Abstract
Orthopedic procedures represent a large expense to the Medicare program, and costs of implantable medical devices account for a large proportion of those procedures’ costs. Physicians have been encouraged to consider costs in the selection of devices, but several factors make acquiring information about costs difficult. To assess physicians’ levels of knowledge about costs, we asked orthopedic attending physicians and residents at seven academic medical centers to estimate the costs of thirteen commonly used orthopedic devices between December 2012 and March 2013. The actual cost of each device was determined at each institution; estimates within 20 percent of the actual cost were considered correct. Among the 503 physicians who completed our survey, attending physicians correctly estimated the cost of the device 21 percent of the time, and residents did so 17 percent of the time. Thirty-six percent of physicians and 75 percent of residents rated their knowledge of device costs “below average” or “poor.” However, more than 80 percent of all respondents indicated that cost should be “moderately,” “very,” or “extremely” important in the device selection process. Surgeons need increased access to information on the relative prices of devices and should be incentivized to participate in cost-containment efforts.
As the United States struggles to contain the growth in health care spending, the costs associated with providing medical care have been a focus of attention. Physicians, the primary gatekeepers of the health care system, control or influence at least 60 percent of health care costs.1 However, they currently receive little training in, or information about, how to contain these costs.1
More than $150 billion is spent each year on medical devices in the United States.2 Orthopedic and cardiac procedures together account for nearly all of Medicare’s device-related expenditures, yet most of the recent increases in such expenditures have been a result of increased utilization of orthopedic devices.3
In 2006 Medicare paid hospitals more for orthopedic procedures than for any other diagnosis-related group.4 The device is often is the single largest contributor to the cost of an orthopedic procedure—in some cases, accounting for up to 87 percent of the cost.5
The devices used in these procedures often differ substantially in cost.5 Since there is little indication that they yield different clinical outcomes, the choice of device can result in cost savings.6–9 Thus, orthopedic surgeons have been encouraged to help manage scarce resources by considering cost in their selection of devices.10
However, several barriers make it difficult for physicians to acquire information about the cost of devices. First, many medical device companies regard pricing information as confidential, and most contracts with hospitals include clauses restricting cost disclosure.3,11 Second, the price of a given device may vary widely from one hospital to another.5,11 Third, device costs can fluctuate substantially over time, despite the fact that hospital purchasing agreements often span multiple years. Fourth, and perhaps most important, most orthopedic surgeons have no incentive to learn the costs of the devices they use, because those costs do not directly affect the care they provide to patients or their own reimbursement.12
It is not clear how much physicians know about the costs of the devices they implant. The study reported here was designed both to assess the extent to which orthopedic surgeons are able to estimate the cost of commonly used orthopedic devices and to determine the factors associated with knowledge about device costs.
Study Data And Methods
Participants
Our study was conducted between December 2012 and March 2013 in the orthopedic departments of the medical centers at the following seven institutions: Duke University, Harvard University, the University of Maryland, Mayo Clinic, the University of Pennsylvania, Stanford University, and Washington University in St. Louis. All orthopedic attending physicians and residents at these institutions were invited to participate.
The invitations were sent via e-mail and consisted of an introductory cover letter and a link to an online survey. Respondents were informed that their participation was voluntary and that their responses would remain confidential and would be reported only in the aggregate. All elements of the study were approved by the University of Maryland’s Institutional Review Board.
Survey
Our study involved thirteen common orthopedic devices. Seven of these were stand-alone devices: a total hip arthroplasty device, a distal radius locking plate, a suture anchor, a tibial intramedullary nail, a spine pedicle screw construct, an external fixator construct, and a ring external fixator construct.
We also asked respondents to consider three pairs of devices. The first item in each pair represented a lower-cost alternative to the second item: a nonlocking plate versus a precontoured anatomic locking plate applied to the distal fibula, a nonlocking plate versus a locking plate applied to the humerus, and a sliding hip screw with side plate versus a cephalomedullary nail applied to the proximal femur. We asked respondents to estimate the cost of each device in the pair, and we compared their estimates to determine their assessment of the cost difference between the paired devices. For a select group of fractures, the higher-cost devices may be more appropriate. However, most clinicians in the field would agree that both devices are equally effective for the majority of fractures treated with these implants. Based on their average prices, we grouped devices into the following three categories of cost: low (less than $500), medium ($500–5,000), and high (greater than $5,000).
For each of the thirteen devices, we chose the brand made by the most common vendor of that device at each institution. The device companies represented in our study were Arthrex (Naples, Florida), DePuy (Warsaw, Indiana), Smith and Nephew (Memphis, Tennessee), Stryker (Kalamazoo, Michigan), Synthes (West Chester, Pennsylvania), and Zimmer (Warsaw, Indiana).
The actual cost of each device, defined as the amount paid by the institution to the vendor for the device, was determined institution by institution. The institutional cost was generally dictated by a contract between the institution and the vendor. Thus, the cost of each device often varied considerably across institutions. The costs of disposable items and other items not implanted in the patient during surgery were not considered in this study.
In the survey, each device was identified with an x-ray (or photograph, in the case of the external fixators) and a list of included components. Respondents were asked to estimate the cost of each device and were instructed to assume that cost was defined as “the contract price representing the amount your institution currently pays the vendor for the implant construct.” They were also told to consider only the implanted components shown in the x-ray or photograph and included in the list of components.
Respondents were then asked about factors that might affect their knowledge of the costs of devices, including prior participation in device cost activities at their institution—such as participation on a committee to review device purchasing – and their professional experience (the number of years in practice for attending physicians and the postgraduate year for residents). Respondents were also asked to indicate which of the devices they used on a regular basis (defined as at least once every two months). Finally, using five-point Likert scales, respondents were asked to rate their own knowledge of device costs and to indicate the role that they thought cost should play in the selection of medical devices.
Analysis
We compared respondents’ estimated costs to the actual costs of devices at their institution. Estimates that were within plus or minus 20 percent of the actual cost were considered correct. For example, in the case of a device with an actual cost of $2,500, estimates between $2,000 and $3,000 were considered correct. This range was chosen to facilitate comparisons with prior studies of estimated medical costs.13
To determine overall device cost knowledge, we calculated the proportion of costs that each respondent estimated correctly. To determine which factors were associated with device cost knowledge, we performed logistic regression for each question (as opposed to each respondent). This allowed us to incorporate respondents’ familiarity with devices, which varied by device. Factors found to be significant in the univariate analysis were retained in the multivariate model.
In the multivariate analysis, all variables were simultaneously entered into the model, with each variable adjusted for all of the others. Associations were estimated on the basis of odds ratios and 95 percent confidence intervals. P values of less than 0.05 were considered to indicate statistical significance. All reported p values were two-sided and were not adjusted for multiple comparisons. Kanu Okike performed the statistical analysis using the statistical software SAS, version 9.
Limitations
The results of our study must be considered in the context of our study design. The study was conducted among orthopedic attending physicians and residents at large academic medical centers, which may limit its generalizability to physicians in other settings.
In addition, some of the factors that we found to be predictive of device cost knowledge in the multivariate analysis and that achieved statistical significance were associated with only small absolute differences in responses to questions on the survey.
Finally, our investigation focused on device costs to the hospital because it is hospitals that purchase the devices from manufacturers. Patient costs were not considered in this study.
Study Results
The response rate for the survey was 96 percent (503 out of 522 attending physicians and residents at the institutions we studied). Of the 503 respondents, 217 were attending physicians, and 286 were residents. Thirty percent of attending physicians (66 out of 217) had previously participated in device cost activities at their institution, as compared to 5 percent of residents (14 out of 286). Among all respondents, the percentage of device costs estimated correctly was 19 percent (95% confidence interval: 18, 20).
Attending physicians correctly estimated the cost of the device 21 percent of the time (95% CI: 19, 23). Of the responses, 42 percent were underestimates, and 38 percent were overestimates. For all devices, attending physicians’ estimates ranged from 1.8 percent of the actual price to 24.6 times the actual price.
For the paired devices, attending physicians nearly always correctly identified the more expensive alternative (99.4 percent). However, they correctly estimated the difference in cost between the two devices in a pair only 15 percent of the time. The difference was underestimated 65 percent of the time and overestimated 20 percent of the time.
In the multivariate analysis, a physician’s prior participation in device cost activities at his or her institution was associated with greater device cost knowledge (Exhibit 1). Cost knowledge was greater for devices that the physicians used regularly. Cost knowledge also varied with the cost of the device. In particular, physicians tended to overestimate the price of low-cost devices and to underestimate the price of high-cost ones (Exhibit 2).
Exhibit 1.
Factors Associated With Knowledge Of Device Costs Of Attending Orthopedic Surgeons At Seven Academic Medical Centers, December 2012 – March 2013
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | Costs correctly estimated | OR | 95% CI | OR | 95% CI |
| Site | |||||
| A | 21.0% | —a | —a | —a | —a |
| B | 24.1 | 1.20 | 0.83, 1.72 | —b | —b |
| C | 19.2 | 0.90 | 0.61, 1.33 | —c | —c |
| D | 19.1 | 0.89 | 0.61, 1.30 | —b | —b |
| E | 23.5 | 1.16 | 0.84, 1.60 | —b | —b |
| F | 17.5 | 0.80 | 0.59, 1.09 | —b | —b |
| G | 23.6 | 1.17 | 0.83, 1.64 | —b | —b |
| Years in practice | |||||
| 10 or fewer | 20.6 | —a | —a | —c | —c |
| 11–24 | 23.2 | 1.16 | 0.95, 1.42 | —b | —b |
| 25 or more | 17.2 | 0.80 | 0.62, 1.04 | —b | —b |
| Prior participation in device cost activities at the institution | |||||
| No | 19.5 | —a | —a | —a | —a |
| Yes | 23.9 | 1.30*** | 1.07, 1.57 | 1.30*** | 1.07, 1.58 |
| Device regularly used | |||||
| No | 18.3 | —a | —a | —a | —a |
| Yes | 26.8 | 1.64**** | 1.36, 1.98 | 1.65**** | 1.36, 2.01 |
| Device cost | |||||
| Low (<$500) | 12.8 | —a | —a | —a | —a |
| Medium ($500–$5,000) | 26.9 | 2.52**** | 1.94, 3.27 | 2.59**** | 1.99, 3.36 |
| High (>$5,000) | 17.9 | 1.49*** | 1.12, 1.96 | 1.61*** | 1.20, 2.15 |
SOURCE Authors’ calculations.
NOTES The seven sites are listed in the text. Specific sites are not identified here to protect respondents’ confidentiality. OR is odds ratio. CI is confidence interval.
Reference category.
[Please provide].
Not [please provide].
p < 0.01
p < 0.001
Exhibit 2.
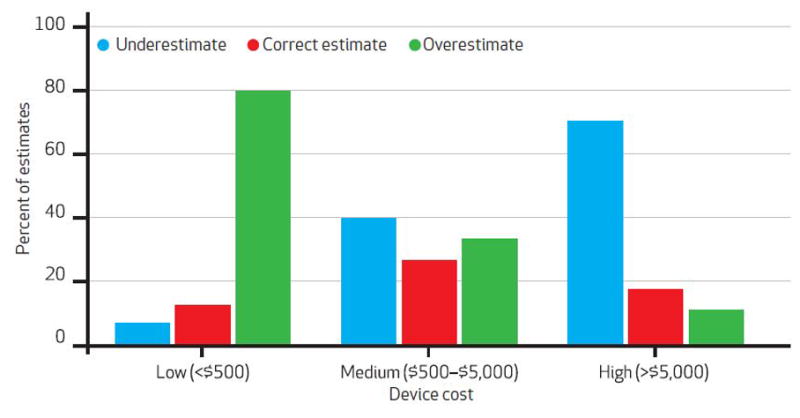
Attending Orthopedic Surgeons’ Estimates Of Device Costs, By Device Cost
SOURCE Authors’ calculations.
Residents correctly estimated the cost of the device 17 percent of the time (95% CI: 16, 18; difference from the attending physicians, p < 0.001). Of the responses, 50 percent were underestimates, and 33 percent were overestimates. For all devices, residents’ estimates ranged from 1.8 percent of the actual price to 54.1 times the actual price.
For the paired devices, residents—like the attending physicians—nearly always correctly identified the more expensive alternative (97.2 percent). However, residents underestimated the difference in cost between the two devices 73 percent of the time.
In the multivariate analysis, the factors associated with residents’ cost knowledge were postgraduate year and device cost (Exhibit 3). Like the attending physicians, residents also overestimated the price of low-cost devices while underestimating the price of high-cost ones (p = 0.02).
Exhibit 3.
Factors Associated With Knowledge Of Device Costs Of Orthopedic Residents At Seven Academic Medical Centers, December 2012 – March 2013
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | Costs correctly estimated | OR | 95% CI | OR | 95% CI |
| Site | |||||
| A | 18.9% | —a | —a | —a | —a |
| B | 19.1 | 1.01 | 0.71, 1.46 | —b | —b |
| C | 13.5 | 0.67 | 0.45, 1.01 | —c | —c |
| D | 19.0 | 1.01 | 0.71, 1.44 | —b | —b |
| E | 18.4 | 0.97 | 0.69, 1.37 | —b | —b |
| F | 14.4 | 0.72 | 0.51, 1.02 | —b | —b |
| G | 16.0 | 0.81 | 0.56, 1.18 | —b | —b |
| Postgraduate year | |||||
| 1 or 2 | 14.6 | —a | —a | —a | —a |
| 3 or higher | 18.3 | 1.31*** | 1.09, 1.57 | 1.24** | 1.01, 1.50 |
| Prior participation in device cost activities at the institution | |||||
| No | 16.7 | —a | —a | —c | —c |
| Yes | 20.3 | 1.27 | 0.88, 1.84 | —b | —b |
| Device regularly used | |||||
| No | 15.3 | —a | —a | —a | —a |
| Yes | 19.2 | 1.31*** | 1.10, 1.56 | 1.17 | 0.97, 1.42 |
| Device cost | |||||
| Low (<$500) | 16.1 | —a | —a | —a | —a |
| Medium ($500-$5,000) | 19.8 | 1.29** | 1.03, 1.60 | 1.29** | 1.04, 1.60 |
| High (>$5,000) | 13.3 | 0.80 | 0.62, 1.03 | 0.82 | 0.63, 1.05 |
SOURCE Authors’ calculations. NOTES The seven sites are listed in the text. Specific sites are not identified here to protect respondents’ confidentiality. OR is odds ratio. CI is confidence interval.
Reference category.
[Please provide].
Not [please provide].
p < 0.10
p < 0.01
When asked about the importance of cost in the selection of orthopedic devices, 8 percent of all of the respondents said it was “extremely important,” and 30 percent said “very important.” Forty-eight percent and 13 percent said it was “moderately important” or “slightly important,” respectively. Less than 1 percent of respondents believed that cost was “not at all important” in the selection of devices.
Fifty percent of the attending physicians who responded to the survey assessed their device cost knowledge as “average,” while 36 percent rated it as “below average” or “poor” (Exhibit 4). Attending physicians’ self-assessed knowledge was correlated with their actual knowledge (p < 0.001 for trend). Seventy-five percent of the residents who responded rated their device cost knowledge as “below average” or “poor.” Residents’ self-assessed knowledge was not correlated with their actual knowledge (p = 0.62 for trend).
Exhibit 4.
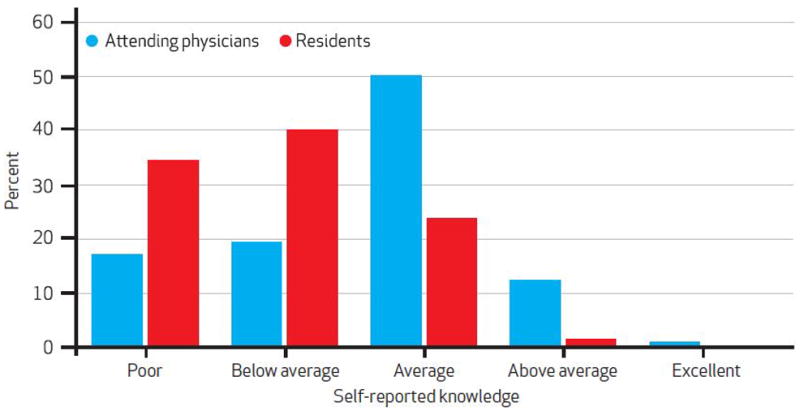
Attending Physicians’ And Residents’ Self-Rated Knowledge Of Device Costs
SOURCE Authors’ calculations. NOTE Categories represent responses to the following question: “How would you rate your knowledge of orthopedic implant costs at your institution?”
Discussion
We are aware of one prior study that assessed surgeons’ knowledge of device costs. In particular, Streit and colleagues surveyed surgeons at two academic medical centers and documented a low level of device cost knowledge. While this study had important strengths, it featured a small sample size (15 responses from attending physicians) as well as a low response rate (53%), and lacked a consistently applied definition of actual cost.
Prior research has also sought to assess awareness of medication costs among physicians.14–17 In a recent systematic review of studies of medication cost knowledge, Michael Allan and colleagues13 found that cost accuracy was generally low, with only 31 percent of physicians’ estimated drug costs falling within 20–25 percent of the actual cost. However, many of these prior studies were limited by ambiguous definitions of actual cost, since the price of a medication often varies by location as well as by prescription plan.
In our study, physicians tended to underestimate the cost of expensive devices while overestimating the cost of inexpensive devices, which has important practical implications. A physician in clinical practice often has a choice between two equally effective devices that differ in cost. By undervaluing high-cost items and overvaluing low-cost items, physicians may underestimate the amount that could be saved by choosing the lower-cost alternative. In the paired constructs in our study, attending physicians and residents consistently underestimated the difference in cost between the high- and low-cost options.
Our study benefited from the fact that we were able to determine the actual contract cost of each device at each participating institution, a challenging task due to the variety of non-dislosure clauses in effect at each institution. In addition, we chose common devices and incorporated institution-specific vendor preferences to ensure that respondents were familiar with the devices used in the survey. In addition, our study was conducted at seven institutions that were distributed throughout the country, which might increase the generalizability of our results.
Finally, our survey had a high response rate, which might reduce the potential for nonresponder bias.18 To achieve this response rate, we employed a variety of strategies. We made sure that respondents were familiar with the research team by—for example—having one coauthor at each study site who served as a local champion for the project. We also designed the survey to be brief and easy to complete, yet interesting; and we sent requests to participate at a time when surgeons were likely to be reachable but not too busy, typically in the early evening.
As the primary gatekeepers of the US health care system, physicians are well positioned to play a key role in controlling costs. While there may be many items in the hospital for which physicians do not know the costs, we believe that a knowledge of implantable medical device costs is particularly important because they represent a convenient target for cost containment.
As noted above, orthopedic procedures topped the list of Medicare payments to hospitals by diagnosis-related group in 2006,4 and implantable devices account for a disproportionately large share of the procedure costs.5 Little evidence demonstrates the superiority of one vendor’s device over another, but wide variation in cost is common.5 Previous studies in the orthopedic setting have shown that it is possible to achieve substantial reductions in implant costs without decreasing the quality of care, primarily by choosing less-expensive implants.6–9
Previous research has also demonstrated the effectiveness of initiatives to educate physicians on the costs of the items they order. Several studies have shown that informing physicians about the cost of diagnostic tests at the time of ordering results in decreased use of tests as well as in decreased charges.19–24
Unfortunately, this strategy has not been transferable to implantable medical devices because of the confidentiality clauses that commonly accompany the contracts between hospitals and vendors of devices.11 The specific provisions of these confidentiality clauses vary widely, but widespread dissemination of device prices is not an option at many institutions.
Medical device manufacturers strive to keep their prices confidential so that they may sell the same implant at a different price to different health care institutions. The amount a company charges for a particular device may vary widely from one site to another depending on a number of factors, including procedure volume, institutional bargaining power, the actual costs of sales and service at the site, and the company’s ability to market its products to hospitals and surgeons. For example, a company might be willing to accept a smaller profit margin at an academic institution than at a private practice because having trainees at the academic center use a device might make it more likely that they would continue to do so in their future practices.
How, then, are surgeons to be educated about the costs of the devices they implant, without running the risk of violating confidentiality clauses in hospital purchasing agreements? One possibility is publicizing relative cost information. The University of Maryland Division of Orthopaedic Trauma is in the process of implementing such a strategy to decrease device costs and increase value. The division categorizes devices used in commonly performed procedures as red, yellow, or green based on their relative prices, and this information is posted in the operating room as a guide for the surgeon.
Of course, efforts to increase surgeons’ knowledge of device costs would be considerably more successful in the absence of confidentiality clauses. Recognizing the cost implications of these clauses, Sen. Chuck Grassley (R-IA) sought to mandate public disclosure of implantable device prices with the Transparency in Medical Device Pricing Act, a bill that was introduced in Congress in 2007 but that did not move beyond the committee stage. Implementing that requirement would not only have increased surgeons’ access to device costs, but it would also have given hospitals the ability to negotiate more uniform prices, thus reducing the wide variation across institutions.3,11 We found that some devices cost almost three times as much at one institution than at another.
However, knowledge of device costs might not be enough for surgeons to participate in cost containment. Surgeons must also be incentivized to make clinical decisions informed, in part, by knowledge of costs. Most orthopedic surgeons have no financial incentive to learn the costs of the devices they implant because that information does not influence patient care or their own reimbursement.
Over the past few decades, many hospitals have sought to incentivize orthopedic surgeons to participate in cost-containment initiatives through various ways of aligning surgeons’ and hospitals’ incentives, such as the gain-sharing model.25–27 In this model, surgeons who generate cost savings for their institution are rewarded—for example, with a new piece of equipment, financial support for a physician assistant, or research funds. Aligning incentives has great potential to motivate surgeons to participate in cost-saving measures, including the selection of lower-cost devices when medically appropriate.
The use of such models with orthopedists is still sporadic. Yet there is reason to believe that this situation may change with the implementation of accountable care organizations under health reform.28,29
It is widely expected that these organizations—in which physicians will be compensated for care provided to a particular patient population over time, instead of for each procedure performed—will assume a prominent role in the US health care system in the near future. As part of an accountable care organization, a surgeon would be reimbursed not based on the number of procedures he or she performed, but according to the quality of care provided and the ability of the care team to provide cost-efficient care.
Further research is needed to determine the effectiveness of the various strategies for implant cost containment that are being implemented, including relative pricing and gain sharing. In addition, as accountable care organizations become more prevalent, it will be necessary to assess the effect of this shift in incentives, which will affect device costs, quality of care, patients’ and providers’ satisfaction, and overall cost of care to the health care system.
Conclusion
In our multicenter survey of 503 orthopedic attending physicians and residents, most respondents indicated that cost should play an important role in the selection of devices. However, actual knowledge of device costs was low among attending physicians and residents alike. This was true even though our survey asked about common orthopedic devices, used institution-specific vendors and pricing, and excluded disposable components and other nonimplanted items.
Surgeons must be educated about the prices of the devices—or at least the relative prices of various devices—and should be incentivized to learn the prices and to participate in cost-containment efforts. Many surgeons lack such incentives, but that situation is expected to change as accountable care organizations and other models of aligning hospitals’ and surgeons’ incentives become more common.
Acknowledgments
Kanu. Okike reports research support from Depuy, educational meeting support from Stryker and Synthes, and research award support from DePuy. Robert O’Toole reports consultancy fees from Synthes and research support from Synthes and Stryker. Andrew Pollak reports royalties from ExtraOrtho and Zimmer and research support from Smith and Nephew. Julius Bishop reports research support from Covidien, royalties from Innomed, and teaching honoraria from Synthes. Christopher McAndrew reports research support from the National Institutes of Health and teaching honoraria from Synthes. Samir Mehta reports research support from the Department of Defense, the National Institutes of Health, the Foundation for Orthopedic Trauma, and the AO Foundation; royalties from Wolters Kluwer Health; speakers’ bureau fees from Olympus Biotech, Bioventus, Synthes, AO North America, and Smith and Nephew; and consultancy fees from Synthes and Smith and Nephew. William Cross reports consultancy fees from Zimmer and teaching honoraria from Synthes. Grant Garrigues reports consultancy fees from Tornier and educational grants from Don-Joy Orthopedics, Zimmer and Breg. The authors thank all of the surgeons who participated in the study and Harry Rubash, Maureen Kelly Fitzgerald, Trent Pierce, and Karen Rottmann.
Footnotes
Mitchel Harris and Christopher Lebrun report no conflicts of interest.
Contributor Information
Kanu Okike, Email: okike@post.harvard.edu, Adjunct assistant professor of orthopaedic surgery at the University of Maryland School of Medicine in Baltimore, Maryland.
Robert V. O’Toole, Associate professor of orthopedic surgery at the University of Maryland School of Medicine in Baltimore, Maryland
Andrew N. Pollak, Professor of orthopedic surgery at the University of Maryland School of Medicine in Baltimore, Maryland
Julius A. Bishop, Assistant professor of orthopedic surgery at the Stanford University School of Medicine in Redwood City, California
Christopher M. McAndrew, Assistant professor of orthopedic surgery at the Washington University School of Medicine in St. Louis, Missouri
Samir Mehta, Assistant professor of orthopedic surgery at the Hospital of the University of Pennsylvania in Philadelphia, Pennsylvania.
William W. Cross, III, Instructor of orthopedic surgery at Mayo Clinic in Rochester, Minnesota.
Grant E. Garrigues, Instructor of orthopedic surgery at the Duke University School of Medicine in Durham, North Carolina
Mitchel B. Harris, Professor of orthopedic surgery at Brigham and Women’s Hospital in Boston, Massachusetts
Christopher T. Lebrun, Assistant professor of orthopedic surgery at the University of Maryland School of Medicine in Baltimore, Maryland
Notes
- 1.Agrawal S, Taitsman J, Cassel C. Educating physicians about responsible management of finite resources. JAMA. 2013;309(11):1115–6. doi: 10.1001/jama.2013.1013. [DOI] [PubMed] [Google Scholar]
- 2.Donahoe G, King G. Estimates of medical device spending in the United States [Internet] Washington (DC): AdvaMed; 2012. Oct, [cited 2013 Dec 3]. Available from: http://www.scribd.com/doc/111162277/Oct-2012-King-Report-FINAL. [Google Scholar]
- 3.Government Accountability Office. Medicare: lack of price transparency may hamper hospitals’ ability to be prudent purchasers of implantable medical devices” [Internet] Washington (DC): GAO; 2012. Jan, [cited 2013 Dec 3]. (GAO-12-126). Available from: http://www.gao.gov/assets/590/587688.pdf. [Google Scholar]
- 4.Integrated Healthcare Association. Orthopedics data compendium: use, cost, and market structure for total joint replacement [Internet] Oakland (CA): IHA; 2006. Aug, [cited 2013 Dec 3]. Available from: http://www.iha.org/pdfs_documents/medical_device/07_OrthopedicsDataCompendium.pdf. [Google Scholar]
- 5.Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693–8. doi: 10.2106/JBJS.K.00355. [DOI] [PubMed] [Google Scholar]
- 6.Olson SA, Obremskey WT, Bozic KJ. Healthcare technology: physician collaboration in reducing the surgical cost. Clin Orthop Relat Res. 2013;471(6):1854–64. doi: 10.1007/s11999-013-2828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuckerman JD, Kummer FJ, Frankel VH. The effectiveness of a hospital-based strategy to reduce the cost of total joint implants. J Bone Joint Surg Am. 1994;76(6):807–11. doi: 10.2106/00004623-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Levine DB, Cole BJ, Rodeo SA. Cost awareness and cost containment at the Hospital for Special Surgery. Strategies and total hip replacement cost centers. Clin Orthop Relat Res. 1995;(311):117–24. [PubMed] [Google Scholar]
- 9.Christo AE, Bargar WL, Morris E. Prosthesis cost containment in total joint replacement: a physician-driven free-market approach. Orthopedics. 2000;23(5):439–42. doi: 10.3928/0147-7447-20000501-11. [DOI] [PubMed] [Google Scholar]
- 10.American Association of Orthopaedic Surgeons. Position statement: value driven use of orthopaedic implants [Internet] Rosemont (IL): AAOS; 2009. Feb, [cited 2013 Dec 3]. Available from: http://www.aaos.org/about/papers/position/1104.asp. [Google Scholar]
- 11.Pauly MV, Burns LR. Price transparency for medical devices. Health Aff (Millwood) 2008;27(6):1544–53. doi: 10.1377/hlthaff.27.6.1544. [DOI] [PubMed] [Google Scholar]
- 12.Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57–63. doi: 10.1097/BLO.0b013e31803372e0. [DOI] [PubMed] [Google Scholar]
- 13.Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. doi: 10.1371/journal.pmed.0040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst ME, Kelly MW, Hoehns JD, Swegel JM, Buys LM, Logemann CD, et al. Prescription medication costs: a study of physician familiarity. Arch Fam Med. 2000;9(10):1002–7. doi: 10.1001/archfami.9.10.1002. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman J, Barefield FA, Ramamurthy S. A survey of physician knowledge of drug costs. J Pain Symptom Manage. 1995;10(6):432–5. doi: 10.1016/0885-3924(95)00018-t. [DOI] [PubMed] [Google Scholar]
- 16.Korn LM, Reichert S, Simon T, Halm EA. Improving physicians’ knowledge of the costs of common medications and willingness to consider costs when prescribing. J Gen Intern Med. 2003;18(1):31–7. doi: 10.1046/j.1525-1497.2003.20115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichert S, Simon T, Halm EA. Physicians’ attitudes about prescribing and knowledge of the costs of common medications. Arch Intern Med. 2000;160(18):2799–803. doi: 10.1001/archinte.160.18.2799. [DOI] [PubMed] [Google Scholar]
- 18.Sprague S, Quigley L, Bhandari M. Survey design in orthopaedic surgery: getting surgeons to respond. J Bone Joint Surg Am. 2009;91(Suppl 3):27–34. doi: 10.2106/JBJS.H.01574. [DOI] [PubMed] [Google Scholar]
- 19.Pugh JA, Frazier LM, DeLong E, Wallace AG, Ellenbogen P, Linfors E. Effect of daily charge feedback on inpatient charges and physician knowledge and behavior. Arch Intern Med. 1989;149(2):426–9. [PubMed] [Google Scholar]
- 20.Tierney WM, Miller ME, McDonald CJ. The effect on test ordering of informing physicians of the charges for outpatient diagnostic tests. N Engl J Med. 1990;322(21):1499–504. doi: 10.1056/NEJM199005243222105. [DOI] [PubMed] [Google Scholar]
- 21.Sachdeva RC, Jefferson LS, Coss-Bu J, Done G, Campbell D, Nelson SI, et al. Effects of availability of patient-related charges on practice patterns and cost containment in the pediatric intensive care unit. Crit Care Med. 1996;24(3):501–6. doi: 10.1097/00003246-199603000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Hampers LC, Cha S, Gutglass DJ, Krug SE, Binns HJ. The effect of price information on test-ordering behavior and patient outcomes in a pediatric emergency department. Pediatrics. 1999;103(4 Pt 2):877–82. [PubMed] [Google Scholar]
- 23.Seguin P, Bleichner JP, Grolier J, Guillou YM, Mallédant Y. Effects of price information on test ordering in an intensive care unit. Intensive Care Med. 2002;28(3):332–5. doi: 10.1007/s00134-002-1213-x. [DOI] [PubMed] [Google Scholar]
- 24.Feldman LS, Shihab HM, Thiemann D, Yeh HC, Ardolino M, Mandell S, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903–8. doi: 10.1001/jamainternmed.2013.232. [DOI] [PubMed] [Google Scholar]
- 25.Levin LS, Gustave L. Aligning incentives in health care: physician practice and health system partnership. Clin Orthop Relat Res. 2013;471(6):1824–31. doi: 10.1007/s11999-012-2775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauk JR, Bray TJ. Orthopaedist-hospital alignment in a community setting. Clin Orthop Relat Res. 2013;471(6):1837–45. doi: 10.1007/s11999-013-2805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz DS. Orthopaedic surgeon-hospital alignment at Geisinger Health System. Clin Orthop Relat Res. 2013;471(6):1846–53. doi: 10.1007/s11999-013-2903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Z, Lee TH. The era of delivery system reform begins. JAMA. 2013;309(1):35–6. doi: 10.1001/jama.2012.96870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Academy of Orthopaedic Surgeons. Accountable care organizations: a primer for orthopaedic surgeons. Rosemont (IL): AAOS; [cited 2013 Dec 4]. Available from: http://www.pwrnewmedia.com/2011/aaos/advocacy_now/special_edition/aug30/downloads/Accountable_Care_Organizations_Primer.pdf. [Google Scholar]


