Abstract
In the past two decades, respiratory sinus arrhythmia (RSA)—an index of parasympathetic nervous system (PNS)-mediated cardiac control—has emerged as a reliable peripheral biomarker of emotion regulation (ER). Reduced RSA and excessive RSA reactivity (i.e., withdrawal) to emotional challenge are observed consistently among individuals with poor ER capabilities, including those with various forms of internalizing and externalizing psychopathology, and those with specific psychopathological syndromes, including anxiety, phobias, attention problems, autism, callousness, conduct disorder, depression, non-suicidal self-injury, panic disorder, and trait hostility. Emerging evidence suggests that low RSA and excessive RSA reactivity index poor ER because they are downstream peripheral markers of prefrontal cortex (PFC) dysfunction. Poorly modulated inhibitory efferent pathways from the medial PFC to the PNS result in reduced RSA and excessive RSA reactivity. According to this perspective, RSA is a non-invasive proxy for poor executive control over behavior, which characterizes most forms of psychopathology.
Keywords: emotion regulation, emotion dysregulation, parasympathetic nervous system, psychopathology, internalizing, externalizing
Introduction
Although the study of human emotion can be traced at least as far back as Darwin [1], for much of the 20th Century psychological science was dominated by behavioral and cognitive paradigms that viewed emotional states as subjective, unquantifiable, and unamenable to scientific inquiry [2]. Beginning in the mid-1990s, these paradigms gave way to the contemporary view that emotional experience and expression are integral to both positive and negative psychological adjustment. This paradigm shift was facilitated by several developments, including a 1990, yearlong seminar on emotion held at the National Institute of Mental Health, and a 1991 conference on the development of emotion regulation (ER) that was sponsored by the John D. and Catherine T. MacArthur Foundation Early Childhood Network. From these, landmark volumes on the nature of emotion [3] and ER [4] emerged. Among other important contributions, chapters in these volumes demonstrated how emotional states can be inferred, verified, and quantified by measuring appropriate biological systems [5, 6, 7]. The study of emotion soon became mainstream, and is currently central to the positive and negative valence systems of the Research Domain Criteria (RDoC) [8*].
Emotion Dysregulation and Psychopathology
It is now widely recognized that problems with ER confer vulnerability to a wide range of psychopathological outcomes [9*], and that difficulties with ER characterize almost all forms of psychopathology [10*,11*]. ER can be defined as the set of processes through which emotional experience and expression are shaped in the service of adaptive behavior [12]. Such shaping of emotion may occur through various mechanisms, including attentional, cognitive, social, and behavioral [13*]. In contrast, emotion dysregulation comprises a pattern of emotional experience and/or expression that interferes with appropriate goal directed behavior [14]. In almost all forms of psychopathology, one or more negative emotions (e.g., sadness, anxiety, panic, anger, rage) is experienced either too intensely or too persistently to be adaptive [15*,16*].
Central Nervous System Substrates of Emotion Dysregulation
Recognition that emotion dysregulation plays such a prominent role in vulnerability to psychopathology has led to considerable research on its central nervous system (CNS) substrates. Taken together, this research demonstrates that ER is subserved by top-down, cortical (prefrontal) brain networks that mature into the early 20s [17*,18*,19*]. Trait anxiety, which confers vulnerability to internalizing disorders (e.g., anxiety, depression), is volitionally regulated through prefrontal inhibition of subcortical amygdalar activity and reactivity, whereas trait impulsivity, which confers vulnerability to externalizing disorders (e.g., ADHD, substance use disorders) is volitionally regulated through prefrontal inhibition of subcortical striatal activity and reactivity [20, 21, 22*]. Those with anxiety disorders exhibit less functional connectivity in amygdalar-prefrontal connections than controls [23], and those with externalizing disorders exhibit less functional connectivity in prefrontal-striatal connections than controls [24]. Moreover, deficient top-down control of the amygdala by the PFC, and reduced functional connectivity between the amygdala and the PFC, are observed among those with deficient ER [25].
Peripheral Nervous System Markers of Emotion Dysregulation
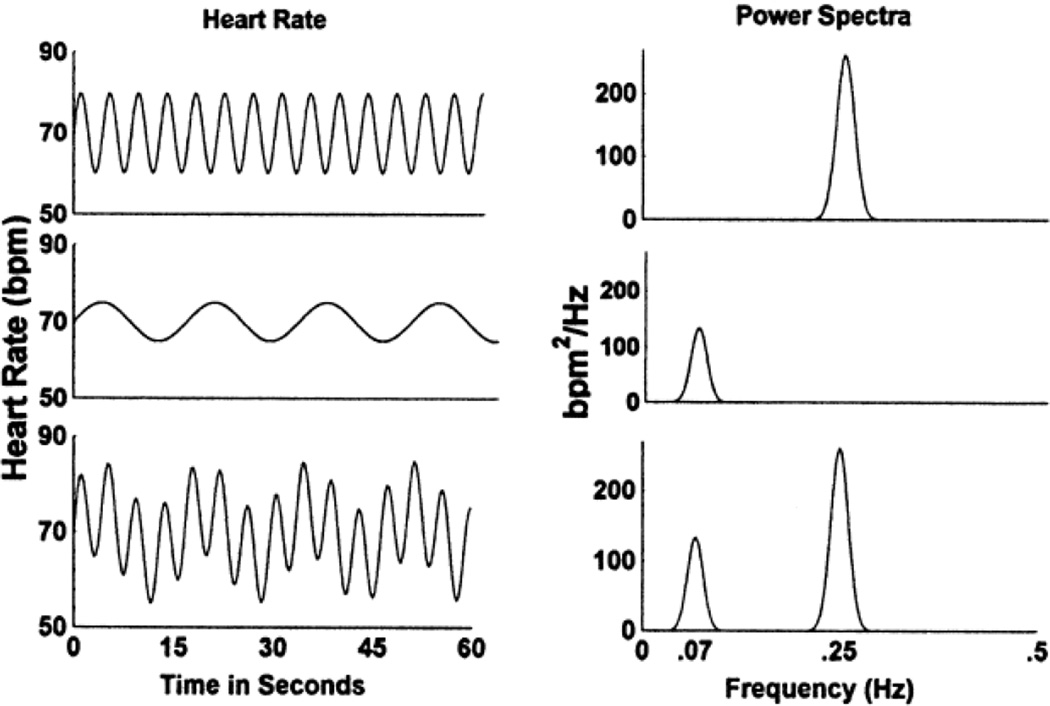
Although the CNS substrates of ER are well characterized [16], measuring CNS processes requires neuroimaging protocols that are expensive, limited in ecological validity, and difficult to use with certain populations, such as young children and those with severe psychopathology. For these reasons and others, many researchers have turned to peripheral nervous system markers of ER, most notably respiratory sinus arrhythmia (RSA). The term RSA refers to ebbing and flowing of heart rate (HR) across the respiratory cycle. This cyclic pattern of HR occurs due to increases in inhibitory parasympathetic efference during exhalation, and decreases in inhibitory parasympathetic efference during inhalation [10, 26*]. RSA can be assessed using any of several methods [27*], the most common being spectral analysis of the electrocardiographic R-wave time series. Spectral analysis isolates parasympathetic influence on the heart—as indexed by RSA—by filtering out low- and mid-frequency heart rate variability (see Figure 1). This is necessary because these frequency bands include parasympathetic, sympathetic, diurnal, and non-neural components [28*].
Figure 1.
Fictitious heart rate (HR) signals and associated power spectra. The top two panels represent pure high-frequency HR variability (0.25 Hz), as associated with RSA. The middle two panels represent low-frequency HR variability (0.07 Hz), which is of parasympathetic, sympathetic, diurnal, and nonneural origin. The bottom panels represent the combined signal including both high- and low-frequency components. Actual heart rate signals include spectral power at additional frequencies.
RSA demonstrates two important qualities that establish its validity as a transdiagnostic biomarker of emotion dysregulation. First, abnormally low resting RSA and/or large reductions in RSA specifically during emotion evocation are associated with symptoms of both internalizing and externalizing psychopathology [29, 30], and with numerous psychopathological syndromes, including anxiety [31], phobias [32], attention problems [33], autism [34], callousness [35], conduct disorder [15], depression [36], non-suicidal self-injury [37], panic disorder [38], and trait hostility [39]. This remarkably long list suggests that RSA marks one or more core self-regulatory functions that are disrupted across diverse forms of psychopathology [10, 11]. Importantly, excessive RSA withdrawal among psychopathological groups is specific to emotion evocation, and is not observed in many other stimulus conditions [40].
Second, mounting evidence suggests that RSA reflects prefrontal cortex (PFC) function, and therefore indexes—albeit peripherally—CNS substrates of ER [11]. This assertion, which is articulated in Thayer’s neurovisceral integration theory [41, 42*], is based on several considerations, including existence of inhibitory neural efferent pathways from the medial PFC to the PNS; (2) positive associations between resting RSA and performance on executive function tasks; and (3) positive correlations between RSA and PFC activity during neuroimaging tasks.
Inhibitory efferent pathways from the medial PFC to the PNS have been characterized for some time [43]. The prefrontal, cingulate, and insular cortices form an interconnected neural network that exhibits feed-forward and feedback connections with the amygdala [41]. Activation of the central nucleus of the amygdala via this network provides inhibition of the nucleus solitary tract, which in turn inhibits vagal motor neurons in the dorsal motor nucleus and the nucleus ambiguus [41]. These structures provide inhibitory input via the PNS to the sinoatrial node [26]. Through this structural network, PFC function is translated into RSA. Since most forms of psychopathology are characterized by PFC dysfunction [44, 45*], they are also characterized by low resting RSA and excessive RSA reactivity, peripheral biomarkers of poor executive control [41].
Consistent with this interpretation, positive associations between resting RSA and performance on executive function (EF) tasks have been reported. For example, among military personnel, those who score high on baseline RSA outperform those who score low on RSA on stimulus detection and addition tasks [46, 46].
Positive correlations between RSA and PFC function have also been demonstrated using positron emission tomography. During emotion-induction, RSA correlates with cerebral blood flow in both the PFC and the anterior cingulate cortex [47*]. Induction of various emotions reduces both RSA and blood flow in these regions.
Taken together, this research provides an explanation for the remarkably consistent findings of low resting RSA and excessive RSA reactivity among those with diverse forms of psychopathology. Non-specific vulnerability to psychopathology is conferred by poor executive (i.e., prefrontal) control over behavior [44, 45], which is reflected in measures of RSA given structural and functional connections between the PFC and PNS efferents to the heart via the vagus nerve. Specific forms of psychopathology are determined by interactions of PFC dysfunction with largely independent subcortical neural circuits that generate approach- and avoidance-related affect. According to this perspective, emotions are generated by phylogenetically old, subcortical neural circuits, but regulated by phylogenetically new, cortical neural circuits [10, 11].
Conclusions
A primary aim of psychophysiological research is to use peripheral measures to make inferences about CNS processes that are difficult and in some cases impossible to index noninvasively [26]. However, much of the literature on RSA-behavior relations is either agnostic with respect to central nervous system substrates of RSA, or implies that the PNS plays a causal role in ER. As reviewed above, it is far more likely that the PNS—via the vagus nerve—mediates links between the PFC and cardiovascular function. In future research, authors should interpret their findings not only at the ANS level, but at the CNS level as well. Furthermore, since cortical PFC dysfunction interacts with subcortical neural systems to confer vulnerability to specific forms of psychopathology, researchers are encouraged to assess multiple CNS and/or autonomic systems concurrently. Doing so provides considerably more specificity in distinguishing among psychiatric disorders [10, 48]. Such an approach is fully consistent with the RDoC initiative [8], which assumes that multiple neurobiological systems interact to affect behavior. By studying such interactions, we are likely to advance our understanding of psychopathology in the upcoming decade [11].
Highlights.
RSA is a valid and reliable biomarker of emotion regulation capacity in humans.
Low tonic RSA and excessive RSA reactivity to emotion evocation mark PFC dysfunction.
Effective cortical regulation of subcortical neural circuits marks psychological health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Darwin CR. The expression of the emotions in man and animals. John Murray; 1872. [Google Scholar]
- 2.Beauchaine TP, Zalewski M. Physiological and developmental mechanisms of emotional lability in coercive relationships. In: Dishion TJ, Snyder JJ, editors. Oxford handbook of coercive relationship dynamics. Oxford University Press; in press. [Google Scholar]
- 3.Ekman P, Davidson RJ. The nature of emotion. Oxford University Press; 1994. [Google Scholar]
- 4.Fox NA, editor. The development of emotion regulation: Biological and behavioral considerations. Monogr Soc Res Child. 1994;59 (2–3, Serial No. 240). [Google Scholar]
- 5.Porges SW, Doussard-Roosevelt JA. Vagal tone and the physiological regulation of emotion. Monogr Soc Res Child. 1994;59:167–186. [PubMed] [Google Scholar]
- 6.Dawson G. Frontal electroencephalographic correlates of individual differences in emotion expression in infants: A brain systems perspective on emotion. Monogr Soc Res Child. 1994;59:135–151. [PubMed] [Google Scholar]
- 7.Panksepp J. The clearest physiological distinctions between emotions will be found in the brain. In: Ekman P, Davidson RJ, editors. The nature of emotion. Oxford University Press; 1994. pp. 258–260. [Google Scholar]
- 8. National Institute of Mental Health. Research Domain Criteria Matrix. Retrieved on 1/18/2015 from http://www.nimh.nih.gov/research-priorities/rdoc/research-domain-criteria-matrix.shtml.. Describes the Research Domain Criteria (RDoC), currently under development by the National Institute of Mental Health as a dimensional, research-based, neuroscience-informed alternative to traditional psychiatric diagnosis.
- 9. Cole PM, Hall SE, Hajal NJ. Emotion dysregulation as a risk factor for psychopathology. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. 2nd ed. Wiley; 2013. pp. 341–373.. Describes how emotion dysregulation in childhood confers transdiagnostic vulnerability to a wide range of psychopathological outcomes later in life.
- 10. Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012.. Describes relations between diverse forms of psychopathology and both tonic and phasic measures of HF-HRV.
- 11. Beauchaine TP, Thayer JF. Heart rate variability: A transdiagnostic biomarker of psychopathology. Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2015.08.004. in press.. Provides an expanded account of links between psychopathology and RSA, and links RSA to prefrontal cortex function.
- 12.Thompson RA. Emotion and self-regulation. In: Thompson RA, editor. Nebraska Symposium on Motivation: Vol 36 Socioemotional development. University of Nebraska Press; 1990. pp. 367–469. [Google Scholar]
- 13. Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion regulation strategies and psychopathology: A meta-analysis. Clin Psychol Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004.. Examines relations between specific emotion-regulation strategies and different subtypes of psychopathology. A key finding is that internalizing disorders are consistently associated with effortful self-regulatory strategies, whereas externalizing disorders are not.
- 14.Beauchaine TP, Gatzke-Kopp LM. Instantiating the multiple levels of analysis perspective in a program of study on externalizing behavior. Dev Psychopathol. 2012;24:1003–1018. doi: 10.1017/S0954579412000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beauchaine TP, Gatzke-Kopp LM, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008.. Describes how dysfunction in subcortical circuits responsible for emotion generation interact with cortical circuits responsible for emotion regulation to result in externalizing psychopathology.
- 16. Beauchaine TP. Future directions in emotion dysregulation and youth psychopathology. J Clin Child Adol Psychol. doi: 10.1080/15374416.2015.1038827. in press.. Reviews the past 20 years of emotion regulation research among children and adolescents with psychopathology, and offers recommendations for future research.
- 17. Beauchaine TP, McNulty T. Comorbidities and continuities as ontogenic processes: Toward a developmental spectrum model of externalizing behavior. Dev Psychopathol. 2013;25:1505–1528. doi: 10.1017/S0954579413000746.. Presents a developmental model of externalizing behavior in which heritable impulsivity interacts with socialized deficiencies in ER across the lifespan to promote increasingly intractable forms of delinquency.
- 18. Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. P Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101.. Describes maturation of the human cortex from preschool to early adulthood, noting that the PFC, which is responsible for ER, matures last.
- 19. Snyder JJ. Coercive family processes in the development of externalizing behavior: Incorporating neurobiology into intervention research. In: Beauchaine TP, Hinshaw SP, editors. Oxford handbook of externalizing spectrum disorders. Oxford University Press; in press.. Describes how aversive family interaction patterns in the homes of externalizing children and adolescents promote emotion dysregulation and alter patterns of prefrontal brain function over time.
- 20.Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol Psychiat. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 21.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heatherton TF. Neuroscience of self and self-regulation. Annu Rev Psychol. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616.. Reviews the neural substrates of self-regulation, including top-down cortical inhibition of subcortically-generated emotions.
- 23.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. JAMA Psychiat. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon KE, Sauder C, Beauchaine TP, Gatzke-Kopp LM. Disrupted effective connectivity between the medial frontal cortex and the caudate in adolescent boys with externalizing behavior disorders. Crim Justice Behav. 2009;36:1141–1157. [Google Scholar]
- 25.Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x.. Describes the phylogeny of sympathetic and parasympathetic innervation of the heart, and their relation to the emergence of complex social behavior.
- 27. Zisner A, Beauchaine TP. Physiological methods and developmental psychopathology. In. In: Cicchetti D, editor. Developmental psychopathology. 3rd ed. Wiley; in press.. Offers comprehensive recommendations for conducting autonomic nervous system assessments in developmental and clinical samples.
- 28. Reyes del Paso GA, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027.. Provides a review of the literature that challenges the assumption that low- and mid-frequency HRV are of pure sympathetic origin, and demonstrates significant parasympathetic influence in these frequency bands.
- 29.Beauchaine TP. Physiological markers of emotion and behavior dysregulation in externalizing psychopathology. Monogr Soc Res Child. 2012;77:79–86. doi: 10.1111/j.1540-5834.2011.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, Gatzke-Kopp LM. Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. J Child Psychol Psyc. 2009;50:1357–1364. doi: 10.1111/j.1469-7610.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- 31.Hastings PD, Sullivan C, McShane KE, Coplan RJ, Utendale WT, Vyncke JD. Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: Direct mothers and moderated fathers. Child Dev. 2008;79:45–64. doi: 10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahs F, Sollers JJ, III, Furmark T, Fredrikson M, Thayer JF. High-frequency heart rate variability and cortico-striatal activity in men and women with social phobia. NeuroImage. 2009;47:815–820. doi: 10.1016/j.neuroimage.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 33.Rash JA, Aguirre-Camacho A. Attention-deficit hyperactivity disorder and cardiac vagal control: A systematic review. Attn Deficit Hyperact Disorders. 2012;4:167–177. doi: 10.1007/s12402-012-0087-1. [DOI] [PubMed] [Google Scholar]
- 34.Neuhaus E, Bernier R, Beauchaine TP. Social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. J Autism Dev Disord. 2014;44:730–737. doi: 10.1007/s10803-013-1923-7. [DOI] [PubMed] [Google Scholar]
- 35.de Wied M, van Boxtel A, Matthys W, Meeus W. Verbal, facial and autonomic responses to empathy-eliciting film clips by disruptive male adolescents with high versus low callous-unemotional traits. Journal Abnorm Child Psych. 2012;40:211–223. doi: 10.1007/s10802-011-9557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rottenberg J. Cardiac vagal tone in depression: A critical analysis. Biol Psychol. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Crowell S, Beauchaine TP, McCauley E, Smith C, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicidal behavior in adolescent girls. Dev Psychopathol. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- 38.Asmundson GJ, Stein MB. Vagal attenuation in panic disorder: An assessment of parasympathetic nervous system function and subjective reactivity to respiratory manipulations. Psychosom Med. 1994;56:187–193. doi: 10.1097/00006842-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Sloan RP, Shapiro PA, Bigger JT, Bagiella M, Steinman RC, Gorman JM. Cardiac autonomic control and hostility in healthy subjects. Am J Cardiol. 1994;74:298–300. doi: 10.1016/0002-9149(94)90382-4. [DOI] [PubMed] [Google Scholar]
- 40.Shahrestani S, Stewart EM, Quintana DS, Hickie IB, Guastella AJ. Heart rate variability during social interactions in children with and without psychopathology: A meta-analysis. J Child Psychol Psyc. 2014;55:981–989. doi: 10.1111/jcpp.12226. [DOI] [PubMed] [Google Scholar]
- 41.Thayer JF, Hansen AL, Saus-Rose E, Helge Johnsen B. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 42. Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav R. 2013;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009.. Summarizes literature that links RSA to PFC and both physical and psychological morbidity.
- 43. Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. NeuroImage. 2007;35:498–708. doi: 10.1016/j.neuroimage.2006.12.027.. Describes relations between PFC function and RSA.
- 44.Maren S, Phan KL, Liberzon I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nature Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003.. Emphasizes the importance of interconnected brain networks for controlling behavior, rather than isolated brain regions.
- 46.Hansen AL, Johnsen BH, Thayer JF. Vagal influence in the regulation of attention and working memory. Int J Psychophysiol. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 47. Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056.. Describes correspondences between (1) PFC activity and reactivity and (2) RSA activity and reactivity during neuroimaging.
- 48.Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. J Abnorm Psychol. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]