 4.92 °/s/cm in the non-MR group; p value = 0.021), but not in the peak twist rate normalized by the LV length (9.97 ± 4.61 °/s/cm in the MR group vs. 12.09 ± 3.42 °/s/cm in the non-MR group; p value = 0.133).
Discussion
MR is increasingly prevalent and represents an important cause of morbidity and mortality. The present study assessed the torsional mechanics of the left human ventricle in patients with chronic severe MR and normal LV systolic function using TDI and compared them with those in non-MR controls. The principal findings of this study were that the LV twist, torsion, untwist, and untwist rates, normalized by the LV length, were significantly lower in the severe MR patients, whereas the twisting rate, normalized by the LV length, did not differ significantly.
Echocardiography is the primary noninvasive method for the detection of MR, elucidation of its mechanism, and quantification of its severity. At present, echocardiography is a useful guide for the timing of valve surgery. In asymptomatic patients, valve surgery is considered when there is progressive LV dilation or EF reduction. Simple, available, accurate, and inexpensive methods for the measurement of LV torsion could facilitate more widespread investigation of LV function in routine clinical application. Using the echocardiographic methods, LV twist was initially measured by studying the rotational motion of the papillary muscles,19 followed by measuring rotational mechanics using TDI,18 2D speckle tracking echocardiography, and velocity vector imaging (VVI). 1, 7, 8, 14 In this study, we used TDI to investigate LV torsional parameters in MR patients and to compare them with those in non-MR controls. Recently, it has been shown that LV torsional deformation may be assessed accurately by TDI with better temporal resolution than MRI and echocardiographic 2D-based methods.18 In this study, we included asymptomatic patients with a normal EF and severe MR. Subclinical LV dysfunction is widely recognized a challenging issue in the management of severe asymptomatic MR and the determination of the appropriate time for surgical intervention. We employed a completely different method, originally based on TDI-derived data, for the detection of torsional abnormalities in these patients.
There are different terms for explaining the wringing motion of the LV.20–22 By all accounts, however, twist and torsion both refer to the same phenomenon in cardiac function and are defined as the base-to-apex gradient in the rotational angle along the longitudinal axis of the LV.2, 23 The present study explained the wringing motion of the LV not only by twist, twist rate, and untwist rate but also by normalized twist, twisting rate, and untwist rate according to the LV length.
Normal LV torsion is a component of systolic function and contributes to an efficient ejection. Limited studies have assessed the impact of chronic pure MR on LV twist and torsion. An experimental canine model showed that progressive MR from acute to chronic could decrease systolic twist24 and suggested that a decrease in systolic twist was due to a decreased leverage of the epicardial fibers relative to the endocardial muscle fibers.2 Borg et al.16 evaluated the correlation between MR severity and speckle-based torsional parameters and found that chronic MR caused significant slowing of LV untwisting and suggested the potential role of torsional variables in assessing the early signs of ventricular dysfunction. Moustafa et al.1 evaluated the effect of MR severity on LV function using VVI and while they displayed a significant difference in the peak twist and torsion of the LV endocardium between the study groups, they found no difference in epicardial LV rotation. Similarly in our study using the TDI method, we found significant differences in the myocardial peak twist, torsion, and untwist rates but no significant differences in the peak twist rate or peak twist rate normalized by the LV length (achieved power = 0.32 and 0.35, respectively).
The Dong et al.25 study demonstrated that an increased preload increased the systolic twist rate. But why does chronic MR tend to reduce systolic twist and torsional parameters? We are inclined to believe that chronic MR correlates with LV adaptive remodeling and this remodeling is associated with subclinical LV dysfunction. Furthermore, as was described previously, the extent of the impairment of these variables depends on the stage of the disease.16 Nakai et al.22 reported that torsion was related to fiber orientation and it, therefore, might depict subclinical abnormality in cardiac function. A decline in these parameters is thought to be secondary to the deterioration of LV function; this notion has been borne out by other studies on patients with impaired LV systolic function. 5, 26–31 Consequently, we would think that ventricular torsion is a sensitive marker of dysfunction and is a useful clinical measure for the early recognition of subclinical LV dysfunction before the other indices of systolic and diastolic functions are impaired. In this study, we assessed the impact of chronic pure MR on the torsional mechanics of the left human ventricle using TDI and evaluated these parameters as a novel index for detecting subclinical latent LV dysfunction in chronic primary MR patients. This could provide a guide to optimize the time of surgery.
In this study, although there were statistically no significant differences in the LV twisting rate and the normalized peak twist rate between the study groups, the differences between the two groups seem to be important clinically (LV twisting rate = 79.53 ± 33.07 °/s in the MR group vs. 95.79 ± 24.87 °/s in the non-MR group and normalized peak twist rate = 9.97 ± 4.61 °/s/cm in the MR group vs. 12.09 ± 3.42 °/s/cm in the non-MR group). The achieved power for the twisting rate and the normalized twisting rate was 0.32 and 0.35, respectively. Nevertheless, these parameters might have been influenced by the small sample size and, thus, need to be evaluated by future studies with larger samples.
The TDI method for the calculation of torsion has been validated against MRI.18 However, it should be noted that the use of the TDI method for torsional analysis is time-consuming. In addition, the reported value of LV twist and torsion might have been slightly underestimated because of the through-plane motion and noise in the velocity data.
Aging in heart failure patients with a normal EF is associated with increased LV torsion secondary to reduced rotational deformation delay and increased peak basal rotation.32 Gustafsson et al.33 demonstrated that there was no significant difference in peak rotation at the basal and apical levels between men and women in healthy individuals. In the present study, we did not evaluate the effects of sex and age on torsional parameters in MR patients and these points require future studies to evaluate the effects of sex and age on torsional parameters in these patients.
Conclusion
The evaluation of LV torsional parameters such as the LV twist, torsion, untwist, and normalized untwist rates in MR patients with a normal LVEF suggests the potential role of these sensitive variables in assessing the early signs of ventricular dysfunction in asymptomatic patients.
Acknowledgments
We would like to thank all the medical staff at the Echocardiography Department of Rajaei Cardiovascular, Medical and Research Center for their invaluable assistance. This study was supported by Iran University of Medical Sciences.
References
- 1. Moustafa SE, Kansal M, Alharthi M, Deng Y, Chandrasekaran K, Mookadam F. Prediction of incipient left ventricular dysfunction in patients with chronic primary mitral regurgitation: a velocity vector imaging study. Eur J Echocardiogr 2011; 12: 291–298. [DOI] [PubMed] [Google Scholar]
- 2. Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. J Am Coll Cardiol 2008; 1: 366–376. [DOI] [PubMed] [Google Scholar]
- 3. Ennis DB, Nguyen TC, Itoh A, Bothe W, Liang DH, Ingels NB, Miller DC. Reduced systolic torsion in chronic pure mitral regurgitation. Circ Cardiovasc Imaging 2009; 2: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otto CM, Bonnow RO. Valvular heart disease: mitral regurgitation. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: a Textbook of Cardiovascular Medicine. 9th ed Philadelphia: Elsevier Science; 2011. p. 1499–1514. [Google Scholar]
- 5. Shawa SM, Foxa DJ, Williams SG. The development of left ventricular torsion and its clinical relevance. Int J Cardiol 2008; 130: 319–325. [DOI] [PubMed] [Google Scholar]
- 6. Thomas JD, Popovic ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol 2006; 48: 2012–2025. [DOI] [PubMed] [Google Scholar]
- 7. Notomi Y, Lysyansky P, Setser RM, Shiota T, Popović ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, Thomas JD. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol 2005; 45: 2034–2041. [DOI] [PubMed] [Google Scholar]
- 8. Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH, Smith HJ, Rosen BD, Lima JA, Torp H, Ihlen H, Smiseth OA. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation 2005; 112: 3149–156. [DOI] [PubMed] [Google Scholar]
- 9. Burns AT, McDonald IG, Thomas J, MacIsaac AI, Prior DL. Doing the twist–new tools for an old concept of myocardial function. Heart 2008; 94: 978–983. [DOI] [PubMed] [Google Scholar]
- 10. Takeuchi M, Otsuji Y, Lang RM. Evaluation of left ventricular function using left ventricular twist and torsion parameters. Curr Cardiol Rep 2009; 11: 225–230. [DOI] [PubMed] [Google Scholar]
- 11. Nakai H, Takeuchi M, Nishikage T, kokumai M, Otani M, Lange R. Effect of aging on twist-displacement loop by 2-dimensional speckle tracking imaging. J Am Soc Echocardiogr 2006; 19: 880–885. [DOI] [PubMed] [Google Scholar]
- 12. Ingels NB, Jr, Hansen DE, Daughters GT, 2nd, Stinson EB, Alderman EL, Miller DC. Relation between longitudinal, circumferential, and oblique shortening and torsional deformation in the left ventricle of the transplanted human heart. Circ Res 1989; 64: 915–927. [DOI] [PubMed] [Google Scholar]
- 13. Gibbons Kroeker CA, Ter Keurs HE, Knudtson ML, Tyberg JV, Beyar R. An optical device to measure the dynamics of apex rotation of the left ventricle. Am J Physiol 1993; 265: 1444–1449. [DOI] [PubMed] [Google Scholar]
- 14. Kim HK, Sohn DW, Lee SE, Choi SY, Park JS, Kim YJ, Oh BH, Park YB, Choi YS. Assessment of left ventricular rotation and torsion with two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr 2007; 20: 45–53. [DOI] [PubMed] [Google Scholar]
- 15. Yoon AJ, Bella JN. New options in noninvasive assessment of left ventricular torsion. Future Cardiol 2009; 5: 51–61. [DOI] [PubMed] [Google Scholar]
- 16. Borg AN, Harrison JL, Argyle RA, Ray SG. Left ventricular torsion in primary chronic mitral regurgitation. Heart 2008; 94: 597–603. [DOI] [PubMed] [Google Scholar]
- 17. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ, American Society of Echocardiography Recommendation for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003; 16: 777–802. [DOI] [PubMed] [Google Scholar]
- 18. Notomi Y, Setser RM, Shiota T, Martin-Miklovic MG, Weaver JA, Popović ZB, Yamada H, Greenberg NL, White RD, Thomas JD. Assessment of left ventricular torsional deformation by Doppler tissue imaging: validation study with tagged magnetic resonance imaging. Circulation 2005; 111: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 19. Rothfeld JM, LeWinter MM, Tischler MD. Left ventricular systolic torsion and early diastolic filling by echocardiography in normal humans. Am J Cardiol 1998; 81: 1465–1469. [DOI] [PubMed] [Google Scholar]
- 20. Buchalter MB, Weiss JL, Rogers WJ, Zerhouni EA, Weisfeldt ML, Beyar R, Shapiro EP. Noninvasive quantification of left ventricular rotational deformation in normal humans using magnetic resonance imaging myocardial tagging. Circulation 1990; 81: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 21. Sorger JM, Wyman BT, Faris OP, Hunter WC, McVeigh ER. Torsion of the left ventricle during pacing with MRI tagging. J Cardiovasc Magn Res 2003; 5: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russel IK, Gotte MJW, Bronzwaer JG, Knaapen P, Paulus WJ, Van Rossum AC. Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. J Am Coll Cardiol 2009; 2: 648–655. [DOI] [PubMed] [Google Scholar]
- 23. Henson RE, Song SK, Pastorek JS, Ackerman JJ, Lorenz CH. Left ventricular torsion is equal in mice and humans. Am J Physiol Heart Circ Physiol 2000; 278: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 24. Van Der Toorn A, Barenbrug P, Snoep G, Van Der Veen FH, Delhaas T, Prinzen FW, Maessen J, Arts T. Transmural gradients of cardiac myofiber shortening in aortic valve stenosis patients using MRI tagging. Am J Physiol Heart Circ Physiol 2002; 283: 1609–1615. [DOI] [PubMed] [Google Scholar]
- 25. Dong SJ, Hees PS, Huang WM, Buffer SA, Weiss JL, Shapiro EP. Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol 1999; 277: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 26. Garot J, Pascal O, Diébold B, Derumeaux G, Gerber BL, Dubois-Randé JL, Lima JA, Guéret P. Alterations of systolic left ventricular twist after acute myocardial infarction. Am J Physiol Heart Circ Physiol 2002; 282: 357–362. [DOI] [PubMed] [Google Scholar]
- 27. Setser RM, Kasper JM, Lieber ML, Starling RC, McCarthy PM, White RD. Persistent abnormal left ventricular systolic torsion in dilated cardiomyopathy after partial left ventriculectomy. J Thorac Cardiovasc Surg 2003; 126: 48–55. [DOI] [PubMed] [Google Scholar]
- 28. Kanzaki H, Nakatani S, Yamada N, Urayama S, Miyatake K, Kitakaze M. Impaired systolic torsion in dilated cardiomyopathy: reversal of apical rotation at mid-systole characterized with magnetic resonance tagging method. Basic Res Cardiol 2006; 101: 465–470. [DOI] [PubMed] [Google Scholar]
- 29. Kroeker CA, Tyberg JV, Beyar R. Effects of ischemia on left ventricular apex rotation. An experimental study in anesthetized dogs. Circulation 1995; 92: 3539–3548. [DOI] [PubMed] [Google Scholar]
- 30. Knudtson ML, Galbraith PD, Hildebrand KL, Tyberg JV, Beyar R. Dynamics of left ventricular apex rotation during angioplasty: a sensitive index of ischemic dysfunction. Circulation 1997; 96: 801–808. [DOI] [PubMed] [Google Scholar]
- 31. Garot J, Pascal O, Diebold B, Derumeaux G, Ovize M, Gueret P. Changes in left ventricular torsion during ischemia–reperfusion. Arch Mal Coeur Vaiss 2002; 95: 1151–1159. [PubMed] [Google Scholar]
- 32. Phan TT, Shivu GN, Abozguia K, Gnanadevan M, Ahmed I, Frenneaux M. Left ventricular torsion and strain patterns in heart failure with normal ejection fraction are similar to age-related changes. Eur J Echocardiogr 2009; 10: 793–800. [DOI] [PubMed] [Google Scholar]
- 33. Gustafsson U, Lindqvist P, Morner S, Waldenstrom A. Assessment of regional rotation patterns improves the understanding of the systolic and diastolic left ventricular function: an echocardiographic speckle-tracking study in healthy individuals. Eur J Echocardiogr 2009; 10: 56–61. [DOI] [PubMed] [Google Scholar]


