Abstract
Objectives
To examine longitudinal changes in movement sequencing in prodromal Huntington’s disease (HD) participants (795 prodromal HD; 225 controls) from the PREDICT-HD study.
Methods
Prodromal HD participants were tested over seven annual visits and were stratified into three groups (low, medium, high) based on their CAG-Age Product (CAP) score, which indicates likely increasing proximity to diagnosis. A cued movement sequence task assessed the impact of advance cueing on response initiation and execution via three levels of advance information.
Results
Compared to controls, all CAP groups showed longer initiation and movement times across all conditions at baseline, demonstrating a disease gradient for the majority of outcomes. Across all conditions, the high CAP group had the highest mean for baseline testing, but also demonstrated an increase in movement time across the study. For initiation time, the high CAP group showed the highest mean baseline time across all conditions, but also faster decreasing rates of change over time.
Conclusions
With progress to diagnosis, participants may increasingly use compensatory strategies, as evidenced by faster initiation. However, this occurred in conjunction with slowed execution times, suggesting a decline in effectively accessing control processes required to translate movement into effective execution.
Keywords: Huntington disease, sequencing, movement, basal ganglia, predictive testing
Introduction
Cross-sectional motor studies in late prodromal Huntington disease (HD) report various motor deficits, including abnormal muscle stretch reflexes, diminished rapid alternating movements, increased movement jerkiness, diminished motor timing, and impaired sequence learning (Kirkwood et al. 2000; Smith et al. 2000; Snowden et al. 2002; Farrow et al. 2006; Feigin et al. 2006; Andrich et al. 2007; Hinton et al. 2007; Doyon 2008; Ghilardi et al. 2008). Longitudinal changes in motor functioning over 2–10 years prior to onset include decline in repetitive alternating movements, psychomotor speed, motor timing, saccades and paced and self-paced finger tapping (Penney et al. 1990; Giordani et al. 1995; Campodonico et al. 1996; Kirkwood et al. 1999; Lemiere et al. 2002, 2004; Witjes-Ane et al. 2007; Solomon et al. 2008; Antoniades et al. 2010; Rowe et al. 2010; Maroof et al. 2011).
The impact of cognitive processing on motor function has not been systematically investigated in HD. Since most day-to-day motor functions are likely to have a cognitive element, the assessment of cognitive-motor functioning may provide an important measure of disease prognosis that warrants further study. Longitudinal changes in cognitive-motor control in prodromal HD in the context of movement sequencing places greater demands on motor programming and online-control processes compared with simple repetitive movements such as finger tapping (see Beglinger et al. 2010). The striatum, which begins atrophy decades before diagnosis (Thieben et al. 2002; Aylward et al. 2004; Ciarmiello et al. 2006; Tabrizi et al. 2011, 2012), may play a key role in programming, along with interconnecting thalamocortical areas comprising the motor circuit (ventrolateral thalamus, supplementary motor area).
The PREDICT-HD study adapted a cued movement-sequencing task developed by Bradshaw et al. (1992) which varied levels of visual cueing (low, moderate, and high levels of advance information). The study demonstrated that “at-risk” individuals were significantly slower than controls with high levels of advance information. The ability to make use of greater levels of advance information requires rapid acquisition and integration of subsets of movements into motor planning (Georgiou et al. 1995). However, at-risk individuals, and especially manifest HD patients, also demonstrated slower movement with low advance information, suggesting difficulty in using internal commands to initiate movements (Bradshaw et al. 1992). The Bradshaw et al. study was the first to suggest that cued movement sequencing could be a sensitive indicator of programming deficits in prodromal HD. Further research found that probability of diagnosis within 5 years was associated with increased mean movement times for both low and moderate levels of advance information (Farrow et al. 2006), suggesting likely cognitive-motor deficits as individuals approach diagnosis. The PREDICT-HD study provides the first opportunity to examine longitudinal changes in programming and online control of motor sequences in a large prodromal HD sample stratified into baseline progression groups. Mutation-positive individuals were grouped into three categories (low, medium, high) based on their CAG-Age Product (CAP) score (Zhang et al. 2011), with mutation-negative controls constituting a fourth group. We aimed to determine whether initial baseline testing and longitudinal (up to 7 years) motor performance differed among the groups, with an emphasis on differences between each CAP group and the controls. Specifically, we predicted that intercepts (initial baseline levels) would vary among the CAP groups with the most progressed group (high group) showing the greatest deterioration. In addition, we predicted that linear slopes would also differ, with the high CAP group showing the greatest change over time.
Methods
Participants
A total of N = 1020 participants (795 prodromal HD, 225 controls) were included in the data analysis. Data were collected at 32 PREDICT-HD sites located in the USA, Canada, Australia, Germany, Spain and the UK. The motor-sequencing task was performed at varying visits throughout the PREDICT-HD study. All participants provided written informed consent in accordance with ethical guidelines in the 1964 Declaration of Helsinki.
Inclusion criteria required independent genetic testing confirming CAG length prior to entry into the PREDICT-HD study. Exclusion criteria were: clinical evidence of unstable medical or psychiatric illness; alcohol or drug abuse within the past year; learning or developmental disability requiring special education; history of another neurological condition; or an unstable psychiatric condition at time of testing.
Participant neurological examination
The Unified Huntington’s Disease Rating Scale (UHDRS; Huntington Study Group 1996) was administered by trained examiners. All participants received a motor examination, which was the basis for the Total Motor Score (TMS) of the UHDRS, and the Diagnosis Confidence Level (DCL). The DCL is the motor examiner’s confidence that the participant exhibits unequivocal signs of HD motor impairment. Diagnosis is defined as DCL = 4, which indicates the examiner has ≥99% confidence that the patient has motor abnormalities that are unequivocal signs of HD. Sixteen participants with DCL = 4 at baseline were not included in any analyses, but 137 individuals received DCL = 4 during the course of the study and were included in the analysis. The possibility of receiving a motor diagnosis was accommodated in the statistical models (see below).
Participant stratification
Gene carriers were stratified into three groups based on their CAG-Age Product (CAP) score using the method developed by Zhang et al. (2011) for PREDICT-HD. The CAP score is computed as CAP = (Age at entry) × (CAG − 33.66) and is similar to the “disease burden” score of Penny et al. (1997). Cut-offs for the three CAP groups (low, medium, and high) were based on an optimization algorithms using the PREDICT-HD participants. Based on stratification the estimated time to diagnosis was > 12.78 years for the low CAP group, between 12.78 and 7.59 for the medium CAP group, and < 7.59 years for the CAP high group. The control group had a parent with HD but did not have the expanded CAG gene. Baseline demographic and clinical data for each sub-group are shown in Table I.
Table I.
Demographic and baseline clinical data for the prodromal HD CAP groups (Low, Medium, High) and Controls.
| Controls (C) | Low (L) | Med (M) | High (H) | P-value and comparisons | |
|---|---|---|---|---|---|
| Count | N = 225 | N = 210 | N = 274 | N = 311 | |
| Demographics | |||||
| Age (Mean ± SD; range) | 43.9 ± 11.5; 19–84 | 35.0 ± 7.7; 20–57 | 41.0 ± 9.5; 26–72 | 44.2 ± 9.8; 18–76 | <.0001; C,H> M>L |
| Gender (count) | 143F, 82M | 142F, 68M | 182F, 92M | 185F, 126M | 0.1988 |
| Education (Mean years; range) | 14.7; 9–20 | 14.5; 8–20 | 14.4; 8–20 | 14.1; 8–20 | 0.1346 |
| CAG Repeats (mean; range) | N/A | 40.8; 38–45 | 42.2; 38–47 | 43.8; 39–61 | <.0001; L< M< H |
| Estimated IQ (Mean ± SD; range) | 113.4 ± 7.4; 90–133 | 112.5 ± 7.5; 75–127 | 112.1 ± 7.9; 84–127 | 111.8 ± 8.3; 75–128 | 0.1406 |
| UHDRS Total Motor Score (TMS) | |||||
| BASELINE (Mean ± SD; range) | 2.7 ± 3.4; 0–22 | 2.9 ± 3.3; 0–18 | 4.1 ± 4.3; 0–25 | 6.8 ± 6.3; 0–35 | <.0001; C,L< M< H |
| UHDRS Diagnosis Confidence Level (DCL) | |||||
| BASELINE (Mean; range) | 0.4; 0–2 | 0.6; 0–2 | 0.7; 0–3 | 1.1; 0–3 | <.0001; C,L< M< H |
| BDI–II | |||||
| BASELINE (Mean ± SD; range) | 4.5 ± 5.3; 0–32 | 7.3 ± 8.3; 0–46 | 9.0 ± 9.1; 0–47 | 7.7 ± 8.7; 0–48 | <.0001; C< L,H< M |
Note: CAG, cytosine-adenine-guanine repeat (number of repeats > 40 is full penetrance); Estimated IQ, estimated full scale IQ; UHDRS, Unified Huntington’s Disease Rating Scale, Motor Score has max score 124; UHDRS Confidence Ratings (max score 4); BDI–II, Beck Depression Inventory II score (max score is 63, normal range 0–9); P-values are based on the test appropriate for the type of variable in question (e.g., chi-squared tests for categorical variables and ANOVA for continuous variables; see text).
Baseline CAP group comparisons of the demographic and clinical data were performed using ANOVA with post-hoc pairwise t-tests for the continuous variables, and a chi-squared test for gender. The control and high CAP groups were significantly older than the medium and low CAP groups (pairwise P values < 0.001). The medium CAP group was significantly older than the low CAP group (pairwise P < 0.001). There were no significant differences in gender, education or estimated IQ. The medium CAP group had significantly higher depression scores than other groups (pairwise P values < 0.001). Low and high CAP groups had significantly higher depression scores than the control group (pairwise P values < 0.001). UHDRS DCL and TMS means were in the expected direction, with the high group scoring significantly highest (omnibus P < 0.0001).
Neuropsychological assessments
To estimate premorbid intellectual functioning at baseline, participants in the USA, Canada, and Australia completed the American National Adult Reading Test (Gladsjo et al. 1999); participants in the UK completed the National Adult Reading Test (NART-2; Nelson and Willison 1991); Spanish participants completed the Word Accentuation Test (Del Ser et al. 1997); and German participants completed the Wortschatztest (WST; Schmidt and Metzler 1992). Estimated premorbid verbal intelligence quotient (IQ) was calculated on raw test scores and possible variation in IQ among countries is acknowledged. Participants completed the Beck Depression Inventory-II (BDI–II; Beck et al. 1996) because depression may be associated with increased motor slowing (Rogers et al. 2002).
Computerized cued movement sequence task
Apparatus
The computerized cued movement sequence task (Stout et al. 2011), based on a previous paradigm (Bradshaw et al. 1992; Georgiou et al. 1993), was adapted for a touch-screen monitor by the Indiana University Clinical Cognitive Neuroscience Laboratory. The task was administered on an Athlon 900MHz computer running Windows 98, 2nd edition (Microsoft, Seattle, WA). The computer monitor was a KDS Pixel Touch, 17 FST Capacitive PC Touch Monitor (Ontario, CA) using interface software from Microsoft (TouchWare for Windows, Version 5.4, Methuen, MA). The screen was enclosed in a wooden box inclined 38°. The monitor displayed a series of blue “buttons” arranged, in 10 vertical pairs, in a line across the screen, with two “start” buttons, placed horizontally to the left of the first vertical pair, and a “finish” button to the right. Each button was ~13 mm in diameter; distance between buttons was ~30 mm diagonally and 18 mm horizontally.
Procedure
To initiate each trial, participants touched the white colored start button on the left with the index finger of their dominant hand. During each trial, one button from every pair, randomly generated, turned white in a sequential manner down the pathway (i.e., left to right). The task consisted of three conditions that systematically manipulated the amount of advance information available for movement preparation. This was achieved by illumination of the buttons (i.e., change to white) in one of three ways. Condition 1 (Low Advance Information), the next target button was not illuminated until the current button was released; each movement therefore had to start without prior knowledge of the next cued button. Condition 2 (Moderate Level of Advance Information), the next button was illuminated as the current button was depressed; therefore, one position was cued ahead of each movement, allowing preparation of the next move. Condition 3 (High Level of Advance Information), the start button and the white button in the first column were presented simultaneously; the next target button was illuminated as a button was released, such that two positions were cued ahead of each movement.
The participant was instructed to touch the white button in each pair quickly and accurately. Illumination of a button was extinguished once depressed. The participant continued to touch each illuminated button in the 10-button sequence; the trial was completed when the button to the right of the last column was depressed. The computer recorded the time each button was held down (initiation time or down time) and the time between the release of one button and the depression of the next (movement time). Errors were recorded and occurred if a non-cued button was depressed, if a single button was depressed twice, or if no response had been made after 1000 ms of illumination; a text box appeared on the screen instructing the participant to restart the trial by returning to the start button. During baseline, participants were allowed a maximum of 28 attempts to get 16 trials correct for each of the three conditions, which were presented in a pseudorandom order across participants. A preliminary trial analysis after the initial baseline visit suggested that for conditions 1 and 2 the number of attempts and the number of trials could be reduced to 20 and eight, respectively, for subsequent visits. To match the number of trials between conditions, and between all visits, the first eight trials were selected for each condition during baseline to match the eight trials for all subsequent visits. Similarly for condition 3, the first eight trials were selected for all visits. A correlation analysis performed for condition 3 at baseline on the first eight trials, compared to the total of 16 trials, yielded no evidence of systematic outcome bias due to trial reduction. Consequently, eight trials were averaged for each condition. Participants received two practice trials prior to each condition.
In summary, three types of measurements were recorded: initiation time, movement time, and errors. Each type was recorded for each of the three experimental conditions. Two types of summary scores were computed for initiation and movement times: mean and standard deviation (SD) among trials in a condition. There were 15 outcome variables: initiation time mean and SD for the three conditions (six total), movement time mean and SD for the three conditions (six total), and errors for the three conditions (three total).
Statistical analyses
Linear mixed effects regression (LMER; Verbeke and Molenberghs 2000) was used for the longitudinal analysis. Each outcome variable was analysed in isolation with the predictors of time, CAP group (dummy codes), time by CAP group interactions, and the covariates described below. In the context of longitudinal data, the intercept of the LMER model represents the outcome level at the first time point. The slope of the LMER model represents the annual rate of change. The time by CAP group interactions indicate that the intercepts and slopes could vary by group. The primary goal of the analysis was to determine if there were statistically reliable group differences in the intercept slopes.
The time metric for the analysis was duration in the study, defined as the current age minus the age at entry into the study. Duration zero indicated the first observation, with the intercept of a group regression line in the LMER model representing the initial or baseline level of the outcome.
For each outcome variable, three models were estimated and their relative fit to the data assessed. Model 1 was a null model that did not have any group intercept or slope differences, with each group having the same starting level and rate of change over time. Model 2 had the predictors of Model 1 plus group intercept differences, but not group slope differences. Thus, each group had a different starting level, but the same rate of change over time. Model 3 had all the effects of Model 2 plus effects and slope differences among the groups. Each group had its unique starting levels as well as its own rate of change over time. All models included the covariates of years of education, age at entry, BDI total score, gender, and a time-varying dummy variable indicating whether a participant was diagnosed with HD (0 = no, 1 = yes). Although the groups did not differ on gender and education, they were still included as covariates in all models (together with age and BDI) so as to rule out any possible influencing effects on the pattern of results. IQ was not included in the model as it is difficult to equate over countries and languages. Moreover, our ancillary analysis (not presented) suggests that education is a superior representative of adult intelligence level at study entry.
LMER has a mix of fixed and random effects, the former being analogous to traditional group-level regression coefficients, and the latter, individual-specific coefficients. The intercept and slope group differences were fixed effects in the LMER models. Individual variability and dependency due to repeated measures were modelled by random intercepts and slopes in all models. Maximum likelihood was used to estimate the parameters, which yields unbiased parameter estimates with missing data under the widely applicable assumption of ignorability (Little and Rubin 2002). Given the number of outcome variables (i.e., 15), the analysis focused on effect sizes derived from the maximum likelihood estimation rather than hypothesis testing. Focus was on global and specific effect sizes. The global effect size indexed any type of intercept or slope difference among the groups. The specific effect size indexed standardized intercept or slope differences between a CAP group and the control group. The control group was the reference group for every specific effect size estimate.
Global effect size was based on Akaike’s Information Criterion (AIC) (Akaike 1973), an index of model fit. The AIC values from the three fitted models for each outcome were scaled by subtracting out the smallest value. The scaling is expressed as ΔAICk = AICk − min(AIC), where AICk is the value for the kth model (k = 1, 2, 3) and min(AIC) is the minimum value of the set of three. ΔAIC is a measure of statistical distance between models and an index of effect size (Long 2012). Values close to zero indicate that two models have very similar fit, whereas values much larger than zero indicate that two models have very different fit (one model being greatly superior to another). ΔAIC of approximately 10 or greater indicates substantial separation and a large effect (Anderson 2008).
The specific effect size indexed an intercept or slope difference between two groups was defined as the difference in parameter estimates divided by the standard error of the difference, and denoted as the Z-ratio. The reference group was always the control group, so that the Z-ratio was the standardized difference between a CAP group and the control group (CAP minus control). Rather than use arbitrary cutoffs, emphasis was on relative magnitudes of Z-ratios as indicators of effect sizes of different effects.
All analyses were performed using the statistical software program R (v2.13.0) (R Development Core Team 2010). LMER models were estimated using the lmer function from the add-on package lme4 (Bates 2005). Graphical and descriptive methods were used to assess consistency with assumptions, especially normality of the random effects and random error. Preliminary results (not presented) indicated reasonable agreement with assumptions.
Results
Table II shows the global fit of each model as indexed by ΔAIC. The best fitting model has ΔAIC = 0 and the other models have ΔAIC > 0, with larger values indicating worse fit relative to the best fitting model. Recall that Model 1 is the null model that has a common intercept and slope among the groups (no group differences), Model 2 has unequal intercepts but a common slope among the groups (intercept differences), and Model 3 has unequal intercepts and slopes among the groups (intercept and slope differences).
Table II.
Global model fit results for initiation time (mean and SD), errors, and movement time (mean and SD) among three conditions.
| Variable | ΔAIC
|
||
|---|---|---|---|
| Model 1 a | Model 2 b | Model 3 c | |
| Condition 1 Initiation Time: Mean | 31.22 | 6.02 | 0 |
| Condition 2 Initiation Time: Mean | 37.64 | 8.61 | 0 |
| Condition 3 Initiation Time: Mean | 31.21 | 7.68 | 0 |
| Condition 1 Initiation Time: SD | 10.67 | 0 | 20.23 |
| Condition 2 Initiation Time: SD | 13.44 | 0 | 10.37 |
| Condition 3 Initiation Time: SD | 14.05 | 0 | 17.77 |
| Condition 1 Movement Time: Mean | 98.98 | 14.66 | 0 |
| Condition 2 Movement Time: Mean | 76.84 | 12.64 | 0 |
| Condition 3 Movement Time: Mean | 56.3 | 16.42 | 0 |
| Condition 1 Movement Time: SD | 72.11 | 0 | 19.26 |
| Condition 2 Movement Time: SD | 96.03 | 0 | 25.6 |
| Condition 3 Movement Time: SD | 57.14 | 0 | 18.08 |
| Condition 1 Errors | 45.19 | 0 | 19.44 |
| Condition 2 Errors | 5.37 | 0 | 17.43 |
| Condition 3 Errors | 20.11 | 0 | 13.69 |
Note: ΔAIC = 0 indicates the best fitting model in a row.
Model 1 has no group differences;
Model 2 has group intercept differences;
Model 3 has group intercept and slope differences.
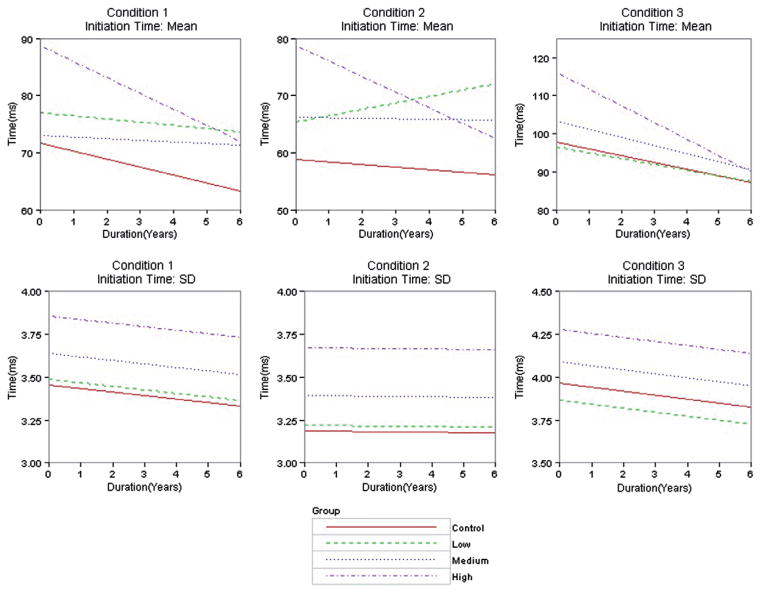
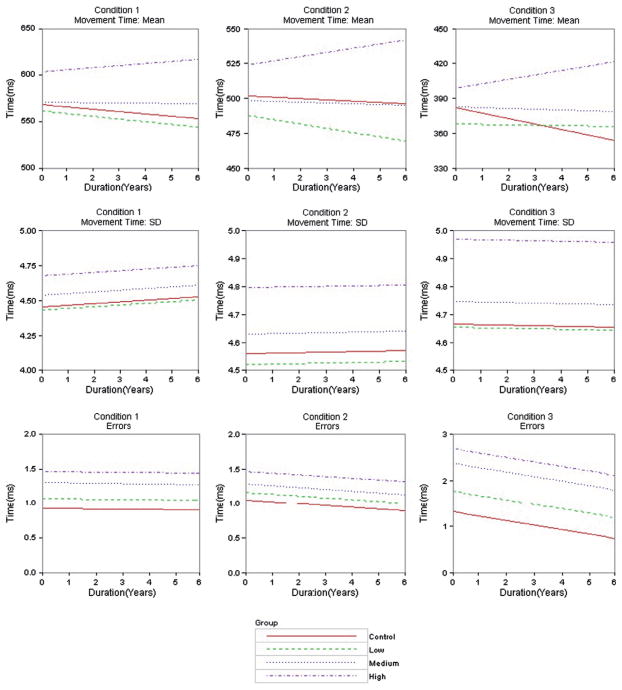
Table III shows estimated intercepts, slopes, associated standard errors (SEs) and Z value [Z] of the best fitting model for each outcome according to Table II. Recall the Z value was the standardized difference between a CAP group (low, medium, high) and the control group (CAP minus control). When the outcome had Model 3 as the best fitting (initiation time mean, movement time mean), the intercept and slope estimates varied among the groups. When the outcome had Model 2 as the best fitting (initiation time SD, movement time SD, errors), intercept estimates varied among the groups, but the slope estimate was constant. To facilitate interpretations, Figure 1 shows graphs of the group regression curves based on the parameter estimates in Table III. All results were adjusted for the covariates, but specific information about the covariates is omitted for clarity (graphs in Figure 1 were constructed fixing the covariates at their mean values).
Table III.
Estimated intercept and slope (SE)[Z-value] of CAP groups (Low, Medium, High) and controls (Control) for the best fitting model of each outcome (see Table II).
| Outcome | Best Model a | Intercept
|
Slope
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Low | Medium | High | Control | Low | Medium | High | ||
| Condition 1 Initiation Time: Mean | 3 | 71.67 (10.03) | 77.05 (9.40)[1.11] | 73.01 (9.71)[0.31] | 88.81 (9.80)[4.00] | −1.40 (1.12) | −0.56 (0.98)[0.56] | −0.28 (0.84)[0.80] | −2.81 (0.85)[−1.00] |
| Condition 2 Initiation Time: Mean | 3 | 58.79 (9.58) | 65.35 (9.00)[1.41] | 66.22 (9.30)[1.76] | 78.69 (9.38)[4.84] | −0.43 (1.35) | 1.11 (1.18)[0.86] | −0.09 (0.99)[0.20] | −2.71 (1.01)[−1.35] |
| Condition 3 Initiation Time: Mean | 3 | 97.86 (10.19) | 96.44 (9.56)[−0.28] | 103.24 (9.85)[1.16] | 116.03 (9.96)[4.02] | −1.78 (1.02) | −1.50 (0.89)[0.20] | −2.13 (0.77)[−0.27] | −4.34 (0.79)[−1.98] |
| Condition 1 Initiation Time: SD | 2 | 3.46 (0.23) | 3.49 (0.21)[0.31] | 3.64 (0.22)[2.02] | 3.86 (0.22)[4.58] | −0.02 (0.01) | −0.02 (0.01) | −0.02 (0.01) | −0.02 (0.01) |
| Condition 2 Initiation Time: SD | 2 | 3.19 (0.26) | 3.22 (0.24)[0.30] | 3.39 (0.25)[1.97] | 3.67 (0.26)[4.73] | −0.002 (0.02) | −0.002 (0.02) | −0.002 (0.02) | −0.002 (0.02) |
| Condition 3 Initiation Time: SD | 2 | 3.96 (0.2) | 3.87 (0.18)[−1.13] | 4.09 (0.19)[1.57] | 4.28 (0.19)[4.08] | −0.02 (0.01) | −0.02 (0.01) | −0.02 (0.01) | −0.02 (0.01) |
| Condition 1 Movement Time: Mean | 3 | 568.3 (15.18) | 561.66 (14.18)[−0.97] | 571.2 (14.67)[0.47] | 603.35 (14.83)[5.79] | −2.43 (1.39) | −2.90 (1.19)[−0.26] | −0.37 (1.02)[1.20] | 2.22 (1.04)[2.68] |
| Condition 2 Movement Time: Mean | 3 | 501.52 (15.17) | 488.01 (14.22)[−1.90] | 498.52 (14.71)[−0.46] | 524.18 (14.85)[3.61] | −0.90 (1.85) | −3.12 (1.61)[−0.91] | −0.57 (1.35)[0.14] | 2.99 (1.38)[1.69] |
| Condition 3 Movement Time: Mean | 3 | 381.88 (18.48) | 368.01 (17.31)[−1.57] | 382.53 (17.88)[0.08] | 399.12 (18.09)[2.19] | −4.69 (1.94) | −0.33 (1.70)[1.69] | −0.58 (1.47)[1.69] | 3.80 (1.50)[3.46] |
| Condition 1 Movement Time: SD | 2 | 4.45 (0.07) | 4.43 (0.07)[−0.77] | 4.54 (0.07)[3.00] | 4.68 (0.07)[8.13] | 0.01 (0.004) | 0.01 (0.004) | 0.01 (0.004) | 0.01 (0.004) |
| Condition 2 Movement Time: SD | 2 | 4.55 (0.07) | 4.52 (0.06)[−1.29] | 4.63 (0.07)[2.53] | 4.80 (0.07)[8.80] | 0.002 (0.004) | 0.002 (0.004) | 0.002 (0.004) | 0.002 (0.004) |
| Condition 3 Movement Time: SD | 2 | 4.67 (0.10) | 4.66 (0.10)[−0.23] | 4.75 (0.10)[1.98] | 4.97 (0.10)[7.48] | −0.002 (0.005) | −0.002 (0.005) | −0.002 (0.005) | −0.002 (0.005) |
| Condition 1 Errors | 2 | 0.93 (0.21) | 1.08 (0.2)[1.59] | 1.30 (0.21)[4.47] | 1.47 (0.21)[6.53] | −0.004 (0.02) | −0.004 (0.02) | −0.004 (0.02) | −0.004 (0.02) |
| Condition 2 Errors | 2 | 1.05 (0.25) | 1.16 (0.24)[0.96] | 1.29 (0.25)[2.37] | 1.47 (0.25)[4.27] | −0.03 (0.02) | −0.03 (0.02) | −0.03 (0.02) | −0.03 (0.02) |
| Condition 3 Errors | 2 | 1.33 (0.70) | 1.78 (0.65)[1.47] | 2.38 (0.68)[3.78] | 2.70 (0.69)[5.00] | −0.10 (0.04) | −0.10 (0.04) | −0.10 (0.04) | −0.10 (0.04) |
A Z-value represents a standardized comparison with the control group. All estimates were adjusted for the covariates of years of education, age at study entry, BDI–II total score, gender, and motor diagnosis.
Note:
Model 2 had intercept differences among groups, but a common slope; Model 3 had intercept and slope differences among groups.
Figure 1.
Regression curves of outcome variables over time by group type. Rows represent outcome variable and columns represent information condition. Curves are based on the parameter estimates in Table III.
Initiation time mean
Table II shows that Model 3 had the best fit for initiation time mean in all conditions. Model 2 was the second best fitting in all conditions (ΔAIC ranged from 6.02 to 8.61), followed by Model 1 (ΔAIC ranged from 31.21 to 37.64). Table III shows the Model 3 estimated intercept and slope for each group along with SEs and the Z values (controls are the reference group). In all three conditions, there was a relatively large standardized intercept difference (Z ≥ 4) between the high CAP group and controls, with the high CAP group having a much higher starting time. The high CAP group also had the largest standardized slope difference indicating a faster decline than the controls. The high CAP group standardized slope difference increased in absolute value with condition (Z = −1.00, −1.35, −1.98, respectively) with the third condition showing a Z value that was almost twice that of the first condition. The fitted regression curves for the groups are shown in the first row of Figure 1, with the estimated intercept increasing as the group increased from control to high CAP Group; with one exception (condition 2 low CAP group), all the groups had a decreasing linear trend over time, but the high CAP group always had the fastest rate of decline within a condition.
Initiation time SD
Table II indicates that Model 2 was the best fitting for initiation time SD in all conditions. The intercept estimates and common slope estimate are shown in Table III. In all conditions the standardized intercept difference relative to controls increased as CAP group increased, with the high CAP group having the largest difference (all Z values > 4). The second row of Figure 1 shows fitted regression lines for the three conditions. In all conditions, the intercept increased as the group increased (control to high CAP group), and the common slope for the groups was negative (showing a decline), but decline was faster in conditions 1 and 3.
Movement time mean
As seen in Table II, the best fitting model for movement time mean in all conditions was Model 3. The estimated intercepts and slopes are shown in Table III, and graphs of the fitted regression lines in the fourth row of Figure 1. The estimated intercept increased as CAP group increased (low to high), but the controls had a higher intercept than the low group in all conditions. The high CAP group had the largest standardized intercept difference with the control group in all conditions (Z values > 2). The high CAP group had a positive slope (indicating slowing) in all conditions, whereas all the other groups had negative slopes (indicating decline). The standardized slope difference between the high CAP group and the controls was strongest for the third condition (Z = 3.46), followed by the first condition (Z = 2.68) and then the second condition (Z = 1.69). The fitted regression lines illustrate the contrast of the high CAP group’s positive linear trend in all conditions versus the negative linear trend of the other groups.
Movement time SD
Model 2 was the best fitting for movement time SD in all conditions (see Table II). The estimated intercepts and common slope are shown in Table III and the fitted regression lines are listed in the fifth row of Figure 1. The estimated intercept increased as CAP group increased, but the controls had a larger intercept than the low group in all conditions. The standardized intercept difference for the high CAP group had the highest effect sizes of any outcomes (Z values > 7). As the fitted regression curves show, the common slope was slightly positive for conditions 1 and 2, and slightly negative for condition 3.
Errors
Table II shows that Model 2 had the best fit for errors in all conditions. The estimated intercepts and common slope are listed in Table III. The standardized intercept difference increased as CAP increased with the high CAP group difference being the largest (Z values > 4). The third row of Figure 1 shows the fitted regression lines. In all conditions, the estimated intercept increased as group increased, and the common slope was negative, but decline increased as condition increased.
Discussion
This is the first study to investigate sequential motor performance for detecting disease progression in the largest sample of prodromal HD participants and over such an extended period of time. Baseline progression (at time of entry) was indexed by CAP group membership. Results showed that as CAP group increased from low to high, initiation times and movement times (means, SDs, and errors) were longer across all conditions at initial baseline testing (apart from a few instances where the low/medium CAP groups showed faster times) demonstrating an apparent disease gradient for intercepts for the majority of outcomes. A more complex gradient was observed for change over time, with initiation time showing more rapid decline as CAP group increased, but movement times becoming slower. Across all conditions, the slopes for mean movement time were indicating slowing only for the high CAP group who demonstrated an increase in movement time (see Figure 1). This is in accord with our prediction that closer estimated proximity to diagnosis would be a significant predictor of longitudinal change. Interestingly, although initiation time mean for the high CAP group was overall higher at initial baseline (followed in the most part by the medium CAP group), compared with all other groups (with the minor exception of condition 3 where the low CAP group was faster than controls), there was a substantial slope difference relative to controls, with faster decreases in initiation times across all conditions (initiation time SD remained fairly constant; see Table III and Figure 1). These findings suggest that in the high CAP group, although movement times were becoming slower across the sequential biennial follow-ups, initiation times were getting faster. Although medium and high CAP groups made more errors at baseline (and across all conditions), compared with other groups, the slope for all groups was close to zero suggesting a relatively constant level of errors across follow-ups (see Figure 1).
Impairments in the programmed control of sequential movement, especially for those individuals with greater baseline progression (high CAP group), suggests a likely increase in the amount of ongoing and online, non-automatic, control of their movements, compared with low and medium CAP groups. This suggests a progressive impairment in the execution of sequential motor programs, and a greater reliance upon the ongoing deliberate control of movement, manifest during the “in-flight” or execution phase when a switch is required between motor segments (reflecting movement time). This may also explain why this and many previous studies have reported impaired movement times, rather than initiation times, in symptomatic HD (Hefter et al. 1987; Girotti et al. 1988; Agostino et al. 1992; Bradshaw et al. 1992; Georgiou et al. 1995; Farrow et al. 2006; Yágüez et al. 2006). This pattern of deficit may represent an early dysfunction in the motor circuit in the high CAP group as they approach diagnosis with respect to phasic activity that may impair the cue necessary to start the pre-movement activity for the next switch movement in the sequence (Tanji and Kurata 1985; Brotchie et al. 1991; Boecker et al. 1998; Elsinger et al. 2006; Lehéricy et al. 2006). Bradshaw et al. (1992) showed that “at-risk” individuals, especially those already diagnosed with HD, demonstrated increased movement times with low advance information (condition 1). Farrow et al. (2006) also demonstrated that probability of 5-year diagnosis was associated with increased movement times only for conditions 1 and 2. We showed that although the high CAP group demonstrated the strongest effect for slopes, this effect was consistent across all three conditions, whereas in contrast, error levels remained relatively stable across follow-up sessions. Therefore, although the movement sequencing task was sensitive overall, the type of advance information did not appear to be differentially sensitive in the high CAP group.
Of particular interest is the disease gradient for slope, characterized by the mean initiation times declining faster as CAP group increased across all conditions. For condition 3, the low CAP group slope was higher than controls (less negative). However, the medium and high CAP groups had a less steep slope that was not consistent for conditions 1 and 2. One possible explanation for the shorter initiation times in the high CAP group (across all conditions) is increased disinhibition during the prodromal HD phase. For example, the high CAP group may have a tendency to react first and think afterwards; in other words, to initiate the response context (lift off the current button) as soon as possible, but decide exactly what to do (which next button to press) “in flight” and during the initiation/preparation phase, therefore “hovering” in mid-air; this explanation supports the increased movement times in the high CAP group over the course of the study. Therefore the slowed movement times evident in the high CAP group (in the absence of IQ differences) may reflect a slowness in movement and/or a slowed thinking process, which may be more apparent in this group as they are approaching onset where subcortical changes are likely to be more significant. Previous studies have noted aberrant frontal disinhibition behaviours on the FrSBe in prodromal HD as they approach onset (Duff et al. 2010). Duff et al. suggest that prodromal individuals may require more effort to initiate an act, inhibit an unwanted response or solve particular problems. The results from this cued movement sequence task suggest that prodromal HD can engage in faster initiation times at the expense of execution times. The slow evolution of the disease may allow for the formation of adaptive compensation mechanisms. The high CAP group may be more likely to use a compensatory strategy as part of the initiation phase but then be unable to effectively access control attention processes required to translate the movement into execution.
During symptomatic HD a range of motor parameters become more notably impaired, including simple and complex (simultaneous and sequential) movements of both the arm and hand, as well as simple timed tasks such as finger dexterity, movement between two points, and walking (Garcia et al. 2002; Saft et al. 2006; Paulsen et al. 2008; Antoniades et al. 2010). Tapping rate (alternating tapping between two buttons) was studied in prodromal and symptomatic HD, although no significant longitudinal changes were reported (Antoniades et al. 2010). Three separate PREDICT-HD investigations reported a range of motor deficits in prodromal HD. Stout et al. (2011) showed that tests assessing psychomotor performance, emotion recognition and working memory were the most sensitive, and that speeded finger tapping and self-timed finger tapping showed the largest effect sizes in individuals near to diagnosis. Rowe et al. (2010) showed that precision of self-paced timing was significantly poorer in prodromal HD with increased proximity to onset. Harrington et al. (2012) also reported that the motor planning/speed factor (comprising a range of motor tasks) and a sensory-perceptual processing factor were the strongest unique predictors of time to diagnosis. Together with the present findings, cognitive-motor functioning appears to be an important measure of disease prognosis.
We systematically investigated the control of cued movement sequencing in one of the largest samples of prodromal HD individuals from the PREDICT-HD study. Notably we reported a disease gradient for intercepts, which increased as the CAP group increased. There was also a disease gradient for slopes, but the pattern was more complex. Initiation time decreased as CAP group increased, but movement time increased. The increased movement times for the high CAP group across all conditions may suggest a failure to execute sequential information, subsequently resulting in problems in selecting appropriate movement parameters via the basal ganglia (e.g., extent, direction) to plan for new and upcoming situations or demands and/or slowness in thinking about which button to press in the sequence. The slow evolution of the disease may allow for the formation of adaptive compensation mechanisms whereby individuals approaching onset (high CAP group) may be more likely to use compensatory strategies when sequencing movements, evident by faster initiation times but with an accompanying inability to effectively access control attention processes required to translate the movement into actual execution. Our findings have important clinical implications with regard to possible links to functional impairment in daily activities, and demonstrate that progressive bradykinesia (but perhaps not akinesia) in those approaching HD onset is likely to manifest with more complex sequential movements that require ongoing monitoring and control. Future research should therefore incorporate a range of motor assessments addressing differential aspects of more complex movements, as functional markers more closely relating to aspects and activities of daily life.
Acknowledgments
This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (NS40068) and CHDI Foundation, Inc.
Footnotes
Statement of Interest
None to declare.
References
- Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115(5):1481–1495. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- Akaike H. Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory. Budapest: Akademiai Kiado; 1973. pp. 267–281. [Google Scholar]
- Anderson DR. Model based inference in the life sciences: A primer on evidence. New York: Springer; 2008. [Google Scholar]
- Andrich J, Saft C, Ostholt N, Muller T. Assessment of simple movements and progression of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2007;78(4):405–407. doi: 10.1136/jnnp.2006.105338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades C, Xu Z, Mason S, Carpenter R, Barker R. Huntington’s disease: changes in saccades and hand-tapping over 3 years. J Neurol. 2010;257(11):1890–1898. doi: 10.1007/s00415-010-5632-2. [DOI] [PubMed] [Google Scholar]
- Aylward EHP, Sparks BFB, Field KMB, Yallapragada VB, Shpritz BDO, Rosenblatt AM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- Bates DM. Fitting linear mixed models in R. R News. 2005;5:27–30. [Google Scholar]
- Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI–II) San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- Beglinger LJ, O’Rourke JF, Wang C, Langbehn DR, Duff K, Paulsen JS. Earliest functional declines in Huntington disease. Psychiatry Res. 2010;178(2):414–418. doi: 10.1016/j.psychres.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker H, Dagher A, Ceballos-Baumann AO, Passingham RE, Samuel M, Friston KJ, et al. Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: investigations with H2 15O PET. J Neurophysiol. 1998;79(2):1070–1080. doi: 10.1152/jn.1998.79.2.1070. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Phillips JG, Dennis C, Mattingley JB, Andrewes D, Chiu E. Initiation and execution of movement sequences in those suffering from and at-risk of developing Huntington’s disease. J Clin Exp Neuropsychol. 1992;14(2):179–192. doi: 10.1080/01688639208402822. [DOI] [PubMed] [Google Scholar]
- Brotchie P, Iansek R, Horne MK. Motor function of the monkey globus pallidus. Brain. 1991;114(4):1685–1702. doi: 10.1093/brain/114.4.1685. [DOI] [PubMed] [Google Scholar]
- Campodonico JR, Codori AM, Brandt J. Neuropsychological stability over two years in asymptomatic carriers of the Huntington’s disease mutation. J Neurol Neurosurg Psychiatry. 1996;61(6):621–624. doi: 10.1136/jnnp.61.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47(2):215–222. [PubMed] [Google Scholar]
- Del Ser T, Gonzalez-Montalvo JI, Martinez-Espinosa S, Delgado-Villapalos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain Cogn. 1997;33(3):343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21(4):478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Wang C, Stout JC, et al. “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci. 2010;22(2):196–207. doi: 10.1176/appi.neuropsych.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinger CL, Harrington DL, Rao SM. From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. NeuroImage. 2006;31(3):1177–1187. doi: 10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Farrow M, Chua P, Churchyard A, Bradshaw JL, Chiu E, Georgiou-Karistianis N. Proximity to clinical onset influences motor and cognitive performance in presymptomatic Huntington disease gene carriers. Cogn Behav Neurol. 2006;19(4):208–216. doi: 10.1097/01.wnn.0000213914.64772.b6. [DOI] [PubMed] [Google Scholar]
- Feigin A, Ghilardi M-F, Huang C, Ma Y, Carbon M, Guttman M, et al. Preclinical Huntington’s disease: compensatory brain responses during learning. Ann Neurol. 2006;59(1):53–59. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Vanhoutte P, Pages C, Besson M-J, Brouillet E, Caboche J. The mitochondrial toxin 3-nitropropionic acid induces striatal neurodegeneration via a c-jun N-terminal kinase/c-jun module. J Neurosc. 2002;22(6):2174–2184. doi: 10.1523/JNEUROSCI.22-06-02174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Phillips JG, Bradshaw JA, Chiu E. Advance information and movement sequencing in Gilles de la Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1995;58(2):184–191. doi: 10.1136/jnnp.58.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of internal cues in the pathogenesis of Parkinsonian hypokinesia. Brain. 1993;116(6):1575–1587. doi: 10.1093/brain/116.6.1575. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Silvestri G, Feigin A, Mattis P, Zgaljardic D, Moisello C, et al. Implicit and explicit aspects of sequence learning in pre-symptomatic Huntington’s disease. Parkinsonism Rel Disord. 2008;14(6):457–464. doi: 10.1016/j.parkreldis.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani B, Berent S, Boivin MJ, Penney JB, Jr, Lehtinen S, Markel D, et al. Longitudinal neuropsychological and genetic linkage analysis of persons at risk for Huntington’s disease. Arch Neurol. 1995;52(1):59–64. doi: 10.1001/archneur.1995.00540250063014. [DOI] [PubMed] [Google Scholar]
- Girotti F, Marano R, Soliveri P, Geminiani G, Scigliano G. Relationship between motor and cognitive disorders in Huntington’s disease. J Neurol. 1988;235(8):454–457. doi: 10.1007/BF00314246. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Heaton RK, Palmer BW, Taylor MJ, Jeste DV. Use of oral reading to estimate premorbid intellectual and neuropsychological functioning. J Int Neuropsychol Soc. 1999;5(3):247–254. doi: 10.1017/s1355617799533079. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Smith MM, Zhang Y, Carlozzi NE, Paulsen JS Predict-HD Investigators of the Huntington Study Group. Cognitive domains that predict time to diagnosis in prodromal Huntington disease. J Neurol Neurosurg Psychiatry. 2012;83(6):612–619. doi: 10.1136/jnnp-2011-301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefter H, Homberg V, Lange HW, Freund H-J. Impairment of rapid movement in Huntington’s disease. Brain. 1987;110(3):585–612. doi: 10.1093/brain/110.3.585. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Paulsen JS, Hoffmann RG, Reynolds NC, Zimbelman JL, Rao SM. Motor timing variability increases in preclinical Huntington’s disease patients as estimated onset of motor symptoms approaches. J Int Neuropsychol Soc. 2007;13(3):539–543. doi: 10.1017/S1355617707070671. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Kirkwood SC, Siemers E, Hodes ME, Conneally PM, Christian JC, Foroud T. Subtle changes among presymptomatic carriers of the Huntington’s disease gene. J Neurol Neurosurg Psychiatry. 2000;69(6):773–779. doi: 10.1136/jnnp.69.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood SC, Siemers E, Stout JC, Hodes ME, Conneally PM, Christian JC, et al. Longitudinal cognitive and motor changes among presymptomatic Huntington disease gene carriers. Arch Neurol. 1999;56(5):563–568. doi: 10.1001/archneur.56.5.563. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Bardinet E, Tremblay L, Van de Moortele P-F, Pochon J-B, Dormont D, et al. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cerebr Cortex. 2006;16(2):149–161. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- Lemiere J, Decruyenaere M, Evers-Kiebooms G, Vandenbussche E, Dom R. Longitudinal study evaluating neuropsychological changes in so-called asymptomatic carriers of the Huntington’s disease mutation after 1 year. Acta Neurol Scand. 2002;106(3):131–141. doi: 10.1034/j.1600-0404.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- Lemiere J, Decruyenaere M, Evers-Kiebooms G, Vandenbussche E, Dom R. Cognitive changes in patients with Huntington’s disease (HD) and asymptomatic carriers of the HD mutation – a longitudinal follow-up study. J Neurol. 2004;251(8):935–942. doi: 10.1007/s00415-004-0461-9. [DOI] [PubMed] [Google Scholar]
- Little RJ, Rubin DB. Statistical analysis with missing data. 2. New York: John Wiley; 2002. [Google Scholar]
- Long JD. Longitudinal data analysis for the behavioral sciences using R. Thousand Oaks, CA: Sage Publications; 2012. [Google Scholar]
- Maroof DA, Gross AL, Brandt J. Modeling longitudinal change in motor and cognitive processing speed in presymptomatic Huntington’s disease. J Clin Exp Neuropsychol. 2011;33(8):901–909. doi: 10.1080/13803395.2011.574606. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J. The National Adult Reading Test (NART): Test manual. 2. Windsor, UK: NFER Nelson; 1991. [Google Scholar]
- Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, et al. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63(6):883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB, Jr, Young AB, Shoulson I, Starosta-Rubenstein S, Snodgrass SR, Sanchez-Ramos J, et al. Huntington’s disease in Venezuela: 7 years of follow-up on symptomatic and asymptomatic individuals. Mov Disord. 1990;5(2):93–99. doi: 10.1002/mds.870050202. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Rogers MA, Bradshaw JL, Phillips JG, Chiu E, Mileshkin C, Vaddadi K. Mental rotation in unipolar major depression. J Clin Exp Neuropsychol. 2002;24(1):101–106. doi: 10.1076/jcen.24.1.101.974. [DOI] [PubMed] [Google Scholar]
- Rowe KC, Paulsen JS, Langbehn DR, Duff K, Beglinger LJ, Wang C, et al. Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology. 2010;24(4):435–442. doi: 10.1037/a0018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saft C, Andrich J, Meisel N-M, Przuntek H, Müller T. Assessment of simple movements reflects impairment in Huntington’s disease. Mov Disord. 2006;21(8):1208–1212. doi: 10.1002/mds.20939. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Metzler P. Wortschatztest (WST) Weinheim: Beltz Verlag; 1992. [Google Scholar]
- Smith M, Brandt J, Shadmehr R. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature. 2000;403(6769):544–549. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Craufurd D, Thompson J, Neary D. Psychomotor, executive, and memory function in preclinical Huntington’s disease. J Clin Exp Neuropsychol: Off J Int Neuropsychol Soc. 2002;24(2):133–145. doi: 10.1076/jcen.24.2.133.998. [DOI] [PubMed] [Google Scholar]
- Solomon AC, Stout JC, Weaver M, Queller S, Tomusk A, Whitlock KB, et al. Ten-year rate of longitudinal change in neurocognitive and motor function in prediagnosis Huntington disease. Mov Disord. 2008;23(13):1830–1836. doi: 10.1002/mds.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, et al. Neurocognitive signs in prodromal huntington disease. Neuropsychology. 2011;25(1):1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Kurata K. Contrasting neuronal activity in supplementary and precentral motor cortex of monkeys. I. Responses to instructions determining motor responses to forthcoming signals of different modalities. J Neurophysiol. 1985;53(1):129–141. doi: 10.1152/jn.1985.53.1.129. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Reilmann R, Roos RAC, Durr A, Leavitt B, Owen G, et al. Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 2012;11:42–53. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, et al. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain. 2002;125(8):1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000. [Google Scholar]
- Witjes-Ane M-NW, Mertens B, van Vugt JPP, Bachoud-Levi A-C, van Ommen G-JB, et al. Longitudinal evaluation of “presymptomatic” carriers of Huntington’s disease. J Neuropsychiatry Clin Neurosci. 2007;19(3):310–317. doi: 10.1176/jnp.2007.19.3.310. [DOI] [PubMed] [Google Scholar]
- Yágüez L, Lange H, Hömberg V. Differential effect of Huntington’s and Parkinson’s diseases in programming motor sequences of varied lengths. J Neurol. 2006;253(2):186–193. doi: 10.1007/s00415-005-0951-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS, et al. Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B: Neuropsychiatric Genet. 2011;156(7):751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]